Summary
Nikkomycin Z (NZ) is a competitive inhibitor of chitin synthase III in the yeast Saccharomyces cerevisiae. Myosin type II-deficient yeast strains (myo1) display a dramatic reduction in growth when chitin synthase III activity is inhibited by NZ, supporting the contention that actomyosin motility plays an important role in maintaining cell wall integrity. A proposed inhibitor of cortical actin polymerization in vitro, 2,3-butanedione monoxime (BDM), also inhibits growth of wild-type yeast strains at a concentration of 20 mM. In this study, we assayed for potential in vivo interplay between BDM-sensitive cell functions and cell wall chitin synthesis by testing for increased sensitivity to NZ during co-treatment with BDM at sub-inhibitory concentrations. Our results show that BDM can increase the sensitivity of yeast cells to Nikkomycin Z.
Keywords: BDM, chitin, myo1, Nikkomycin Z, Saccharomyces cerevisiae, yeast
Introduction
Chitin synthesis is essential for maintaining the integrity of the fungal cell wall in most fungi. Chitin is a component of the cell wall that accounts for 1–2% of the dry weight of the budding yeast Saccharomyces cerevisiae. The catalytic subunit of S. cerevisiae chitin synthase III (CSIII) Chs3p is responsible for approximately 90% of all cell wall chitin synthesis (Shaw et al. 1991). Deletion of the myosin type II heavy chain gene (myo1) in S. cerevisiae strains disables actomyosin-driven cytokinesis and proper primary septum formation (Schmidt et al. 2002). This causes increased deposition of cell wall chitin by CSIII, particularly at the abnormal septum to prevent cell lysis (Rodríguez & Paterson 1990; Schmidt et al. 2002).
Nikkomycin Z (NZ), a nucleoside–peptide antibiotic with antifungal properties that functions as a competitive inhibitor of CSIII (Gaughran et al. 1994), is capable of inhibiting chitin synthesis in several medically important fungi. Its usefulness in the treatment of Blastomyces dermatitidis (Clemons & Stevens 1997), Candida albicans, Aspergillus fumigatus, Rhizopus sp., and Coccidioides immitis (Stevens 2000) has been demonstrated. Co-treatment of NZ with other synergistic antifungal compounds (e.g. Echinocandin) can further increase the susceptibility of fungal cells to NZ (Stevens 2000).
Recent in vitro studies prove that the compound 2,3- butanedione monoxime (BDM), can act as an inhibitor of retrograde movement by inhibition of actin nucleation and polymerization at the leading edge in mammalian tissue culture cells (Yarrow et al. 2003). Studies with the fission yeast Schizosaccharomyces pombe demonstrated that BDM at a sub-lethal concentration of 10 mM BDM, disorganizes the fission yeast cell wall and inhibits cytokinesis (Steinberg & McIntosh 1998). A study byChon et al. (2001) showed that 20 mM BDM completely inhibits growth in S. cerevisiae. Others have shown that 20 mM BDM inhibits actin polymerization at the cell cortex by inhibiting the activity of type I nonmuscle myosins, Myo3p, and Myo5p (Lechler et al. 2000). The effects of BDM on growth of S. cerevisiae at sub-inhibitory concentrations (defined here as concentrations lower than 20 mM) have not been described.
We hypothesized that cell wall weakening effects in myo1 strains can be indirectly monitored by measuring the relative sensitivity to NZ. To test this hypothesis, we designed an in vivo functional assay to screen for effects of chemical compounds on cell wall integrity in a wildtype test strain and used myo1 and chs3 strains as controls. In this study we tested whether cell wall integrity could be disrupted by sub-inhibitory concentrations of BDM. We show that co-treatment with 2 mM BDM significantly increased sensitivity to NZ in a wild-type test strain.
Materials and methods
Strains and growth conditions
A myosin type II heavy chain deficient (myo1) strain, YJR6 (myo1D: HIS5) was generated from wild-type strain MGD353-46D (MATa trp-289 ura3-52 leu2-3, 112 his3D1 cyhR ADE+ARG+) by targeted inactivation (details are available on request). A chs3 strain was obtained from ATCC (Strain # 4003160, YFR1). A myo1chs3 strain (YJR7) was constructed by crossing the YFR1 and YJR6 haploid strains and standard tetrad dissection. Sister haploid strains of wild-type (YJR12), myo1 (YJR13), and chs3 (YJR14), were also isolated from this cross. Complete synthetic medium (CSM) was used to grow the wild-type strain and complete synthetic medium without histidine (CSM/HIS-) was used to grow YJR6 in broth cultures. Kanamycin resistance was used as a selection for the chs3 strain. 2% (w/v) glucose was used as the carbon source and nitrogen base without amino acids as the nitrogen source for these media. Cultures were harvested at maximum optical density (OD) at 600 nm of between 0.5 and 1.0.
Sensitivity of the myo1 strain to NZ was measured as described previously (Popolo et al. 1997) with certain modifications. A cell suspension containing 2×105 cells/ ml was added to an equal amount of CSM containing specified dilutions of NZ. Cell suspensions were dispensed in a 96-well microtiter plate. Final concentrations of NZ ranging between 0 and 50 µM were tested with untreated controls in triplicate experiments. The plate was incubated at 25 °C with vigorous shaking for 36 h and turbidity of the culture was read at 595 nm in an ELISA reader and the readings averaged. The experiments shown in Figures 1b, c, and 2 were conducted in 10 ml broth culture medium with 200 rev/min rotation at 25 °C for 28, 48, and 24 h respectively.
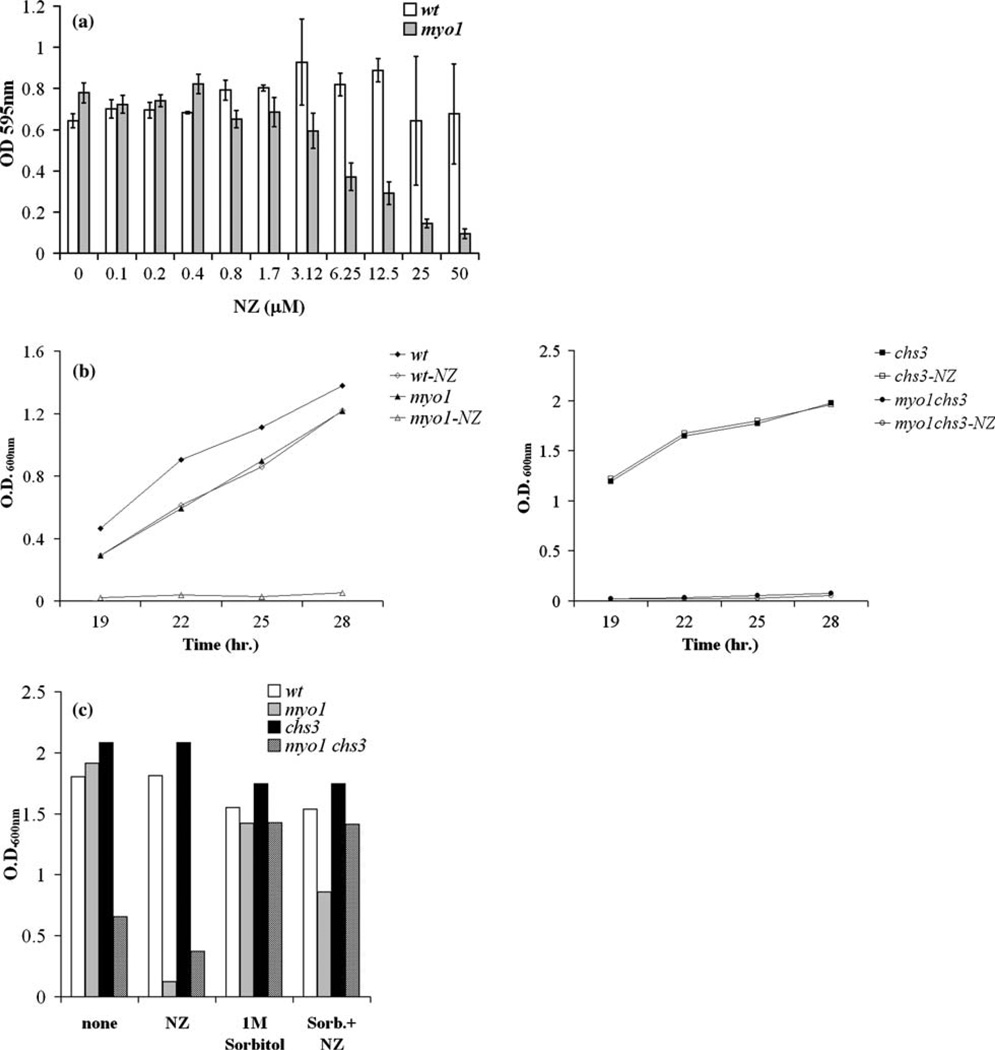
Figure 1.
Graph of the response of yeast strains to treatment with NZ. (a) To determine the dose–response to NZ by wild-type (wt) and myo1 strains, these were cultured in 300 µl microwell plates with NZ for 36 h at 25 °C and treated with a range of concentrations of NZ. Growth was monitored by OD595 nm readings as described in the Materials and methods section. The IC50 for NZ was established at 6.25 µM. (b) Growth rates for the wild-type (wt), myo1, chs3, and myo1chs3 strains in standard broth culture conditions were monitored in the presence or absence of 6.25 µM NZ at four time points taken during a time interval between 19 and 28 h by OD600 nm readings. (c) Cell growth was monitored for 48 h under standard broth culture conditions as described in Figure 1b for wild-type (wt), myo1, chs3, and myo1chs3 strains over a period of 45 h in untreated cultures (none), with 6.25 µM NZ (NZ), with 1 Msorbitol, or with a combination of 1 Msorbitol+6.25 µMNZ (Sorb+NZ) added to the culture medium.
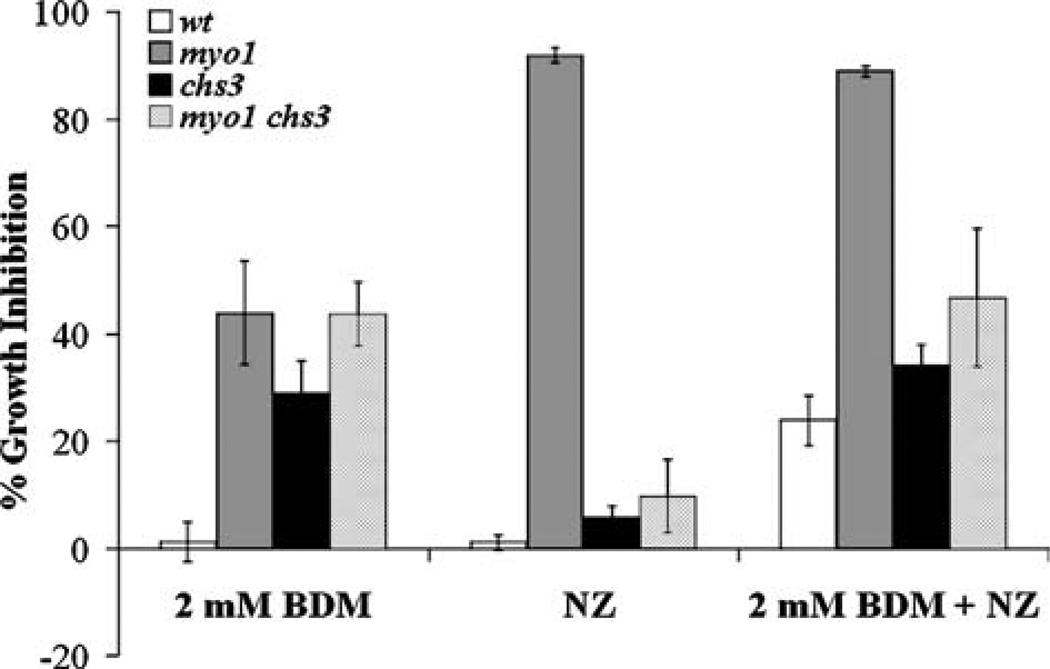
Figure 2.
Graph of the response of yeast strains to a combined treatment with BDM and 6.25 µM NZ. Treatment with 2 mM BDM, 6.25 µM NZ (NZ), and a combined treatment of 2 mM BDM and 6.25 µM NZ (2 mM BDM+NZ) were performed on wild-type (wt), myo1, chs3, and myo1chs3 strains for 24 h. Monitoring of cell growth and calculation of the percentage of growth inhibition are as described in the Materials and methods section.
For BDM sensitivity assays, a fresh stock solution of 0.5 M BDM was prepared in 95% ethanol. The OD600 nm of the starting cultures used for each strain was between 0.5 and 1.0. For each strain, a 20 ml culture with a concentration of 1.0×105 cells /ml was prepared and divided in four cultures of 5 ml each. Three of the cultures were treated with BDM for 24 h in triplicate experiments as described, and one was used as the untreated control. The averaged growth inhibitions from three experiments were calculated as follows [OD600 nm untreated)OD600 nm treated/OD600 nm untreated]×100. The average deviation was calculated.
Results and discussion
To assay for integrity of the cell wall in wild-type and myo1 strains, the dose–response of these strains to treatment with NZ was tested as described in the Materials and methods. We determined the inhibitory concentration (IC50) of the myo1 strain for NZ at 6.25 µM. We observed that the myo1 strain (YJR6) exhibited sensitivity to NZ at the ‡ 6.25 µM range while the wild-type strain first exhibited a reduction in growth potential at concentrations ‡ 25 µM NZ. This interpretation was derived from an increase in the standard deviations of OD600 nm readings average from the cultures treated with 25 and 50 µM NZ (Figure 1a).
Figure 1b (left panel) shows that the inhibitory effect of 6.25 µM NZ on the myo1 strain was stably maintained even up to 28 h. The relative insensitivity of the wild-type strain to 6.25 µM NZ is also demonstrated (Figure 1b left panel). The inhibitory effect of NZ on the growth rate of the myo1 strain was similar in magnitude to the effect of CSIII deficiency caused by chs3 deletion in the myo1 strain genetic background (Figure 1b right panel). Treatment with 6.25 µM NZ alone produced no effect on the growth rate of a chs3 strain, which is in agreement with the finding that NZ specifically inhibits CSIII in vivo (Gaughran et al. 1994). Growth in myo1 cultures was restored to approximately 50% of the level of 1 M sorbitol-treated control cultures after 48 h of exposure to 6.25 µM NZ and 1 M sorbitol. This supports that cell lysis is a major contributing factor in reducing cell viability in the NZ-treated myo1 cultures (Figure 1c). The double mutant strain experienced a measurable degree of non-specific toxicity to NZ with longer periods of exposure.
To establish the minimal concentration of BDM needed to inhibit cell growth under conditions where CSIII activity was blocked or inhibited; we tested a range of concentrations between 1 and 10 mM BDM over a period of 24 h using a chs3 test strain that lacks CSIII activity. We found that a minimum concentration of 2 mM BDM significantly increased the percentage of growth inhibition in the chs3 strain relative to the wild-type strain. Morever, this concentration did not produce any effect on growth of the treated wild-type strain in comparison to the untreated control strain (data not shown).
To test our hypothesis that BDM can affect the integrity of the cell wall, we assayed the effect of combined treatments of 2 mM BDM and 6.25 µM NZ over a 24-h period on wild-type, chs3, myo1, and myo1chs3 strains. Treatment with 2 mM BDM alone produced less than 5% growth inhibition in the wild-type strain and an average of 30–40% growth inhibition in the chs3, myo1, and myo1chs3 strain cultures (Figure 2, labeled 2 mM BDM). The percentages of growth inhibition obtained for the chs3 and myo1 strains were almost similar to the growth inhibition observed for the myo1chs3 strain. This result indicates that any interaction between the individual genetic mutations and putative BDM-sensitive cell wall functions is not summative in the double mutant strain. This observation suggests that both mutations may exert a common effect on cell wall function and that BDM therefore similarly affects the viability of these strains.
As predicted from our previous experiments, the myo1 positive control strain treated with 6.25 µM NZ alone experienced a significant increase in the percentage of growth inhibition whereas the wild-type, chs3, or myo1chs3 strains were relatively unchanged after 24 h (Figure 2, labeled NZ). We observed 25% increase in the percentage of growth inhibition upon exposure of these strains to a combined treatment with 2 mM BDM and 6.25 µM NZ when compared to wild-type cultures treated with each of these compounds singularly (Figure 2, labeled 2 mM BDM+NZ). The percentage of growth inhibition observed for the myo1 strain exposed to the combined treatment was attributed to the effect of the 6.25 µMNZ treatment alone. The chs3 and myo1chs3 strains both exhibited the same percentage of growth inhibition, which could be attributed to the effect of the 2 mM BDM treatment. From these results, we infer that treatment with 2 mMBDMincreases the requirement for CSIII-dependent chitin synthesis in the wild-type strain. Possibly, the magnitude of the percentage of growth inhibition in the wild-type strain under the combined treatment was not as high as that of the myo1 strain treated with 6.25 µM NZ due to the lack of specificity of BDM for non-muscle myosin type II (Ostap 2002) and the reversible nature of this inhibition (Chon et al. 2001).
Micrographic imaging of cell wall chitin distribution by fluorescence staining with Calcofluor White (Santos & Snyder 2000) revealed that the chitin synthesis and deposition in the cell wall were not visibly inhibited by treatment with 2 mM BDM in wild-type or myo1 strains (Figures 3a, b respectively, labeled BDM) in comparison to untreated controls (Figures 3a, b respectively, labeled untreated). Treatment with 6.25 µM NZ produced a pronounced reduction in the level of Calcofluor White fluorescence in both strains, which is consistent with the inhibition of CSIII catalytic activity in vivo (Figure 3a, b respectively, labeled NZ). The combined treatment with 2 mM BDM and 6.25 µM NZ did not change this chitin distribution or further reduce the intensity of the Calcofluor White fluorescence, suggesting that there are no apparent differences between the combined treatment vs. treatment with 6.25 µM NZ alone (Figure 3a, b respectively, labeled BDM+NZ). On basis of these observations we speculate that 2 mM BDM must therefore interfere with cell function(s) at a level that is not directly related to cell wall chitin synthesis and deposition.
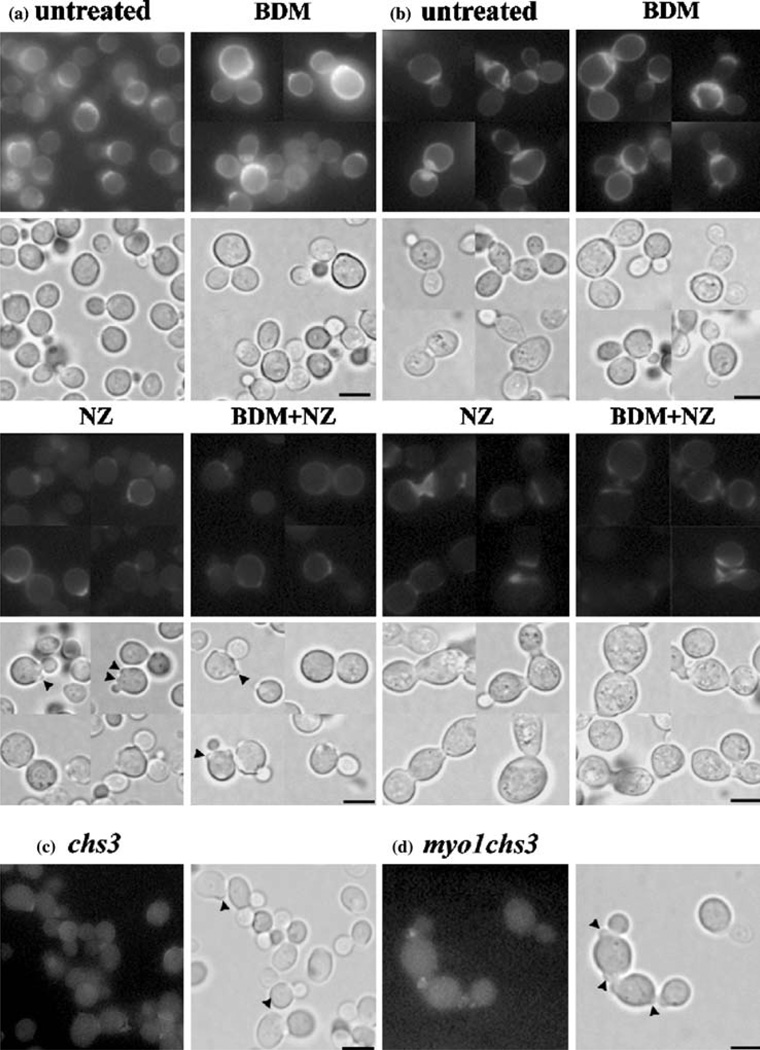
Figure 3.
Micrographic imaging of cell wall chitin distribution by Calcofluor White staining. Wild-type (a) and myo1 (b) strain cultures were treated with 2 mM BDM, 6.25 µM NZ, or treated simultaneously with 2 mM BDM and 6.25 µM NZ (BDM+NZ) for 24 h and later stained with Calcofluor White to visualize chitin deposition. Untreated control cultures of wild-type (a), myo1 (b), chs3 (c), and myo1chs3 (d) strains are shown. Upper panels represent a collage of u.v. fluorescence micrographs and the lower panels represent the corresponding light micrographs for each group. Arrowheads indicate putative aborted bud structures. All images were captured at the same exposure time and magnification (1000×). Bar=5 µm.
Light micrographs of the wild-type control cultures treated with NZ and BDM+NZ indicated the appearance of aborted bud structures previously described in chs3 strains (Santos & Snyder 2000) (arrowheads, Figures 3a, NZ and 3A BDM+NZ, respectively). Untreated control cultures of chs3 (Figure 3c) and myo1chs3 (Figure 3d) strains demonstrate that they were devoid of CSIII activity. Punctate fluorescence observed near the neck of the bud in some control cells was attributed to chitin synthase I and/or II enzyme activities that were intact in these strains.
Yeast cells can escape cell lysis when placed under conditions that affect cell wall integrity by increasing CSIII-dependent cell wall chitin synthesis up to fourfold (Popolo et al. 1997; Osmond et al. 1999; Valdivieso et al. 2000). Because activation of CSIII expression is vital for this cell wall integrity response and because co-treatment with BDM at sub-inhibitory concentrations for growth can increase sensitivity to NZ in wildtype strains by as much as four-fold in vivo, we propose that BDM may act by compromising cell wall integrity. These results warrant further testing of BDM as a potential enhancing agent for NZ in the treatment of other fungal species.
Conclusions
Saccharomyces cerevisiae myo1 strains are hypersensitive to 6.25 µM NZ, a competitive inhibitor of CSIII. An isogenic wild-type strain was relatively resistant to 6.25 µM NZ but 2 mM BDM caused a significant reduction in the viability of this strain when applied simultaneously with NZ. These results suggest that treatment with 2 mM BDM causes weakening of the fungal cell wall thereby increasing the requirement for CSIII-dependent chitin synthesis to reinforce it. The use of BDM as a potential enhancing agent for NZ is proposed.
Acknowledgements
We thank Laura M. Bretaña for editorial assistance. This research was supported by an NIH grant awarded to the University of Puerto Rico Medical Sciences Campus S06 GM08224 with partial support from grants G12 RR03051 and R25 GM61838.
References
- Chon K, Hwang HS, Lee JH, Song K. The myosin ATPase inhibitor 2,3-butanedione-2-monoxime disorganizes microtubules as well as F-actin in Saccharomyces cerevisiae. Cell Biology and Toxicology. 2001;17:383–393. doi: 10.1023/a:1013748500662. [DOI] [PubMed] [Google Scholar]
- Clemons KV, Stevens DA. Efficacy of nikkomycin Z against experimental pulmonary blastomycosis. Antimicrobial Agents and Chemotherapy. 1997;41:2026–2028. doi: 10.1128/aac.41.9.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughran JP, Lai MH, Kirsch DR, Silverman SJ. Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isozyme Chs3 in vitro and in vivo. Journal of Bacteriology. 1994;176:5857–5860. doi: 10.1128/jb.176.18.5857-5860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Schevchenko A, Li R. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. Journal of Cell Biology. 2000;148:363–373. doi: 10.1083/jcb.148.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond BC, Specht CA, Robbins PW. Chitin synthase III: synthetic lethal mutants and “stress related” chitin synthesis that bypasses the CSD3/CHS6 localization pathway. Proceedings of the National Academy of Sciences USA. 1999;96:11206–11210. doi: 10.1073/pnas.96.20.11206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostap EM. 2,3-Butanedione monoxime (BDM) as a myosin inhibitor. Journal of Muscle Research and Cell Motility. 2002;23:305–308. doi: 10.1023/a:1022047102064. [DOI] [PubMed] [Google Scholar]
- Popolo L, Gillardelli D, Bonfante P, Vai M. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1D mutant of Saccharomyces cerevisiae. Journal of Bacteriology. 1997;179:463–469. doi: 10.1128/jb.179.2.463-469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JR, Paterson BM. Yeast myosin heavy chain mutant: Maintenance of the cell type specific budding pattern and the normal deposition of chitin and cell wall components requires an intact myosin heavy chain gene. Cell Motility and the Cytoskeleton. 1990;17:301–308. doi: 10.1002/cm.970170405. [DOI] [PubMed] [Google Scholar]
- Santos B, Snyder M. Sbe2p and Sbe22p, Two homologous Golgi proteins involved in yeast cell wall formation. Molecular Biology of the Cell. 2000;11:435–452. doi: 10.1091/mbc.11.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Bowers B, Varma A, Roh DH, Cabib E. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. Journal of Cell Science. 2002;115:293–302. doi: 10.1242/jcs.115.2.293. [DOI] [PubMed] [Google Scholar]
- Shaw JA, Mol PC, Bowers BB, Silverman SJ, Valdivieso MH, Duran A, Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. Journal of Cell Biology. 1991;114L:111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G, McIntosh JR. Effects of the myosin inhibitor 2,3-butanedione monoxime on the physiology of fission yeast. European Journal of Cell Biology. 1998;77:284–293. doi: 10.1016/S0171-9335(98)80087-3. [DOI] [PubMed] [Google Scholar]
- Stevens DA. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (Nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrobial Agents and Chemotherapy. 2000;44:2547–2548. doi: 10.1128/aac.44.9.2547-2548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivieso M-H, Ferrario L, Vai M, Duran A, Popolo L. Chitin synthesis in a gas1 mutant of Saccharomyces cerevisiae. Journal of Bacteriology. 2000;182:4752–4757. doi: 10.1128/jb.182.17.4752-4757.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarrow JC, Lechler T, Li R, Mitchinson TJ. Rapid delocalization of actin leading edge components with BDM treatment. BioMed Central Cell Biology 4,5. (e-journal) 2003 doi: 10.1186/1471-2121-4-5. ( http://www.biomedcentral.com/1471-2121/4/5) [DOI] [PMC free article] [PubMed] [Google Scholar]