Abstract
Objective
Depression and anxiety and are associated with cognitive deficits and brain changes, especially in older adults. Despite the frequent co-occurrence of these conditions, cognitive neuroscience studies examining comorbid depression and anxiety are limited. The goal of the present study was to examine the unique and combined effect of depressive and anxiety symptoms on cognitive and brain functioning in young and older adults.
Methods
Seventy-one healthy, community-dwelling adults between the ages of 18 and 81 were administered a neuropsychological battery and completed the Center for Epidemiologic Studies Depression Scale (CES-D) and the trait form of the State-Trait Anxiety Inventory (STAI-T). A subset of 25 participants also underwent functional magnetic resonance imaging (fMRI) scanning while completing the n-back working memory task.
Results
Total depressive symptoms, depressed mood symptoms, and somatic symptoms were associated with deficits in speed, working memory and executive functions, especially in older adults. Symptoms of lack of well-being were not associated with any neuropsychological test. Anxiety was associated with better attention and working memory. Moreover, anxiety modified the relationship between depressive symptoms and executive functioning in older adults, as elevated depressive symptoms were associated with worse performance at low levels of anxiety, but not at higher anxiety levels. Similarly, analysis of fMRI data showed that total depressive symptoms and depressed mood symptoms were associated with decreased activity in the superior frontal gyrus at low anxiety levels, but not at high anxiety levels.
Conclusion
Results confirm previous reports that subthreshold depression and anxiety impact cognitive and brain functioning and suggest that the interaction of depression and anxiety results in distinct cognitive and brain changes. Findings highlight the importance of assessing and controlling for symptoms of depression and anxiety in research studies of either condition.
INTRODUCTION
The frequent co-occurrence of depression and anxiety has long been recognized. As many as 40–50% of patients with major depression have comorbid anxiety disorders [1–5]. Evidence suggests that the combination of depression and anxiety leads to worse outcomes and response to treatment compared to either disorder alone [6–11], highlighting the importance of research that examines the interactive effect of depression and anxiety on the broad spectrum of possible outcomes. This may be particularly pertinent for older adults, as research has shown that in many cases, the adverse impact of depression and anxiety is greater at older ages [12–14]. Moreover, the co-occurrence of depression and anxiety may be even higher in older adults. According to a recent report, anxiety symptoms are present in 67% of older adults with subthreshold depression and 87% of those with clinical depression [15].
Both depression and anxiety are associated with cognitive deficits and changes in brain structure and function. Numerous studies have documented reduced cognitive functioning in major depression compared to controls, as well as a linear relationship between higher depressive symptoms, even at a subthreshold level, and lower cognitive functioning [16–21]. Deficits are most consistently seen on tasks of episodic memory, working memory, attention, and executive functioning, and are often seen exclusively or disproportionately in older adults compared to young adults [13, 14]. Corresponding with these cognitive changes are findings from the neuroimaging literature that document depression-related structural and functional brain changes in a network of frontolimbic regions, which underlie performance on memory, attention, and executive tasks. Findings include reduced regional brain volumes, altered functional activity, and increases in white matter lesions [22–26].
Findings on the relationship between anxiety and cognitive performance are mixed [27–33]. Investigations comparing patients diagnosed with anxiety disorders, such as obsessive-compulsive disorder, generalized anxiety disorder, and post-traumatic stress disorder typically find anxiety-related attentional biases, executive dysfunction, and memory deficits [34, 35]. In contrast, although some studies have reported a linear relationship between subthreshold anxiety and cognition in older adults [32, 36, 37], many studies in young and older adults show an inverted U-shaped function, such that an intermediate level of anxiety symptoms is associated with optimal cognitive performance, while low and high severity are related to worse functioning [16, 38, 39]. Neuroimaging studies of clinical and subthreshold anxiety suggest that increased anxiety is associated with decreased volumes in the hippocampus and other temporal regions [40, 41], heightened amygdala and insular activity and reduced prefrontal and temporal activity [42–47].
Despite the frequent comorbidity of depression and anxiety, few studies have examined the unique and interactive effect of the two on cognitive and brain functioning. There is evidence that mixed depression and anxiety in late life is associated with worse visual memory compared to controls [48], and a longitudinal study [49] found greater decline in memory over 4 years in anxious depression compared to non-anxious depression. In another study, general cognitive status was better predicted by combined depression and anxiety than by either variable alone [37]. Similarly, studies in young adults show memory deficits in mixed depression and anxiety compared to controls [50, 51], as well as poor verbal fluency, speed, and executive functioning in depression with comorbid anxiety compared to non-anxious depression [52]. Functional imaging studies in older adults have found that compared to depression without anxiety, mixed depression and anxiety is associated with great activation in the right hemisphere dorsal anterior cingulate cortex (ACC), posterior cingulate, and precentral gyrus during an executive task, and with lower default mode network activation in the rostral ACC, medial prefrontal cortex (PFC), and orbitofrontal cortex [53, 54]. This parallels functional studies in young adults during emotion processing tasks, which have documented increased activation in middle frontal regions and the insula as a function of heightened anxiety in mixed major depression/generalized anxiety disorder patients [55], as well as reduced activity in the hippocampus [40].
The goal of the present study was to add to the limited cognitive neuroscience research on the combined effect of depression and anxiety in late life. In a sample of young and older adults, we examined the unique and combined effect of subthreshold symptoms of depression and anxiety on neuropsychological test performance and brain activity measured with functional magnetic resonance imaging (fMRI) during a working memory task. We also examined the impact of age on the relationships of depressive and anxiety symptoms with cognitive and brain functioning. We predicted that depressive symptoms would have a linear relationship with deficits in memory, attention, and executive functions, and that this relationship would be modified by symptoms of anxiety. For the fMRI data, based on previous findings of heightened frontal activity during working memory tasks in depression [56, 57], we hypothesized that depressive symptoms would be associated with hyperactivity in frontal brain regions, and this effect would be accentuated in individuals with high symptoms of anxiety.
METHODS
Participants
The cognitive sample included 71 healthy, community dwelling adults between the ages of 18 and 81; the neuroimaging sample included a subset of 25 adults between the ages of 18 and 75 who were enrolled after the neuroimaging substudy began, had no MRI contraindications, and were interested in participating in the fMRI portion of the study (Figure 1). Participants were recruited from the University of Florida and the surrounding area via the Claude D. Pepper Registry for Research, local flyer and newspaper advertisements, and public service announcements. All participants were right-handed, native English speakers with normal or corrected-to-normal vision, and at least 9 years of education. Exclusionary criteria included history of major medical or neurological illness, head trauma, learning disorder, current antiepileptic or antipsychotic use, and language comprehension difficulties. Participants were given the mood disorders, psychotic screener, substance use disorders, and anxiety disorders modules of the Structured Clinical Interview for DSM-IV Axis I Disorders [SCID-IV; 58] to assess the presence of major psychopathology, other than unipolar depression or anxiety disorders, that would be an exclusionary criteria. All subjects scored higher than 30 on the Telephone Interview for Cognitive Status [TICS; 59], which has 94% sensitivity and 100% specificity for differentiating demented and cognitively intact individuals at this cutoff score. Participants who underwent fMRI met standard magnetic resonance imaging (MRI) safety criteria. All participants gave written and verbal consent to participate in the study, and the protocol was approved by the University of Florida Health Science Center Institutional Review Board. Sample demographic data are presented in Table 1.
Figure 1.
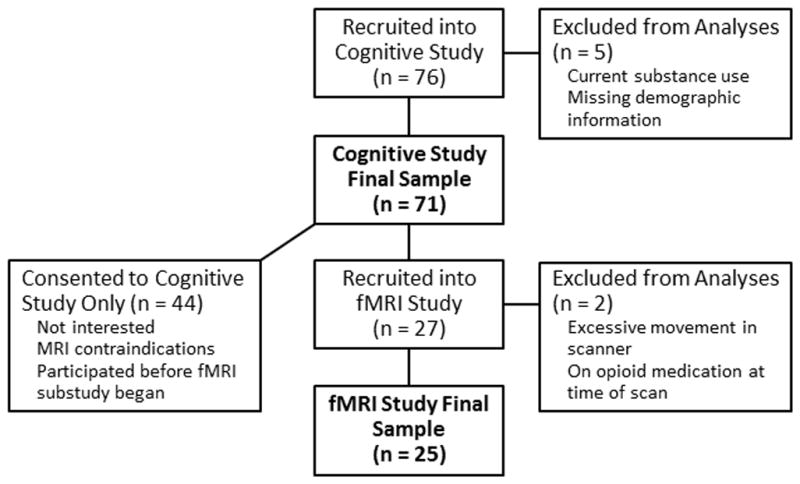
Flow chart of participant enrollment for the cognitive and neuroimaging samples.
Table 1.
Sample characteristics
| Cognitive Sample | fMRI Sample | |
|---|---|---|
| Age (years) | 38.3 (±22.4) | 48.2 (±21.0) |
| Education (years) | 14.8 (±2.0) | 15.2 (±2.2) |
| Gender (male/female) | 21/50 | 9/16 |
| Race (white/non-white) | 60/11 | 20/5 |
| CES-D Total Score | 10.6 (±11.0) | 8.5 (±11.1) |
| Depressed Mood Subscale Score | 3.2 (±4.3) | 2.4 (±3.9) |
| Somatic Symptoms Subscale Score | 4.0 (±3.3) | 3.4 (±3.5) |
| Lack of Well-being Subscale Score | 2.8 (±3.2) | 2.3 (±3.4) |
| STAI-Trait Total Score | 37.9 (±12.7) | 36.7 (±12.6) |
Note: The Lack of Well Being and Total CES-D scores were missing for one participant. CES-D = Center for Epidemiologic Studies Depression Scale; STAI = State-Trait Anxiety Inventory
Measures
Symptoms of depression were assessed with the Center for Epidemiologic Studies Depression Scale [CES-D; 60], a 20-item self-report measure of the frequency and severity of depressive symptoms experienced in the past week. This widely-used measure has been validated in both young and older community-dwelling adults [61, 62] and has a well-replicated factor structure that includes subscales for depressed mood, somatic symptoms, lack of well-being, and interpersonal symptoms [63]. Statistical analyses used continuous measures of CES-D total and subscale scores. The interpersonal symptoms scale was not analyzed due to the restricted range of the scale, which includes only 2 items.
The State-Trait Anxiety Inventory [STAI; 64] was administered to measure symptoms of anxiety. The STAI is a 40-item questionnaire that measures situational (i.e., at the time of testing) and dispositional symptoms of anxiety, and yields separate scores for state and trait anxiety, respectively. Only the trait score (STAI-T) was used in the current analyses due to missing state scores at the time of fMRI scanning for some participants.
Participants were administered a neuropsychological battery that focused on measures of episodic and working memory, attention, and executive functions, which are known to be impacted by depression. Measures of episodic memory included the immediate recall and delayed recall scores from the Hopkins Verbal Learning Test, Revised [HVLT-R; 65] and Brief Visual Memory Test, Revised [BVMT-R; 66]. Attention, working memory, and executive functions were measured by the total scores on the Digit Span and Letter-Number Sequencing (LNS) subtests of the Wechsler Adult Intelligence Scale, Third Edition [67], and time to completion on the Trailmaking Test [68] parts A (TMT-A) and B (TMT-B).
Neuroimaging Procedure
During fMRI scanning, participants completed a letter variant version of the n-back task [69]. Participants were shown individual letters and were asked to indicate whether the stimuli were targets (33% of trials) or non-targets by pressing one of two buttons. Four separate conditions were presented: 0-back (target stimulus is the letter “X”), 1-back (target was any letter that was the same as the previous letter), 2-back (target was any letter that matched the letter two back from itself), and 3-back (target was any letter that matched the letter three back from itself). This paradigm allows for working memory to be examined over increasingly difficult trial conditions while maintaining consistency in all other stimulus processing and response demands. Each letter was presented for 750 ms with a fixed inter-stimulus interval of 3250 ms. Prior to each task block, instructions were read aloud to the participant. A fixation cross (“+”) presented for 16000 ms preceded the onset of the first letter in each block. Four blocks of 32 trials were presented in each condition and the order of the conditions was pseudo-randomized across participants. Stimuli were presented using an Integrated Functional Imaging System hardware system (IFIS; Psychology Software Tools, Pittsburgh, PA) and a LCD screen viewed by participants via reflective mirrors positioned at the back of the magnet bore. Subject responses were made via an MRI-compatible keypad. Both accuracy and response latency were recorded by the IFIS system. Prior to entering the fMRI scanner, all participants practiced one block of each task condition (0- through 3-back) to ensure that they fully understood the task requirements. If accuracy was below 80 percent on any practice block, the block was repeated.
Image Acquisition and Preprocessing
MRI data was collected using a Philips 3-Tesla scanner at the University of Florida’s McKnight Brain Institute. Participants lay supine on the scanner bed and a standard 8-channel head radio-frequency coil was placed over their head. Foam pads were used to minimize head motion. Functional MR images were acquired in 35 axial slices with approximately 30° rotation above the anterior commissure–posterior commissure (AC–PC) line using a T2*-weighted echo planar imaging (EPI) pulse sequence [repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle (FA) = 90°; field of view (FOV) = 24 cm; 64 × 64 voxels at 3.75 mm3 with .4 mm slice gap]. Seventy-four volumes were acquired per run. Prior to acquiring functional data, a T1-weighted Magnetization Prepared Rapid Gradient Echo (MP-RAGE) high-resolution 3D anatomical scan (170 1-mm thick slices; TR = 2000 ms; TE = 4.13 ms; FA = 8°; FOV = 24 cm; 256 × 256 voxels at 3.75 mm3) was performed to enable standardization of brain space on which to overlay functional data (Talairach and Tournoux, 1988).
Preprocessing and statistical analysis of MRI data was performed using BrainVoyager software (BVQX 2.4.0.2; Brain Innovation, Maastricht, the Netherlands). Functional data were motion-corrected and spatially smoothed with a Gaussian kernel (8 mm full width half-maximum [FWHM]), followed by low-frequency non-linear drifts removal with a temporal high-pass filter of frequencies below 3 cycles per time course. Functional images were aligned with their respective high resolution 3D anatomical volumes using automatic standard BVQX co-registration procedures; manual alignment was subsequently conducted to ensure accurate co-registration. The 3D scans were transformed into Talairach space [70] to allow for group averaging.
Data Analysis
Cognitive data analysis
Cognitive data were analyzed using the PROC GLM procedure in SAS version 9.2 (SAS Institute, Cary, NC). Models included continuous measures of CES-D score, STAI trait score, and age as independent variables and race (white/non-white), years of education and sex as covariates. Independent variables were centered around the variable mean. For graphing purposes, an age cutoff of 50 was used for creating young adult and older adult groups. Separate models were performed for each of the aforementioned cognitive measures and for error rates and reaction times (RTs) excluding non-responses on the n-back task.
fMRI data analysis
Whole brain voxel-wise statistical tests and follow-up contrasts on signal intensity in identified regions of interest (ROI) were examined using a two-step general linear modeling (GLM) approach [71]. First, an individual fixed-effects GLM was determined for every participant for each task. The GLMs utilized four predictors: 0-back, 1-back, 2-back, and 3-back. Subsequently, separate-subject predictors random-effects GLM analyses were performed to determine main effects for the task (z-transformed time-course, in-brain functional image intensity threshold = 300). Hemodynamic response for each event was estimated by convolving each regressor with a standard two-gamma function (onset = 0, response undershoot ratio = 6, time to response peak = 5 sec, time to undershoot peak = 15 sec, response dispersion = 1, undershoot dispersion = 1) [72]. This shifted the predicted hemodynamic response approximately 5 sec to account for expected delay. Parameter estimates (β) were generated, which indicated relative strength of covariance between the data and the hemodynamic response function (HRF). We used a 3D spatial contiguity threshold of 20 voxels and a statistical significance threshold of p ≤ .005 to decrease the likelihood of spurious findings. Localization of suprathreshold clusters of brain activity was verified using the “nearest gray matter” search function provided by Talairach Daemon software [73; http://www.talairach.org].
To examine whether anxiety and depressive symptoms impacted the observed working-memory related brain activity, we conducted ROI GLMs over contiguous voxels centering on the areas of activation identified in the whole-brain analysis, from which beta weights were obtained. GLMs that paralleled the cognitive data analyses were conducted on the beta weights for each region.
RESULTS
Cognitive Results
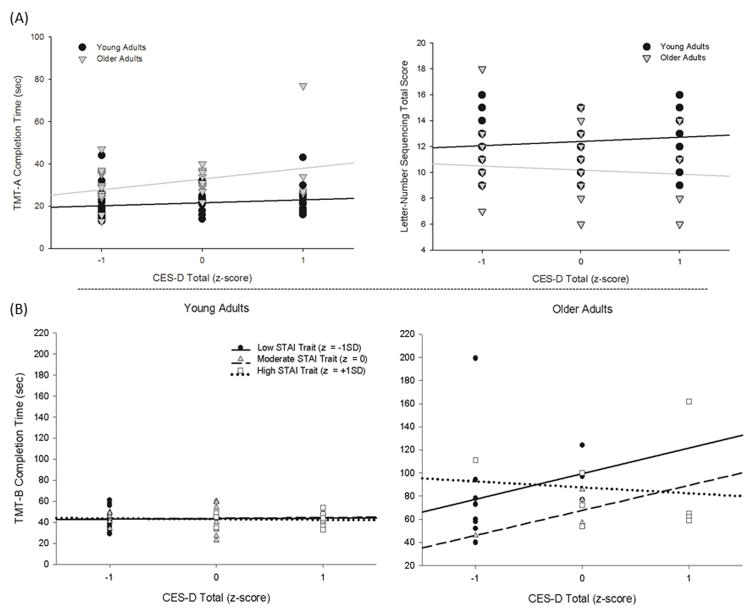
Mean performance data for the cognitive tests are presented in Table 2; results of the cognitive data analyses are summarized in Table 3. An age × CES-D effect was observed for TMT-A [F(1,59) = 4.61, p =0.04] and LNS [F(1,59) = 6.19, p = 0.02] (Figure 2a). For both measures, higher CES-D scores were associated with worse performance in older adults, but with stable or better performance in young adults. Performance on Digit Span improved as a function of higher STAI-T scores, but this effect was greater in older adults [age × STAI-T; F(1, 59) = 4.79, p = 0.03] (Figure 3). Similarly, older adults with higher STAI-T scores performed better on LNS, although young adults did not show this association [age × STAI-T; F(1,59) = 6.90, p = 0.01] (Figure 3). We observed an age × CES-D × STAI-T effect for TMT-B [F(1,58)= 4.83, p=0.03] (Figure 2b). In older adults, higher CES-D scores were associated with worse performance only in those with lower STAI-T scores, while depressive and anxiety symptoms were not associated with TMT-B scores in young adults.
Table 2.
Descriptive statistics for cognitive measures
| Test | Mean (SD) |
|---|---|
| HVLT-R Immediate Recall | 27.66 (4.13) |
| HVLT-R Delayed Recall | 9.68 (1.90) |
| BVMT-R Immediate Recall | 28.39 (5.64) |
| BVMT-R Delayed Recall | 10.68 (1.69) |
| TMT-A (seconds) | 25.28 (10.09) |
| TMT-B (seconds) | 57.63 (29.43) |
| Digit Span (total score) | 19.10 (3.91) |
| LNS (total score) | 11.54 (2.63) |
| N-back Error Rate | .06 (.05) |
| N-back Reaction Time (sec) | 714.01 (147.37) |
Note: N-back performance data were missing for two participants. HVLT-R = Hopkins Verbal Learning Test, Revised; BVMT-R = Brief Visual Memory Test, Revised; TMT = Trail Making Test; LNS = Letter-Number Sequencing.
Table 3.
Regression Coefficients (standard error) in the Neuropsychological Test Analyses
| TMT-A | TMT-B | BVMT-R immediate recall | BVMT-R delayed recall | HVLT-R immediate recall | HVLT-R delayed recall | Digit Span | LNS | |
|---|---|---|---|---|---|---|---|---|
| CES-D Total Score model | ||||||||
| Sex | 3.616 (2.310) | −0.750 (6.164) | −0.816 (1.441) | −0.236 (0.451) | −3.549 (1.043)** | −1.187 (0.524)* | −0.693 (1.029) | −1.371 (0.628)* |
| Race | 2.487 (2.828) | 3.215 (7.544) | −0.902 (1.765) | 0.196 (0.552) | −2.049 (1.277) | −0.884 (0.642) | −1.612 (1.259) | −1.179 (0.769) |
| Education | −0.594 (0.540) | −0.197 (1.447) | 0.375 (0.337) | 0.087 (0.105) | 0.276 (0.244) | 0.110 (0.123) | 0.556 (0.240)* | 0.389 (0.147)* |
| Age | 0.347 (0.068)** | 1.210 (0.180)** | −0.141 (0.042)** | −0.025 (0.013) | −0.106 (0.031)** | −0.035 (0.015)* | −0.060 (0.030) | −0.089 (0.018)** |
| STAI-Trait | 0.152 (0.292) | 0.138 (0.786) | −0.065 (0.182) | 0.030 (0.057) | 0.172 (0.132) | 0.093 (0.066) | −0.186 (0.130) | −0.133 (0.079) |
| Age x STAI-Trait | −0.007 (0.006) | −0.010 (0.016) | 0.001 (0.004) | −0.001 (0.001) | −0.001 (0.003) | −0.002 (0.001) | 0.006 (0.003)* | 0.004 (0.002)* |
| CES-D | −0.277 (0.328) | −0.932 (0.872) | −0.089 (0.205) | −0.079 (0.064) | −0.102 (0.148) | −0.074 (0.074) | 0.167 (0.146) | 0.137 (0.089) |
| Age x CES-D | 0.019 (0.009)* | 0.046 (0.024) | 0.001 (0.006) | 0.002 (0.002) | −0.001 (0.004) | 0.002 (0.002) | −0.005 (0.004) | −0.006 )0.002)* |
| STAI-Trait x CES-D | 0.003 (0.014) | 0.050 (0.037) | 0.006 (0.009) | 0.003 (0.003) | −0.010 (0.006) | −0.004 (0.003) | 0.002 (0.006) | −0.002 (0.004) |
| Age x STAI-Trait x CES-D | −0.000 (0.000) | −0.002 (0.001)* | −0.000 (0.000) | −0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) |
|
| ||||||||
| Depressed Mood model | ||||||||
| Sex | 2.804 (2.244) | −1.414 (5.685) | −0.490 (1.362) | −0.189 (0.424) | −2.953 (1.030)** | −0.982 (0.504) | −0.675 (0.987) | −1.144 (0.627) |
| Race | 3.267 (2.853) | 5.673 (7.225) | −1.173 (1.732) | 0.127 (0.539) | −2.362 (1.310) | −0.992 (0.641) | −1.721 (1.256) | −1.096 (0.797) |
| Education | −0.519 (0.532) | −0.188 (1.355) | 0.344 (0.323) | 0.087 (0.101) | 0.162 (0.244) | 0.067 (0.120) | 0.563 (0.234)* | 0.359 (0.149)* |
| Age | 0.280 (0.064)** | 1.237 (0.161)** | −0.156 (0.039)** | −0.036 (0.012)** | −0.078 (0.029)** | −0.034 (0.014)* | −0.050 (0.028) | −0.070 (0.018)** |
| STAI- Trait | 0.165 (0.251) | 0.274 (0.642) | −0.092 (0.152) | 0.004 (0.047) | 0.162 (0.115) | 0.090 (0.056) | −0.139 (0.111) | −.100 (0.070) |
| Age x STAI-Trait | −0.005 (0.005) | −0.016 (0.0138) | 0.003 (0.003) | 0.001 (0.001) | −0.001 (0.003) | −0.002 (0.001) | 0.005 (0.002)* | 0.003 (0.002)* |
| Mood | −0.448 (0.720) | −3.357 (1.815) | −0.001 (0.437) | −0.050 (0.136) | −0.364 (0.330) | −0.175 (0.162) | 0.309 (0.317) | 0.188 (0.201) |
| Age x Mood | 0.035 (0.022) | 0.166 (0.055)** | −0.012 (0.013) | −0.003 (0.004) | 0.004 (0.010) | 0.002 (0.005) | −0.011 (0.010) | −0.010 (0.006) |
| STAI-Trait x Mood | −0.022 (0.037) | 0.170 (0.095) | 0.005 (0.023) | 0.003 (0.007) | −0.016 (0.017) | −0.011 (0.008) | 0.007 (0.016) | 0.001 (0.010) |
| Age x STAI-Trait x Mood | 0.000 (0.001) | −0.008 (0.003)** | 0.000 (0.001) | 0.000 (0.000) | 0.000 (0.001) | 0.000 (0.000) | −0.000 (0.000) | 0.000 (0.000) |
|
| ||||||||
| Somatic Symptoms model | ||||||||
| Sex | 2.857 (2.375) | −1.343 (6.018) | −0.663 (1.372) | −0.295 (0.417) | −2.950 (1.066)** | −1.032 (0.506)* | −0.505 (0.992) | −1.060 (0.620) |
| Race | 2.728 (2.975) | 2.994 (7.544) | −1.205 (1.719) | 0.102 (0.522) | −1.992 (1.335) | −0.910 (0.634) | −1.678 (1.243) | −1.217 (0.777) |
| Education | −0.368 (0.568) | 0.176 (1.444) | 0.401 (0.328) | 0.121 (0.100) | 0.172 (0.255) | 0.110 (0.121) | 0.503 (0.237)* | 0.305 (0.148)* |
| Age | 0.280 (0.058)** | 1.063 (0.146)** | −0.123 (0.033)** | −0.025 (0.010)* | −0.084 (0.026)** | −0.029 (0.012)* | −0.047 (0.024) | −0.070 (0.015)** |
| STAI-Trait | 0.085 (0.281) | −0.065 (0.721) | −0.053 (0.162) | 0.034 (0.049) | 0.104 (0.126) | 0.076 (0.060) | −0.183 (0.117) | −0.118 (0.073) |
| Age x STAI-Trait | −0.001 (0.005) | 0.002 (0.014) | −0.000 (0.003) | −0.001 (0.001) | −0.001 (0.002) | −0.002 (0.001) | 0.005 (0.002)* | 0.003 (0.001)* |
| Somatic | −0.375 (0.934) | −1.479 (2.368) | −0.480 (0.540) | −0.315 (0.164) | −0.106 (0.419) | −0.216 (0.199) | 0.512 (0.390) | 0.357 (0.244) |
| Age x Somatic | 0.022 (0.021) | 0.064 (0.053) | 0.018 (0.012) | 0.010 (0.004)* | 0.000 (0.009) | 0.008 (0.005) | −0.011 (0.009) | −0.011 (0.006)* |
| STAI-Trait x Somatic | −0.018 (0.048) | 0.101 (0.120) | 0.025 (0.028) | 0.010 (0.008) | −0.032 (0.021) | −0.013 (0.010) | 0.017 (0.020) | 0.001 (0.012) |
| Age x STAI-Trait x Somatic | 0.000 (0.000) | −0.005 (0.003) | −0.001 (0.001) | −0.000 (0.000) | 0.001 (0.000) | 0.000 (0.000) | −0.000 (0.000) | 0.000 (0.000) |
|
| ||||||||
| Lack of Well-being model | ||||||||
| Sex | 3.544 (2.436)** | −0.344 (6.526) | −0.747 (1.461) | −0.199 (0.465) | −3.482 (1.091)** | −1.146 (0.545)* | −0.867 (3.437)** | −1.383 (0.673)* |
| Race | 2.188 (2.949) | 4.294 (7.899) | −0.819 (1.769) | 0.194 (0.563) | −1.903 (1.321) | −0.819 (0.660) | −1.803 (1.265) | −1.265 (0.815) |
| Education | −0.714 (0.576) | −0.227 (1.552) | 0.396 (0.346) | 0.074 (0.110) | 0.284 (0.258) | 0.111 (0.129) | 0.588 (0.247)* | 0.401 (0.159)*0 |
| Age | 0.323 (0.071)** | 0.997 (0.189)** | −0.151 (0.042)** | −0.029 (0.014)* | −0.091 (0.032)** | −0.035 (0.016)* | −0.057 (0.030) | −0.072 (0.020)** |
| STAI-Trait | −0.006 (0.285) | −0.288 (0.771) | −0.149 (0.171) | −0.005 (0.054) | 0.120 (0.128) | 0.024 (0.064) | −0.113 (0.122) | −0.088 (0.079) |
| Age x STAI-Trait | −0.003 (0.006) | 0.014 (0.017) | 0.003 (0.004) | 0.000 (0.001) | −0.001 (0.003) | −0.001 (0.001) | 0.003 (0.003) | 0.002 (0.002) |
| Well-being | −0.214 (1.064) | 0.723 (2.840) | 0.117 (0.639) | −0.058 (0.203) | −0.352 (0.477) | −0.024 (0.238) | 0.152 (0.457) | 0.145 (0.294) |
| Age x Well-being | 0.035 (0.028) | −0.026 (0.075) | −0.007 (0.017) | 0.002 (0.005) | 0.006 (0.012) | 0.001 (0.006) | −0.001 (0.012) | −0.006 (0.008) |
| STAI-Trait x Well-being | 0.019 (0.060) | 0.027 (0.161) | 0.020 (0.036) | 0.009 (0.011) | −0.027 (0.027) | −0.008 (0.013) | 0.013 (0.026) | 0.003 (0.017) |
| Age x STAI-Trait x Well-being | −0.001 (0.001) | −0.002 (0.004) | −0.000 (0.001) | −0.000 (0.000) | 0.000 (0.001) | 0.000 (0.000) | 0.000 (0.001) | 0.000 (0.000) |
Note: STAI-Trait, CES-D, Mood, Somatic, and Well-being variables were all mean-centered prior to entry into the model. The TMT-B score was missing for one participant. The Lack of Well Being and Total CES-D scores were missing for another participant. CES-D = Center for Epidemiologic Studies Depression Scale; STAI-Trait = State-Trait Anxiety Inventory – Trait scale; TMT = Trail Making Test; BVMT-R = Brief Visuospatial Memory Test – Revised; HVLT-R = Hopkins Verbal Learning Test – Revised; LNS = Letter Number Sequencing. Women were coded as 0 and men as 1. Whites were coded as 0 and non-whites as 1.
indicates significance at p <.05.
indicates significance at p <.01.
Figure 2.
Significant results from the analysis of total CES-D and neuropsychological test performance. (A) Age × CES-D effects for TMT-A and Letter-Number Sequencing. (B) Age × CES-D × STAI Trait effect for TMT-B. Age (cutoff of 50 years), CES-D, and STAI scores are presented as groups for ease of display, but were used as continuous variables centered around the mean in all analyses. CES-D = Center for Epidemiologic Studies Depression Scale; STAI= State-Trait Anxiety Inventory; TMT = Trailmaking Test.
Figure 3.
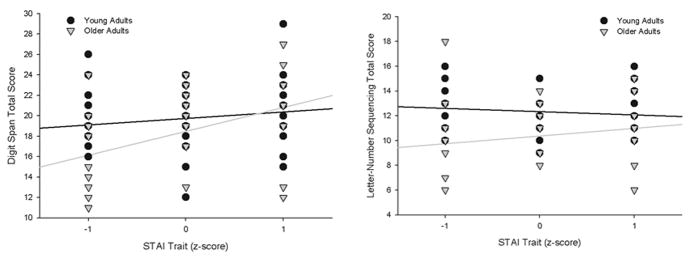
Significant anxiety effects on neuropsychological test performance. Age (cutoff of 50 years) and STAI scores are presented as groups for ease of display, but were used as continuous variables centered around the mean in all analyses. STAI= State-Trait Anxiety Inventory.
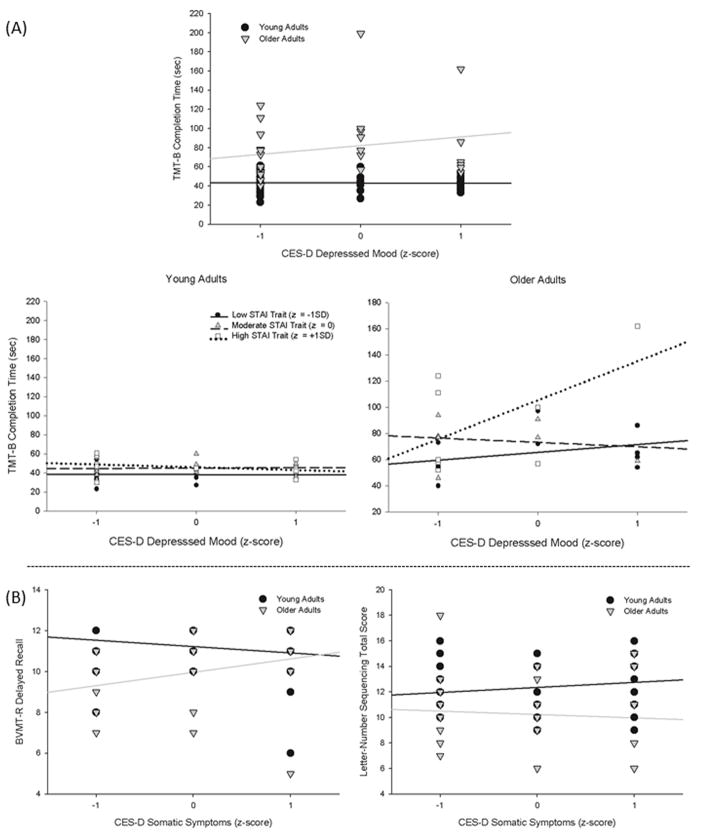
Analysis of CES-D subscales revealed parallel results for depressed mood effects on TMT-B [age × depressed mood [F(1,59) = 9.20, p = 0.00; age × depressed mood × STAI-T [F(1,59) = 7.97, p = 0.01] (Figure 4a). Age differences on LNS were greater in individuals with higher somatic symptoms [F (1,60)= 3.97, p=0.05], but were attenuated on BVMT delayed recall as a function of higher somatic symptoms [F(1,60)= 6.56, p=0.01] (Figure 4b).
Figure 4.
Significant results from the analysis of the CES-D subscale scores and neuropsychological test performance. (A) Age × Mood and Age × Mood × STAI Trait effects for TMT-B. (B) Age × Somatic Symptoms effect for the BVMT-R and Letter-Number Sequencing. Age (cutoff of 50 years), CES-D, and STAI scores are presented as groups for ease of display, but were used as continuous variables centered around the mean in all analyses. CES-D = Center for Epidemiologic Studies Depression Scale; STAI= State-Trait Anxiety Inventory; TMT = Trailmaking Test; BVMT-R = Brief Visual Memory Test, Revised.
fMRI Results
Analysis of activation associated with increasing working memory load on the n-back task revealed increased activity in a cluster in the frontal lobes that included the superior frontal gyrus (SFG; Brodmann area [BA] 10), prefrontal cortex (BA 9), and inferior frontal gyrus (BA 44), with peak activity in the right SFG, and in the left insula (BA 13) extending into the putamen (Figure 5a and 5b). Peak t-values and localization for all observed separable clusters of working memory activation are provided in Table 4.
Figure 5.
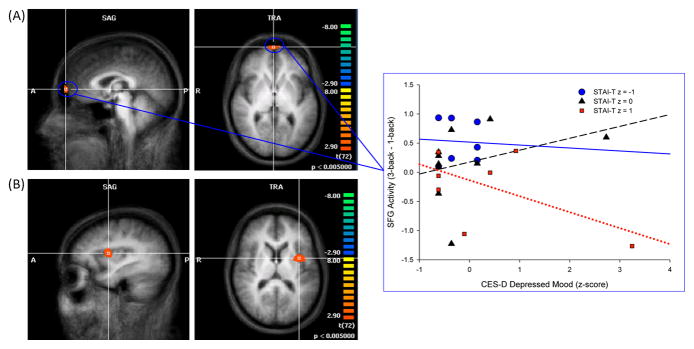
Increased activity across 0- through 3-back trials of the n-back in the SFG (A) and insula (B), and CES-D × STAI Trait effect for the SFG. Statistical maps are superimposed on the three-dimensional structural MRI averaged over all participants. p ≤ .005, cluster contiguity ≥ 20 voxels. A= anterior; P = posterior; R = right; SFG = superior frontal gyrus; CES-D = Center for Epidemiologic Studies Depression Scale; STAI= State-Trait Anxiety Inventory.
Table 4.
Brain regions showing significant working memory-related activity.
| Region | BA | Side | Coordinate*
|
Cluster Size | t | p | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Insula/Putamen | 13 | L | −34 | 4 | 15 | 80 | 4.27 | <.0001 |
| Superior Frontal Gyrus | 10 | R | 5 | 71 | 6 | 26 | 4.78 | <.0001 |
| Prefrontal Cortex | 9 | -- | -- | -- | -- | -- | -- | -- |
| Inferior Frontal Gyrus | 44 | -- | -- | -- | -- | -- | -- | -- |
Note. Talairach coordinates reflect location of peak voxel within cluster. BA = Brodmann’s area
ROI analysis of fMRI activity within clusters identified in the whole-brain analysis revealed a depressed mood × STAI-T effect for the SFG [F(1,17) = 4.83, p = .04] (Figure 5a). Working-memory related activity in the SFG increased on more difficult trials as a function of higher depressed mood scores in individuals with lower STAI-T scores, but decreased as a function of higher depressed mood scores in those with high STAI-T scores. Higher total CES-D and depressed mood scores were associated with increased activity in the insula [CES-D F(1,16) = 5.00, p = . 04; depressed mood F(1,17) = 6.73, p = . 0.02].
N-back Task Results
Performance data for the n-back are presented in Table 2. Data were missing for two participants, thus analyses were performed on 23 participants. Higher total depressive symptoms and depressed mood symptoms were associated with more errors [total CES-D F(1,15) = 7.02, p = 0.02; depressed mood F(1,16) = 9.06, p = 0.01]. Error rates generally increased on more difficult trials (i.e., higher working memory load), but individuals with the highest total CES-D and mood subscale scores made more errors on 1-back compared to 2-back trials [working memory load × CES-D F(3,49) = 2.89, p=0.05; working memory load × depressed mood F(3,48) = 3.09, p = 0.04]. For RTs, higher lack of well-being scores were associated with greater increases in RTs at higher working memory load [F(3,45) = 4.50, p = 0.01], but this effect was reduced at higher STAI-T levels [F(3,45) = 6.93, p = 0.00].
DISCUSSION
This study examined the effects of depressive symptoms, anxiety symptoms, and their interaction on cognitive and brain functioning. We examined healthy young and older adults who presented with a range of depressive and anxiety symptoms, which allowed use to examine subthreshold symptoms. This focus is important as there is increasing recognition that subthreshold mood symptoms are particularly common in older adults and are associated with similar negative sequelae as clinical mood disorders [74, 75]. Despite the frequent comorbidity of depressive and anxiety disorders, cognitive neuroscience studies focusing on the unique and combined effects of depression and anxiety are limited. The present study was designed to address this gap in the literature.
We found effects of both depressive and anxiety symptoms on cognitive and brain functioning. At older ages, total depressive symptoms were associated with slower processing speed, as measured by TMT-A, and with worse working memory performance on LNS. The finding of an interactive effect of age and depressive symptoms is consistent with previous studies showing that older adults may be particularly vulnerable to the adverse impact of depression [12–14]. Indeed, many studies of cognitive functioning in depressed young adults have failed to show effects, while cognitive studies in late-life depression yield more consistent results and generally show deficits in attention, working memory, episodic memory, and executive functions [18, 21].
Examination of the CES-D subscales and neuropsychological test performance revealed effects for the depressed mood and somatic symptoms subscales, but not the lack of well-being subscale, consistent with our previous work [76–78]. Higher depressed mood symptoms were associated with deficits in executive functioning on TMT-B in older adults but not young adults. Additionally, age differences in working memory were greater in individuals with elevated somatic symptoms, but age differences in delayed visual memory were reduced as a function of higher somatic symptoms. Perhaps somatic symptoms in older adults are a reflection of physical changes that are correlated with white matter lesions in the brain, which are known to increase as a function of age and depression [79, 80]. Based on previous evidence that prefrontal white matter is most susceptible to aging and that such lesions are associated with deficits in frontally-mediated cognitive functions [81, 82], somatic symptoms might be expected to have a greater impact on working memory in older adults compared to visual memory.
Regarding anxiety symptoms, we found that higher trait anxiety was associated with better performance on Digit Span and LNS, measures of attention and working memory, and this effect was greater at older ages. Given our non-clinical sample, higher STAI-T scores correspond with moderate levels of anxiety rather than severe levels characteristic of anxiety disorders. Thus, our findings are consistent with studies reporting an inverted U-shape function in the relationship between anxiety and cognitive performance, such that moderate levels of anxiety are associated with better performance, while low and high symptoms are associated with performance deficits [16, 38, 39]. Interestingly, we found that anxiety moderated the impact of depressive symptoms on executive functioning. Higher total CES-D and depressed mood symptoms were associated with deficits on TMT-B at low levels of trait anxiety, but not at higher anxiety levels. Since moderate anxiety is associated with better performance on some cognitive measures, it appears that anxiety counteracted the negative impact of depressive symptoms on executive functioning. This is contrary to previous investigations of mixed depression and anxiety, which have shown that comorbid depression and anxiety are associated with worse outcomes, including cognitive deficits, compared to either condition alone [37, 49, 52]. This discrepancy may be due to our examination of subthreshold depression and anxiety, rather than clinical conditions. Perhaps the unique and interactive effects of depression and anxiety differ as a function of severity level. This is an important area for future research considering the frequent comorbidity of depression and anxiety and the high prevalence of subthreshold conditions.
A subset of our sample underwent fMRI scanning while they performed the n-back working memory task. Consistent with other working memory studies, we found monotonic increases in activity in the PFC, inferior and superior frontal gyri, putamen and insula at higher working memory loads. Higher total depressive symptoms was associated with reduced activity in the SFG at higher working memory loads in individuals with high anxiety, but with increased activity in those with low anxiety. This suggests that anxiety reduced the heightened frontal activity that has previously been shown in depression during working memory tasks [56, 57]. Analysis of n-back task performance showed that higher total depressive symptoms and depressed mood symptoms were associated with more errors on the n-back, consistent with the neuropsychological test results. Given the smaller sample size in the fMRI subsample, we did not examine whether the effect differed in young and older adults. In contrast to the neuropsychological test results, we also found an effect of the lack of well-being subscale on performance. Higher lack of well-being scores were associated with greater increases in RTs at higher working memory load, but higher trait anxiety reduced this effect. This finding parallels the fMRI activation results but should be interpreted with caution due to the small sample size in the neuroimaging subsample.
Taken together, our results confirm previous reports that subthreshold depression and anxiety impact cognitive and brain functioning, add to the growing literature showing that age may moderate these effects, and suggest that the interaction of subthreshold depression and anxiety results in distinct cognitive and brain changes. Findings highlight the importance of assessing and controlling for symptoms of depression and anxiety in research studies of either condition. Results also have implications for clinicians, as they suggest that older adults with comorbid symptoms of depression and anxiety may be at risk for cognitive deficits even in the absence of a diagnosis of major depression or an anxiety disorder. Future work should compare the unique and interactive effect of depression and anxiety on cognitive and brain functioning at both subthreshold and clinical levels, and should examine changes in functioning over time using longitudinal designs.
Acknowledgments
This research was supported by an Age Related Memory Loss award from the McKnight Brain Research Foundation (VMD), and by the National High Magnetic Field Laboratory. This project was performed at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility in the McKnight Brain Institute of the University of Florida.
References
- 1.Fava M, Alpert JE, Carmin CN, Wisniewski SR, Trivedi MH, et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34:1299–308. doi: 10.1017/s0033291704002612. [DOI] [PubMed] [Google Scholar]
- 2.Fava M, Rankin MA, Wright EC, Alpert JE, Nierenberg AA, et al. Anxiety disorders in major depression. Compr Psychiatry. 2000;41:97–102. doi: 10.1016/s0010-440x(00)90140-8. [DOI] [PubMed] [Google Scholar]
- 3.Kvaal K, McDougall FA, Brayne C, Matthews FE, Dewey ME, et al. Co-occurrence of anxiety and depressive disorders in a community sample of older people: results from the MRC CFAS (Medical Research Council Cognitive Function and Ageing Study) Int J Geriatr Psychiatry. 2008;23:229–37. doi: 10.1002/gps.1867. [DOI] [PubMed] [Google Scholar]
- 4.Lenze EJ. Comorbidity of depression and anxiety in the elderly. Curr Psychiatry Rep. 2003;5:62–7. doi: 10.1007/s11920-003-0011-7. [DOI] [PubMed] [Google Scholar]
- 5.Sanderson WC, Beck AT, Beck J. Syndrome comorbidity in patients with major depression or dysthymia: prevalence and temporal relationships. Am J Psychiatry. 1990;147:1025–8. doi: 10.1176/ajp.147.8.1025. [DOI] [PubMed] [Google Scholar]
- 6.Angst J, Vollrath M. The natural history of anxiety disorders. Acta Psychiatr Scand. 1991;84:446–52. doi: 10.1111/j.1600-0447.1991.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 7.Das-Munshi J, Goldberg D, Bebbington PE, Bhugra DK, Brugha TS, et al. Public health significance of mixed anxiety and depression: beyond current classification. Br J Psychiatry. 2008;192:171–7. doi: 10.1192/bjp.bp.107.036707. [DOI] [PubMed] [Google Scholar]
- 8.Fichter MM, Quadflieg N, Fischer UC, Kohlboeck G. Twenty-five-year course and outcome in anxiety and depression in the Upper Bavarian Longitudinal Community Study. Acta Psychiatr Scand. 2010;122:75–85. doi: 10.1111/j.1600-0447.2009.01512.x. [DOI] [PubMed] [Google Scholar]
- 9.Merikangas KR, Zhang H, Avenevoli S, Acharyya S, Neuenschwander M, et al. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Arch Gen Psychiatry. 2003;60:993–1000. doi: 10.1001/archpsyc.60.9.993. [DOI] [PubMed] [Google Scholar]
- 10.Murphy JM, Olivier DC, Sobol AM, Monson RR, Leighton AH. Diagnosis and outcome: depression and anxiety in a general population. Psychol Med. 1986;16:117–26. doi: 10.1017/s0033291700057809. [DOI] [PubMed] [Google Scholar]
- 11.Vollrath M, Angst J. Results of the Zurich cohort study: course of anxiety and depression. Psychiatr Psychobiol. 1989;4:307–313. [Google Scholar]
- 12.Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009;34:367–75. [PMC free article] [PubMed] [Google Scholar]
- 13.Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry. 2008;16:318–30. doi: 10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–26. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 15.Braam AW, Copeland JR, Delespaul PA, Beekman AT, Como A, et al. Depression, subthreshold depression and comorbid anxiety symptoms in older Europeans: Results from the EURODEP concerted action. J Affect Disord. 2013 doi: 10.1016/j.jad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Bierman EJ, Comijs HC, Jonker C, Beekman AT. Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry. 2005;13:686–93. doi: 10.1176/appi.ajgp.13.8.686. [DOI] [PubMed] [Google Scholar]
- 17.Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. 2012;140:113–24. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur J Pharmacol. 2010;626:83–6. doi: 10.1016/j.ejphar.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 19.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisenbach SL, Boore LA, Kales HC. Depression and cognitive impairment in older adults. Curr Psychiatry Rep. 2012;14:280–8. doi: 10.1007/s11920-012-0278-7. [DOI] [PubMed] [Google Scholar]
- 22.Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 25.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Sacher J, Neumann J, Funfstuck T, Soliman A, Villringer A, et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142–8. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Bannon S, Gonsalvez CJ, Croft RJ. Processing impairments in OCD: it is more than inhibition! Behav Res Ther. 2008;46:689–700. doi: 10.1016/j.brat.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Beekman AT, Bremmer MA, Deeg DJ, van Balkom AJ, Smit JH, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13:717–26. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Haikal M, Hong RY. The effects of social evaluation and looming threat on self-attentional biases and social anxiety. J Anxiety Disord. 2010;24:345–52. doi: 10.1016/j.janxdis.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Johnsen GE, Asbjornsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. J Affect Disord. 2008;111:74–82. doi: 10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Johnsen GE, Asbjornsen AE. Verbal learning and memory impairments in posttraumatic stress disorder: the role of encoding strategies. Psychiatry Res. 2009;165:68–77. doi: 10.1016/j.psychres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Mantella RC, Butters MA, Dew MA, Mulsant BH, Begley AE, et al. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15:673–9. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- 33.Schultz SK, Moser DJ, Bishop JR, Ellingrod VL. Phobic anxiety in late-life in relationship to cognition and 5HTTLPR polymorphism. Psychiatr Genet. 2005;15:305–6. doi: 10.1097/00041444-200512000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Ferreri F, Lapp LK, Peretti CS. Current research on cognitive aspects of anxiety disorders. Curr Opin Psychiatry. 2011;24:49–54. doi: 10.1097/YCO.0b013e32833f5585. [DOI] [PubMed] [Google Scholar]
- 35.Potvin O, Hudon C, Dion M, Grenier S, Preville M. Anxiety disorders, depressive episodes and cognitive impairment no dementia in community-dwelling older men and women. Int J Geriatr Psychiatry. 2011;26:1080–8. doi: 10.1002/gps.2647. [DOI] [PubMed] [Google Scholar]
- 36.Beaudreau SA, O’Hara R. The association of anxiety and depressive symptoms with cognitive performance in community-dwelling older adults. Psychol Aging. 2009;24:507–12. doi: 10.1037/a0016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stillman AN, Rowe KC, Arndt S, Moser DJ. Anxious symptoms and cognitive function in non-demented older adults: an inverse relationship. Int J Geriatr Psychiatry. 2012;27:792–8. doi: 10.1002/gps.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bierman EJ, Comijs HC, Rijmen F, Jonker C, Beekman AT. Anxiety symptoms and cognitive performance in later life: results from the longitudinal aging study Amsterdam. Aging Ment Health. 2008;12:517–23. doi: 10.1080/13607860802224276. [DOI] [PubMed] [Google Scholar]
- 39.Salthouse TA. How general are the effects of trait anxiety and depressive symptoms on cognitive functioning? Emotion. 2012;12:1075–84. doi: 10.1037/a0025615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, et al. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67:1002–11. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 41.Yucel K, McKinnon MC, Chahal R, Taylor VH, Macdonald K, et al. Anterior cingulate volumes in never-treated patients with major depressive disorder. Neuropsychopharmacology. 2008;33:3157–63. doi: 10.1038/npp.2008.40. [DOI] [PubMed] [Google Scholar]
- 42.Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–71. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laeger I, Dobel C, Dannlowski U, Kugel H, Grotegerd D, et al. Amygdala responsiveness to emotional words is modulated by subclinical anxiety and depression. Behav Brain Res. 2012;233:508–16. doi: 10.1016/j.bbr.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 44.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43:1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Sehlmeyer C, Dannlowski U, Schoning S, Kugel H, Pyka M, et al. Neural correlates of trait anxiety in fear extinction. Psychol Med. 2011;41:789–98. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- 46.Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, et al. Differential limbic--cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- 47.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Biringer E, Mykletun A, Dahl AA, Smith AD, Engedal K, et al. The association between depression, anxiety, and cognitive function in the elderly general population--the Hordaland Health Study. Int J Geriatr Psychiatry. 2005;20:989–97. doi: 10.1002/gps.1390. [DOI] [PubMed] [Google Scholar]
- 49.DeLuca AK, Lenze EJ, Mulsant BH, Butters MA, Karp JF, et al. Comorbid anxiety disorder in late life depression: association with memory decline over four years. Int J Geriatr Psychiatry. 2005;20:848–54. doi: 10.1002/gps.1366. [DOI] [PubMed] [Google Scholar]
- 50.Airaksinen E, Larsson M, Lundberg I, Forsell Y. Cognitive functions in depressive disorders: evidence from a population-based study. Psychol Med. 2004;34:83–91. doi: 10.1017/s0033291703008559. [DOI] [PubMed] [Google Scholar]
- 51.Kizilbash AH, Vanderploeg RD, Curtiss G. The effects of depression and anxiety on memory performance. Arch Clin Neuropsychol. 2002;17:57–67. [PubMed] [Google Scholar]
- 52.Basso MR, Lowery N, Ghormley C, Combs D, Purdie R, et al. Comorbid anxiety corresponds with neuropsychological dysfunction in unipolar depression. Cogn Neuropsychiatry. 2007;12:437–56. doi: 10.1080/13546800701446517. [DOI] [PubMed] [Google Scholar]
- 53.Andreescu C, Butters M, Lenze EJ, Venkatraman VK, Nable M, et al. fMRI activation in late-life anxious depression: a potential biomarker. Int J Geriatr Psychiatry. 2009;24:820–8. doi: 10.1002/gps.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, et al. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depress Anxiety. 2011;28:202–9. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlund MW, Verduzco G, Cataldo MF, Hoehn-Saric R. Generalized anxiety modulates frontal and limbic activation in major depression. Behav Brain Funct. 2012;8:8. doi: 10.1186/1744-9081-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26:860–9. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 57.Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–66. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- 58.First MB, Gibbon M, Spitzer RL, Williams JB. User’s guide for the Structured clinical interview for DSM-IV-TR axis I disorders SCID-I : research version. New York: Biometrics Research; 2001. p. 132. [Google Scholar]
- 59.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 60.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appli Psychol Meas. 1977;1:385–401. [Google Scholar]
- 61.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, et al. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–5. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 62.Haringsma R, Engels GI, Beekman AT, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–63. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- 63.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–46. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 64.Speilberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 65.Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised. Professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 66.Benedict RH. Brief Visuospatial Memory Test-Revised. Odessa, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 67.Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 68.Reitan R. Trail Making Test: Manual for Administration and Scoring. Tucson, AZ: Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- 69.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, et al. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 70.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 71.Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–72. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- 72.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–21. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lavretsky H, Kumar A. Practical geriatrics: clinically significant nonmajor geriatric depression. Psychiatr Serv. 2003;54:297–9. doi: 10.1176/appi.ps.54.3.297. [DOI] [PubMed] [Google Scholar]
- 75.Royall DR. The “subsyndromal” syndromes of aging. J Am Geriatr Soc. 2004;52:463–5. doi: 10.1111/j.1532-5415.2004.52124.x. [DOI] [PubMed] [Google Scholar]
- 76.Dotson VM, Zonderman AB, Davatzikos C, Kraut MA, Resnick SM. Frontal Atrophy and Attention Deficits in Older Adults with a History of Elevated Depressive Symptoms. Brain Imaging Behav. 2009;3:358. doi: 10.1007/s11682-009-9078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dotson VM, Zonderman AB, Kraut MA, Resnick SM. Temporal relationships between depressive symptoms and white matter hyperintensities in older men and women. Int J Geriatr Psychiatry. 2013;28:66–74. doi: 10.1002/gps.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kirton JW, Resnick SM, Davatzikos C, Kraut MA, Dotson VM. Depressive Symptoms, Symptom Dimensions, and White Matter Lesion Volume in Older Adults: A Longitudinal Study. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol. 2012;98:99–143. doi: 10.1016/j.pneurobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–48. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bolandzadeh N, Davis JC, Tam R, Handy TC, Liu-Ambrose T. The association between cognitive function and white matter lesion location in older adults: a systematic review. BMC Neurol. 2012;12:126. doi: 10.1186/1471-2377-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–17. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]