Abstract
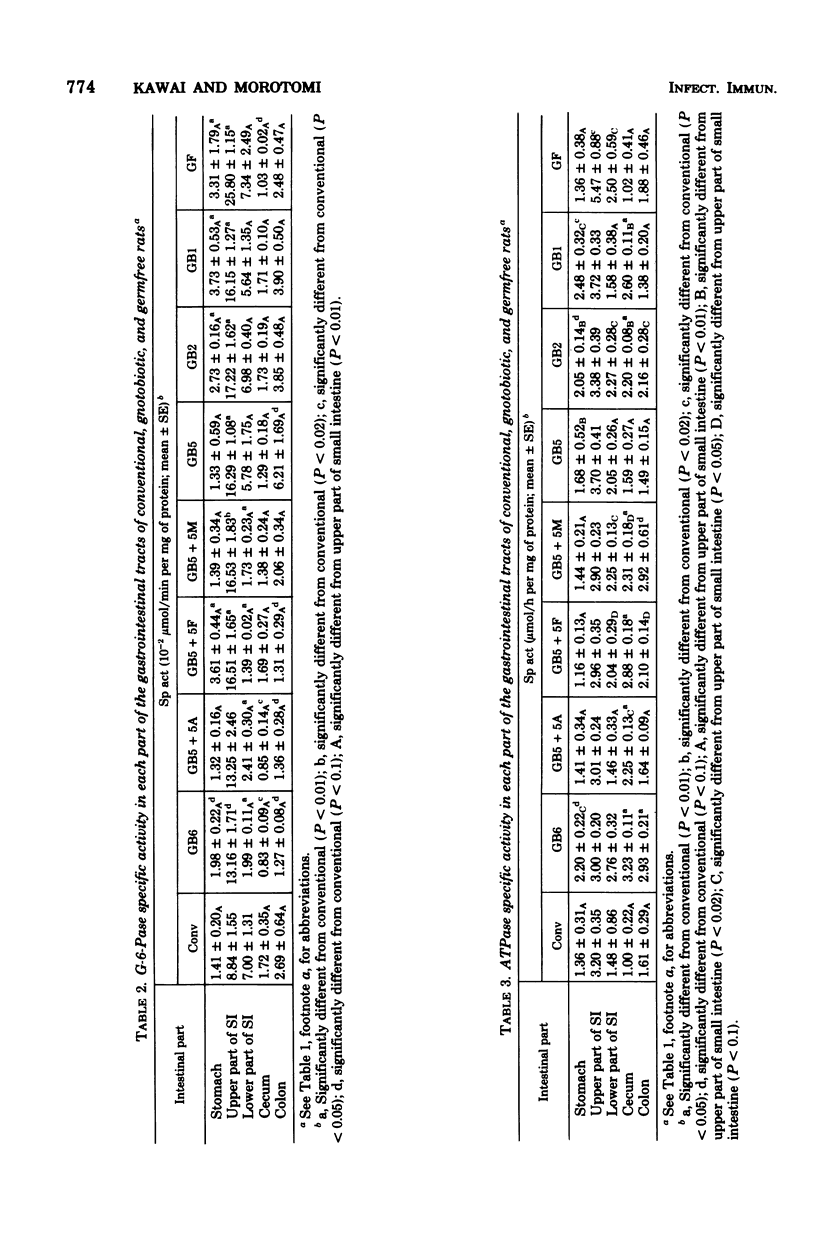
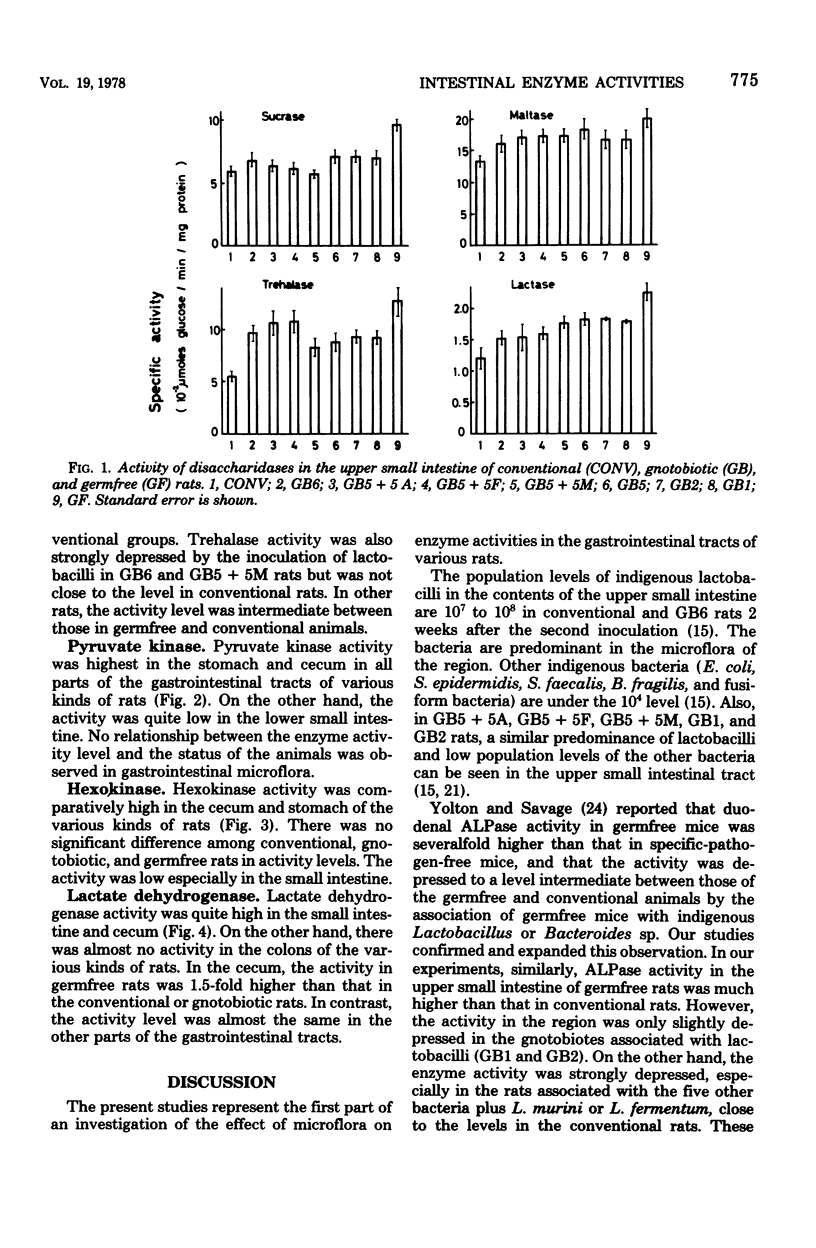
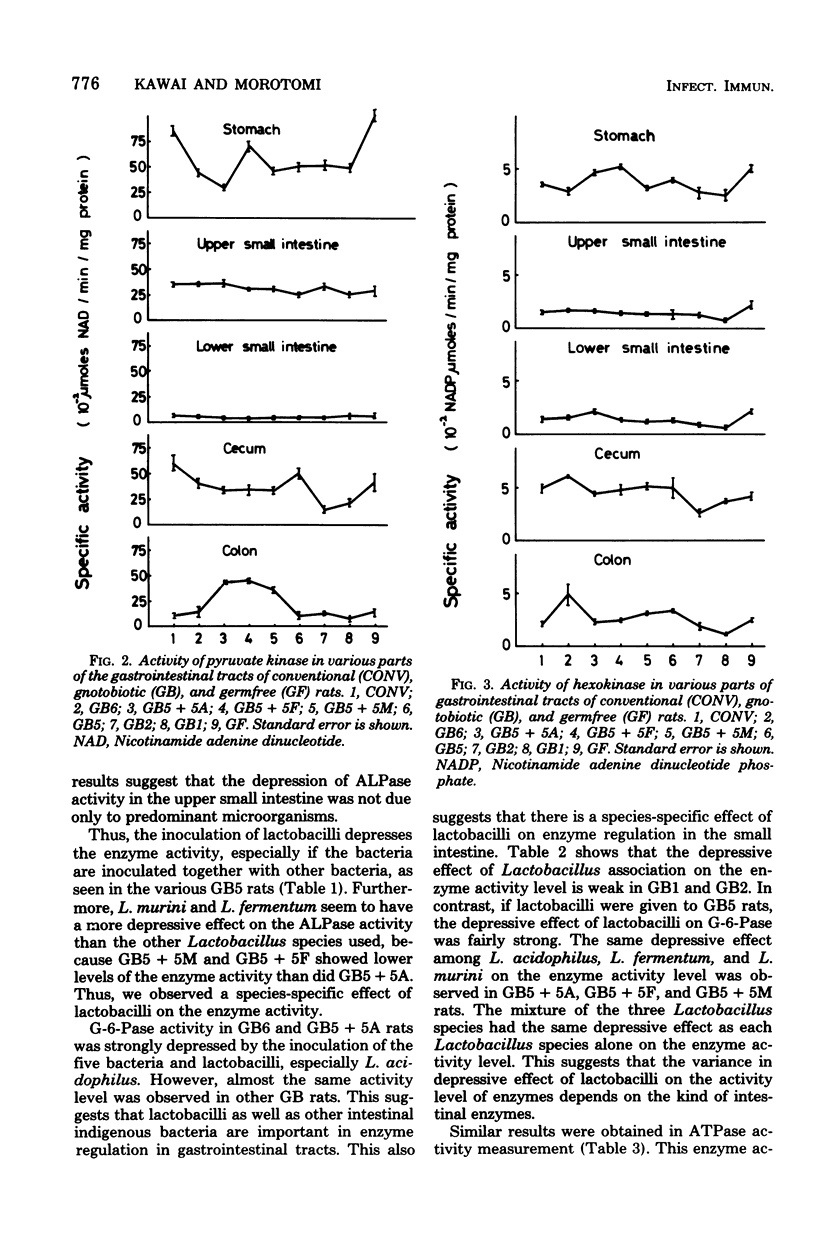
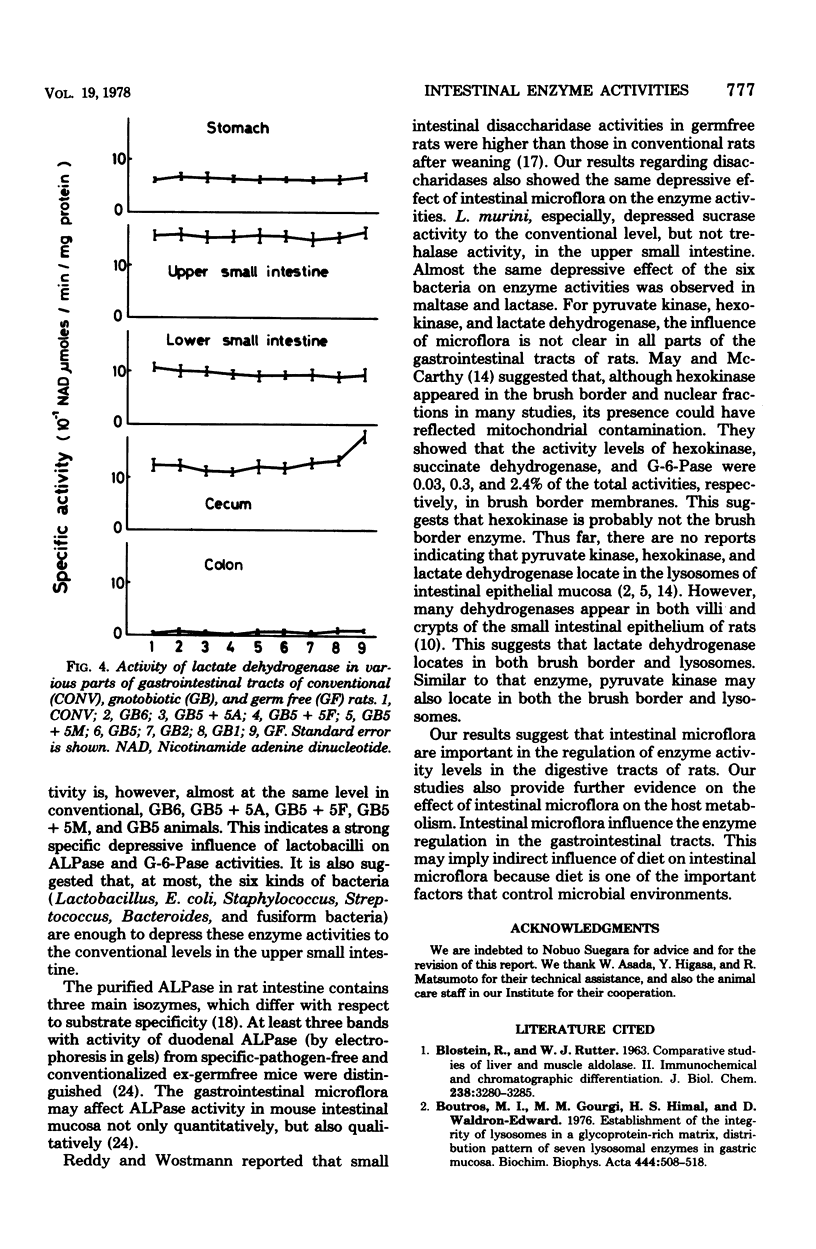
Various enzyme activities involved in the active transport system, glycolysis, and digestion were assayed in various parts of the gastrointestinal tracts of germfree, conventional, and gnotobiotic rats associated with indigenous bacteria. The activity levels of alkaline phosphatase, glucose 6-phosphatase, adenosine triphosphatase, and disaccharidases in the upper small intestine were highest in all parts of the gastrointestinal tracts of various kinds of gnotobiotic, conventional, and germfree rats. Alkaline phosphatase, glucose 6-phosphatase, and adenosine triphosphatase activities in the upper small intestine of germfree rats were, respectively, 2.3-, 2.9-, and 1.7-fold higher than those in conventional rats. Similar to the results of these enzymes, sucrase, maltase, trehalase, and lactase activities in the upper small intestine of germfree rats were, respectively, 1.6-, 1.5-, 2.3-, and 1.8-fold higher than those in conventional rats. In various gnotobiotic rats, enzyme activity levels were intermediate between those in germfree and conventional rats. These findings suggest that those enzymatic activities are strongly depressed by the association with the indigenous microorganisms in the epithelial mucosa of the upper small intestine of rats. The levels of pyruvate kinase, hexokinase, and lactate dehydrogenase activities were highest, respectively, in the stomach, cecum, and the upper small intestine and cecum in all parts of the gastrointestinal tracts in various kinds of gnotobiotic, conventional, and germfree rats. It was also shown that six kinds of gastrointestinal bacteria, including lactobacilli, significantly depressed the enzyme activity levels to levels between those of the germfree and conventional rats in the upper small intestine of gnotobiotic rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOSTEIN R., RUTTER W. J. COMPARATIVE STUDIES OF LIVER AND MUSCLE ALDOLASE. II. IMMUNOCHEMICAL AND CHROMATOGRAPHIC DIFFERENTIATION. J Biol Chem. 1963 Oct;238:3280–3285. [PubMed] [Google Scholar]
- Boutros M. I., Gourgi M. M., Himal H. S., Waldron-Edward D. Establishment of the integrity of lysosomes in a glycoprotein-rich matrix. Distribution pattern of seven lysosomal enzymes in gastric mucosa. Biochim Biophys Acta. 1976 Sep 24;444(2):508–518. doi: 10.1016/0304-4165(76)90394-9. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Fortin-Magana R., Hurwitz R., Herbst J. J., Kretchmer N. Intestinal enzymes: indicators of proliferation and differentiation in the jejunum. Science. 1970 Mar 20;167(3925):1627–1628. doi: 10.1126/science.167.3925.1627. [DOI] [PubMed] [Google Scholar]
- HAENNINEN O., HARTIALA K. STUDIES ON INORGANIC PHOSPHATE, INORGANIC PYROPHOSPHATASE AND ALKALINE NONESPECIFIC PHOSPHOMONOESTERASE LEVELS IN THE GASTROINTESTINAL TRACT OF THE RAT. Acta Chem Scand. 1965;19:817–822. [PubMed] [Google Scholar]
- Henderson J. D., Jr, Titus J. L. Growth rates and morphology of germfree and conventional mice. Mayo Clin Proc. 1968 Jul;43(7):517–529. [PubMed] [Google Scholar]
- Hirschhorn N., Rosenberg I. H. Sodium-potassium stimulated adenosine triphosphatase of the small intestine of man: studies in cholera and other diarrheal diseases. J Lab Clin Med. 1968 Jul;72(1):28–39. [PubMed] [Google Scholar]
- Iemhoff W. G., Hülsmann W. C. Development of mitochondrial enzyme activities in rat-small-intestinal epithelium. Eur J Biochem. 1971 Dec 10;23(3):429–434. doi: 10.1111/j.1432-1033.1971.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Kawachi T., Tanaka N., Kogure K., Sugimura T. Changes in aldolase isozymes of the digestive tract during postnatal development. Biochim Biophys Acta. 1973 Aug 17;320(1):59–63. doi: 10.1016/0304-4165(73)90165-7. [DOI] [PubMed] [Google Scholar]
- LESHER S., WALBURG H. E., Jr, SACHER G. A., Jr GENERATION CYCLE IN THE DUODENAL CRYPT CELLS OF GERM-FREE AND CONVENTIONAL MICE. Nature. 1964 May 30;202:884–886. doi: 10.1038/202884a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- May R. J., McCarthy D. M. Particulate' hexokinase activity in rat intestine. The comparative contributions of mitochondria and brush-border membranes. Biochem J. 1976 Oct 1;159(1):189–192. doi: 10.1042/bj1590189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi M., Watanabe T., Suegara N., Kawai Y., Mutai M. Distribution of indigenous bacteria in the digestive tract of conventional and gnotobiotic rats. Infect Immun. 1975 May;11(5):962–968. doi: 10.1128/iai.11.5.962-968.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. S., Wostmann B. S. Intestinal disaccharidase activities in the growing germfree and conventional rats. Arch Biochem Biophys. 1966 Mar;113(3):609–616. doi: 10.1016/0003-9861(66)90238-4. [DOI] [PubMed] [Google Scholar]
- Saini P. K., Done J. The diversity of alkaline phosphatase from rat intestine. Isolation and purification of the enzyme (s). Biochim Biophys Acta. 1972 Jan 20;258(1):147–153. doi: 10.1016/0005-2744(72)90974-6. [DOI] [PubMed] [Google Scholar]
- Stifel F. B., Rosenweig N. S., Zakim D., Herman R. H. Dietary regulation of glycolytic enzymes. I. Adaptive changes in rat jejunum. Biochim Biophys Acta. 1968 Dec 23;170(2):221–227. doi: 10.1016/0304-4165(68)90001-9. [DOI] [PubMed] [Google Scholar]
- Suegara N., Morotomi M., Watanabe T., Kawal Y., Mutai M. Behavior of microflora in the rat stomach: adhesion of lactobacilli to the keratinized epithelial cells of the rat stomach in vitro. Infect Immun. 1975 Jul;12(1):173–179. doi: 10.1128/iai.12.1.173-179.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G., CANTERO A. Glucose-6-phosphatase activity in normal, pre-cancerous, and neoplastic tissues. Cancer Res. 1955 Feb;15(2):105–108. [PubMed] [Google Scholar]
- Watanabe T., Morotomi M., Suegara N., Kawai Y., Mutai M. Distribution of indigenous Lactobacilli in the digestive tract of conventional and gnotobiotic rats. Microbiol Immunol. 1977;21(4):183–191. doi: 10.1111/j.1348-0421.1977.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Yolton D. P., Savage D. C. Influence of certain indigenous gastrointestinal microorganisms on duodenal alkaline phosphatase in mice. Appl Environ Microbiol. 1976 Jun;31(6):880–888. doi: 10.1128/aem.31.6.880-888.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Savage D. C. Influence of the indigenous gastrointestinal microbial flora on duodenal Mg2+ -dependent and (Na+ + K+) -stimulated adenosine triphosphatase activities in mice. Infect Immun. 1976 Apr;13(4):1193–1198. doi: 10.1128/iai.13.4.1193-1198.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Stanley C., Savage D. C. Influence of the indigenous gastrointestinal microbial flora on duodenal alkaline phosphatase activity in mice. Infect Immun. 1971 Jun;3(6):768–773. doi: 10.1128/iai.3.6.768-773.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



