Abstract
Alcohol abuse is the most common and costly form of drug abuse in the United States. It is well known that alcohol abuse contributes to risky behaviors associated with greater incidence of human immunodeficiency virus (HIV) infections. As HIV has become a more chronic disease since the introduction of antiretroviral therapy, it is expected that alcohol use disorders will have an adverse effect on the health of HIV-infected patients. The biomedical consequences of acute and chronic alcohol abuse are multisystemic. Based on what is currently known of the comorbid and pathophysiological conditions resulting from HIV infection in people with alcohol use disorders, chronic alcohol abuse appears to alter the virus infectivity, the immune response of the host, and the progression of disease and tissue injury, with specific impact on disease progression. The combined insult of alcohol abuse and HIV affects organ systems, including the central nervous system, the immune system, the liver, heart, and lungs, and the musculoskeletal system. Here we outline the major pathological consequences of alcohol abuse in the HIV-infected individual, emphasizing its impact on immunomodulation, erosion of lean body mass associated with AIDS wasting, and lipodystrophy. We conclude that interventions focused on reducing or avoiding alcohol abuse are likely to be important in decreasing morbidity and improving outcomes in people living with HIV/AIDS.
Keywords: AIDS, alcohol, cytokines, immune, muscle wasting, SIV
Introduction
While the number of newly diagnosed cases of human immunodeficiency virus (HIV) has decreased during the past decade, the number of people living with HIV/AIDS (PLWHA) has continued to rise. More than 1.1 million people in the U.S. are currently estimated to live with HIV [1]. Chronic alcohol consumption prevails as the most common and costly form of drug abuse, with approximately 7 percent of the adult U.S. population fulfilling the diagnostic criteria for alcohol abuse and/or alcoholism [2, 3]. The use of antiretroviral therapy (ART) has substantially reduced HIV-associated morbidity and mortality, making HIV a chronic disease during which infected individuals are likely to engage in alcohol abuse at a rate comparable to or greater than those of the non-infected population [4,5]. Thus, the social and behavioral patterns of the population at large are also prevalent in HIV+ individuals [6].
Alcohol and HIV Frequently Coexist
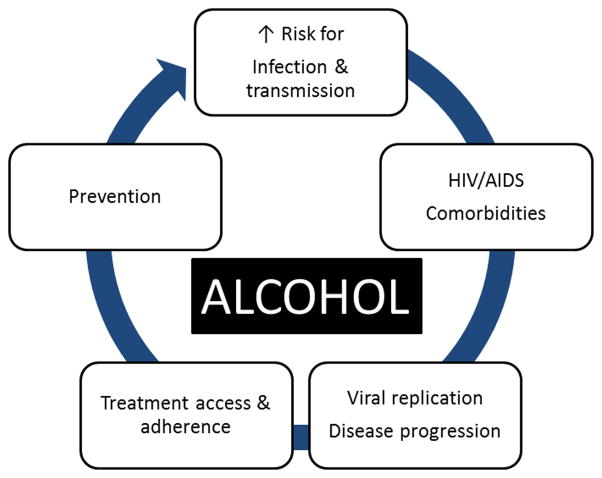
Alcohol abuse and HIV frequently coexist in the same individual [4, 7, 8]. Some studies indicate that approximately 50% of HIV+ patients currently in care self-report consuming any alcohol [8, 9]. Moreover, rates of heavy drinking among PLWHA are higher than those in the general population [8, 10]. Reports range from 9% of PLWHA surveyed engaging in regular binge drinking [11] to some reports of problem drinking in as many as 40 to 50% of patients surveyed [12]. Alcohol use disorders (AUD) have long been recognized as a significant behavioral risk factor conducive to increased incidence of HIV infection. Although the potential interaction between alcohol-related biomedical consequences and the progression of HIV infection has received increased attention, there is a dearth of information on the likelihood of changes in the course of disease progression due to alcohol-associated biomedical derangements [13] (Fig. 1).
Figure 1.
Alcohol has had significant impact on the HIV epidemic. Risky sexual behavior resulting from impaired cognitive and executive function resulting from alcohol abuse increases risk of infection and impacts on disease transmission. However, alcohol produces multisystemic effects that have been demonstrated to impact biological and biochemical aspects of the HIV disease processes, increasing the risk for comorbid conditions and impacting the disease progression. More recently, the potential interferences with treatment and prevention of infection have been reported, particularly as it pertains to access and availability of antiretroviral therapy and its effectiveness in viral control in this patient population. Issues such as access and adherence to therapy as well as their combined toxicity are the focus of health care provider concern.
Alcohol Abuse Contributes to Comorbid Conditions in HIV+
Alcohol abuse has significant multisystemic pathophysiological outcomes including disruption of nutritional, metabolic, oxidative, and neuroendocrine pathways [14]. Due to the chronic nature of the HIV infection and AUD; the heterogeneous patient population; the effects of non-prescription, experimental drugs frequently used by these individuals; and limitations in methodology to investigate the cellular and molecular mechanisms that drive viral kinetics and resulting injury; it is extremely difficult to conduct a controlled study of the alcohol-deranged biological mechanism(s) that can impact the course of HIV infection. Thus, few studies have examined the pathophysiology involved in alcohol-abusing HIV+ patients and even fewer in patients on ART. Most of our knowledge is derived from experimental models, including that of simian immunodeficiency virus (SIV)-infected rhesus macaques, which is known to be the best animal model for studying the pathogenesis of HIV-like infection because of its similarities to HIV/AIDS in humans.
Non-Human Primate Model for the Study of Alcohol Interaction with SIV Disease Progression
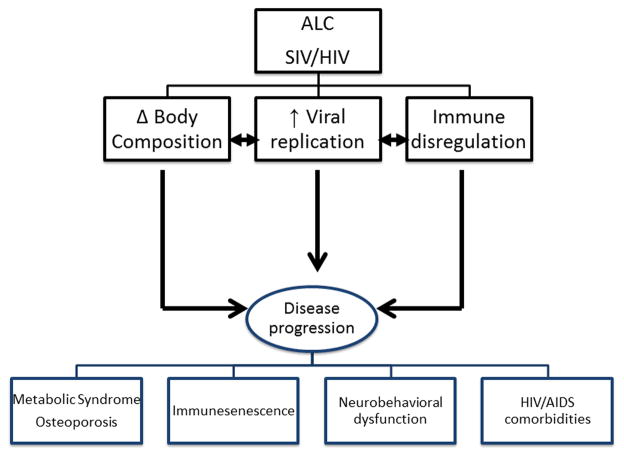
The simian immunodeficiency viruses are considered the closest relatives of the human AIDS viruses because of their genetic, antigenic, and biologic properties [15]. Experimental SIV infection of rhesus macaques results in a disease that is remarkably similar to human HIV/AIDS [16]. Inoculation with SIV results in peak viral load between days 14 and 21, and set point viral load is well established after 30 days. This is followed by an asymptomatic infection period and ultimately culminates in a clinical AIDS stage. Immunologically, SIV infection is characterized by a noteable reduction in CD4+ cells and in the CD4+/CD8+ cell ratio, as well as a substantial increase in the rates of lymphocyte turnover [16]. The final stages of the disease are characterized by diarrhea, weight loss, lymphopenia, thrombocytopenia, and lymphadenopathy/lymphoid hyperplasia, progressing to immunosuppression [17, 18]. The precipitating events that are criteria for euthanasia include enteric disease, diarrhea, weight loss, neurological deficits, and opportunistic infections. Using this clinically relevant model of HIV-like infection, we have provided evidence that chronic alcohol consumption adversely affects the course and progression of the disease, particularly through its effects on the immune and musculoskeletal systems [19, 20, 21]. Our results add to the existing body of knowledge that collectively indicates that alcohol enhances vulnerability to infection and viral replication, discourages adherence to ART, and decreases effectiveness of ART in control of the infection [22] (Fig. 2).
Figure 2.
Key Domains of alcohol abuse interaction with SIV/HIV disease progression. Three salient processes appear to be significantly impacted by chronic alcohol abuse in the SIV-infected macaque: control of body composition, viral replication, and immune responses to infectious challenges. These salient domains are critical in determining disease progression, which in turn is associated with significant loss of functional musculoskeletal mass, immune senescence, neurobehavioral dysfunction and cognitive deficits, and comorbid conditions; all of which are associated with poor outcomes.
Alcohol-Mediated Immunomodulation and its Impact on HIV Disease
The well-known immunosuppressive effects associated with alcohol consumption have been proposed to increase host susceptibility to HIV infection, impairing the immune mechanisms responsible for control of viral load [23]. However, immune activation seen with chronic alcohol consumption is equally as likely to contribute to the impact of immune dysregulation on disease progression and tissue injury. Alcohol facilitates HIV infection of isolated peripheral blood mononuclear cells [24], and oral epithelial cells [25]. Acute alcohol ingestion has also been shown to increase HIV replication in isolated peripheral blood mononuclear cells and to impair stimulated lymphocyte responses [26]. In addition to these in vitro studies, controlled in vivo studies conducted in SIV-infected (SIVmac251 and SIVB670) rhesus macaques have demonstrated that chronic alcohol administration results in higher viral load and decreases time to end-stage disease [19, 20, 27], suggesting the possibility of an alcohol-induced impairment in immunological control of viral replication. However, this has not been consistently demonstrated in our model. Macaques chronically fed alcohol have been shown to have higher viremia despite greater virus-specific cellular immune responses, compared to a sucrose-treated group [28]. An alternative potential mechanism for the enhanced viral replication may be the changes in the gut mucosal immune system. We have found a higher percentage of SIV target cells (CXCR4+CD4) in the gut, coupled with lower percentages of effector memory (CD95+CD28−) CD8 lymphocytes in animals The ineffective control of viral replication could have serious implications on the risk for enhanced transmission rate among HIV+ alcohol users. Opportunistic infections, such as pneumonia, can increase viral replication thereby accelerating progression to AIDS. Our studies [30] have shown that direct intrapulmonary infection with S. pneumoniae leads to prolonged viral replication in infected lungs of SIV-infected alcohol-fed animals as compared to that of SIV-infected controls. The most likely site of viral replication is the alveolar macrophage [31]. These findings are similar to the enhanced localized viral replication elicited by bleomycin in SIV-infected macaques [32]. These findings suggest that HIV+ alcoholic subjects have greater activation of viral replication, or alternatively, decreased capacity to control viral reactivation during opportunistic infections. Increased localized HIV replication, particularly in alveolar macrophages and CD4+ cells, has been reported to increase viral mutations and promote viral escape from latent reservoirs [33]. Thus, factors that preclude effective prevention or resolution of opportunistic infections are likely to endanger the HIV+ host by reactivating the virus or facilitating viral mutations. Acute alcohol abuse has been consistently demonstrated to impair some of the principal factors in the control of infection, including the recruitment of neutrophils to the infection site [34], and to attenuate the granulopoietic response [35]. The implications of alcohol-mediated enhancement of local viral replication are of clinical consequence because higher viral loads may facilitate HIV transmission through increased vaginal shedding [36]. The potential for greater transmissibility of HIV in the alcohol-consuming population therefore warrants further investigation.
Alcohol-mediated alterations in immune function may have additional detrimental effects on vulnerable organ compartments. Gut tissue, liver, and brain have been identified as sites where the impact of alcohol consumption most markedly affects immune cell populations during the acute phase of infection [24]. The resulting tissue inflammation has been proposed to favor a milieu with increased susceptibility to infection, but may also participate in accentuated tissue injury. As demonstrated by studies in rodent models of HIV encephalitis, chronic alcohol feeding results in higher microglial reaction, enhanced oxidative stress, increased viremia, and enhanced neuroinflammation [37]. The synergistic detrimental impact of chronic alcohol exposure and HIV infection has also been demonstrated to result in profound and consistent brain volume deficits, including the cortical mantle, insular and anterior cingulate cortices, thalamus, corpus callosum, and frontal sulci [38]. These alterations in brain inflammatory responses and oxidative stress, as well as the reported morphological changes resulting from chronic alcohol abuse in HIV+ subjects, correlate with clinical manifestations of impaired cognitive function. Chronic alcohol abuse has been reported to exacerbate deficits in motor and visuomotor speed and coordination [39, 40], attention and learning [41], and memory [42] in HIV+ patients. Moreover, depression, the most common psychiatric comorbidity in HIV infection [43], is highly prevalent in HIV+ individuals and alcoholics [44, 45]. Studies from our group have observed similar behavioral deficits in learning and memory formation in SIV-infected chronic alcohol-consuming primates [22]. Whether these findings are a consequence of enhanced neuroinflammation or greater viral load remains to be determined and is the focus of current investigations.
Alcohol and the Musculoskeletal System in HIV
While considerable advances have been made in understanding alcohol-induced injury of the liver, pancreas, heart, and the brain; the metabolic consequences of chronic alcohol abuse have not received the same level of investigation. Chronic alcohol consumption and HIV infection both result in notable changes in metabolic regulation that lead to muscle wasting, loss of bone mineral mass, and lipodystrophy. Muscle wasting, frequently associated with weight loss, is one of the most well-known factors affecting survival from HIV and AIDS. Excess alcohol consumption is consistent with ~50% incidence of skeletal muscle myopathy [46] due to decreased muscle protein synthesis [47, 48, 49] and accelerated muscle proteolysis. Previous studies from our laboratory have shown that chronic binge alcohol consumption causes a greater decrease in lean body mass and an increased incidence of SIV-associated AIDS (SAIDS) wasting. Moreover, we have demonstrated a strong association between the development of SAIDS wasting and decreased time to end-stage disease (from ~900 days in sucrose-fed SIV-infected to 390 days in chronic alcohol-fed SIV-infected macaques) [20]. Similar accentuation of muscle atrophy has been reported in chronic alcohol-fed HIV-1 transgenic rats [50, 51].
Studies suggest that during the initial stage of HIV infection, there is a reduction in lean body mass and preservation of total body fat prior to a decrease in total body weight [52, 53]. Studies in chronic alcohol-fed SIV-infected macaques indicate that rates of skeletal muscle protein synthesis in the fasted and non-intoxicated state are not altered during the asymptomatic or terminal phases of SIV infection [21]. In contrast, reports in the literature indicate that rates of protein synthesis are suppressed following acute and chronic alcohol administration. Moreover, alcohol blunts the muscle anabolic response to the amino acid leucine resulting in “leucine resistance” in the presence of alcohol in the system [54]. The presence of alcohol in the blood at the time of measurement of protein synthesis in our SIV-infected macaques could also unmask alterations in stimulated rates of muscle protein synthesis and contribute to the overall loss in lean body mass has yet to be explored.
The imbalance between increased muscle proteolysis [55] and decreased protein synthesis [56] can be exacerbated by opportunistic infections [57] and attenuated by a reduction of viral load [58]. Thus, decreased lean mass may result from enhanced catabolic processes characteristic of other disease and injury states [59, 60, 61, 62, 63] associated with increased expression of ubiquitin-proteasome pathway elements [64]. This pathway regulates intracellular protein degradation together with lysosomal and non-lysosomal pathways [65] and is modulated by glucocorticoids, catecholamines, and proinflammatory cytokines [66, 67, 68]. Similar increased expression of the genes encoding for the ubiquitin-ATP-dependent proteolytic system has been detected in skeletal muscle of cachectic AIDS patients [69], suggesting that the ubiquitin-proteasome pathway contributes to AIDS-related muscle wasting. We have shown that both alcohol and SIV infection raise levels of ubiquitin-proteasome components as well as proteasomal activity in skeletal muscle mRNA [70]. Moreover, we have found elevated atrogene expression in skeletal muscle of chronic alcohol-fed SIV-infected macaques [71]. Atrogin-1, also known as MAFbx (muscle atrophy F-box protein), has been suggested as a contributing cause of muscle wasting [72, 73].
The increased ubiquitin proteasome pathway activity in skeletal muscle of chronic alcohol-fed SIV-infected (SIVmac251 and SIVB670) animals is associated with marked upregulation of tissue inflammation and oxidative stress and decreased IGF-I expression [70]. Similar derangements in growth factors and proinflammatory cytokine expression have been associated with wasting in AIDS patients [74, 75], as well as in other animal models of chronic alcohol feeding and HIV infection [51, 76, 77]. Proinflammatory cytokines including IL-6 and TNF-α have been determined to exert anti-insulin effects in both skeletal muscle and adipose tissue [78, 79]. Furthermore, it has been shown that myostatin, a negative modulator of skeletal muscle growth [80], is involved in suppression of skeletal muscle growth and in muscle wasting in HIV-infected men [81]. Taken together, our studies and those of others indicate that chronic alcohol consumption promotes an inflammatory and pro-oxidative tissue milieu that precedes upregulation of multiple components of the ubiquitin-proteolytic pathway. In addition to a loss in muscle fiber proteins resulting from activation of the ubiquitin-proteasome system, the autophagy-lysosome pathway may contribute to muscle wasting in chronic disease conditions [82]. Whether this mechanism contributes to alcoholic myopathy or AIDS wasting remains to be determined, but warrants investigation.
An important factor promoting muscle wasting, particularly in chronic alcohol and SIV/HIV infection, is suppressed anabolic (insulin/IGF-I) signaling that can simultaneously favor proteolysis and inhibit anabolism. We have shown that IGF-I and mTOR mRNA expression and PI3-K activity are significantly decreased, while PTP1B expression is significantly increased in skeletal muscle of chronic alcohol-fed SIV-infected animals at end stage [70, 71]. In addition, we have also shown that skeletal muscle of chronic alcohol-fed SIV-infected animals showed significantly increased atrogin-1 mRNA expression [70]. Thus, our findings coincide with previous clinical and pre-clinical reports indicating that inflammation impacts the responsiveness of muscle tissue to an anabolic stimulus and is associated with marked alterations of protein metabolism [54, 83, 84, 85]. It appears likely that the accentuated loss of muscle mass at SAIDS in chronic alcohol-fed animals is caused by a proinflammatory, pro-oxidative, and anabolic imbalance favoring overall catabolic processes leading to loss of muscle mass associated with impaired muscle growth.
Several reports strongly suggest that chronic alcohol intake disrupts multiple neuroendocrine signaling pathways responsible for control of metabolism [13, 21, 154]. Thus, it is possible that in addition to altering caloric and nutrient intake, alcohol disrupts the neuroendocrine response to feeding, thus further compounding the problem by interfering with the feeding-induced anabolic response. The loss in lean body mass is known to be a comorbidity factor for chronic diseases including cancer and AIDS. Interventions to reduce alcohol consumption or modify nutritional intake should lead to preservation of lean mass. Published studies suggest that even reduction of alcohol drinking without achieving complete abstinence is sufficient to improve muscle strength and decrease myopathy [86].
Alcohol and SIV/HIV Promote Bone Loss
Chronic alcohol consumption is correlated with changes in bone metabolism, decreased bone mineral density, and greater risk of fractures [86, 87, 88] even in the absence of liver failure [89]. Overall, it has been estimated that osteoporosis is prevalent in over 40% of alcoholics [90]. The detrimental effect of chronic alcohol consumption on bone mineral density and bone mineral mass [91, 92] is multifactorial [93]. Impaired bone formation has been proposed as a principal mechanism for increased fracture rates in chronic alcoholics [94, 95], and this has been reported to be restored following relatively short periods of abstinence [96]. Whether the relationship of alcohol consumption with fracture risk, bone mineral density, or osteoporosis is dose-dependent remains unclear and merits further study [97].
High prevalence of osteopenia and osteoporosis is a result of marked alterations in bone metabolism, which is also consistent with HIV infection. [98]. Risk factors for the development of osteopenia include the use of protease inhibitors, duration of HIV infection, high viral load, high lactate levels, low bicarbonate levels, elevated alkaline phosphatase levels, and lower body weight prior to initiation of antiretroviral therapy [99]. Individuals treated with ART and protease inhibitors have a higher prevalence of reduced bone mineral density and osteoporosis compared to untreated HIV-infected patients [100, 101].
The anabolic hormones, particularly testosterone, are among the most important elements regulating bone, adipose, and skeletal muscle mass. Androgens maintain a critical role in the control of bone remodeling by suppressing osteoclastogenesis and promoting osteoblastogenesis, which results in protection of bone mass and mineral density [102]. In addition, androgen-mediated anabolic effects on bone are also attributed to the inhibitory action on proinflammatory cytokines and the promotion of osteoprotegerin synthesis, reducing the activation and maturation of osteoclasts [103,104]. The contribution of proinflammatory cytokines to bone loss and myopathy has been suggested by several studies [84,105, 106, 107]. Bone resorption is associated with an increased expression of proinflammatory cytokines, particularly in androgen-or IGF-I-deficient states [108, 109, 110]. Studies have reported that HIV infection is associated with decreased circulating levels of testosterone and in addition, some have reported a beneficial effect of treatment with the hormone. Whether alcohol further accentuates deficits in anabolic steroid production and function is not clear. However, studies show that chronic alcohol consumption leads to suppression of the hypothalamic-pituitary-gonadal axis resulting in decreased circulating levels of testosterone [111, 112, 113], suggesting a possible endocrine mechanism for alcohol’s effects on bone metabolism.
Alcohol and HIV Dysregulate Adipose Tissue
ART is associated with metabolic dysregulation characterized by dyslipidemia, insulin resistance, and lipodystrophy/lipoatrophy [114], as well as altered adipokine profiles. Adipose tissue and circulating levels of adiponectin are lower in HIV-infected ART patients [115]. Although the pattern of leptin alterations is less clear, recently identified peptides such as visfatin and retinol-binding protein 4 (RBP-4) are altered in HIV-infected individuals. Visfatin expression in adipose tissue correlates with insulin resistance [116]; elevated levels of RBP-4 in obese and diabetic patients correlate with indices of metabolic syndrome, including increased body mass index (BMI), triglycerides, and systolic blood pressure [117]. In addition to changes in circulating markers of metabolic dysregulation, adipose tissue obtained from HIV-infected individuals displays greater proinflammatory cytokine expression, apoptosis, fibrosis, vessel density, and macrophage infiltration, as well as lower adiponectin and leptin mRNA levels than that of control subjects [118]. The elevated expression of proinflammatory cytokines and adipokines, as well as lipodystrophic fat distribution, has been linked to metabolic alterations in HIV+ ART-treated patients [119, 120]. There has been no systematic investigation of the impact of chronic alcohol abuse on adipose tissue mass and phenotype has not been investigated in a systematic way. An association between chronic alcohol consumption and decreased fat mass has been determined [121], and this is considered to be a result of altered neuroendocrine function, resulting in increased cortisol release [122]. In contrast, other studies have determined a tendency of dyslipidemia and increased fat mass in alcoholics, with over 20% of patients fulfilling criteria for metabolic syndrome [123]. However, some new literature suggests that beyond altering fat mass, alcohol may disrupt adipokine profiles such as that of leptin [124] and adiponectin [125] and promote macrophage infiltration into adipose tissue. These changes in adipose tissue phenotype are thought to be the result of oxidative stress resulting from alcohol metabolism [126] and are associated with hepatic and adipose tissue insulin resistance [127]. It is a likely possibility that excessive chronic alcohol consumption exacerbates the alterations in adipose tissue mass, distribution, and phenotype associated with HIV infection and ART, and this is an area of current investigation.
Potential Mechanisms for Alcohol-Mediated Effects on Body Composition
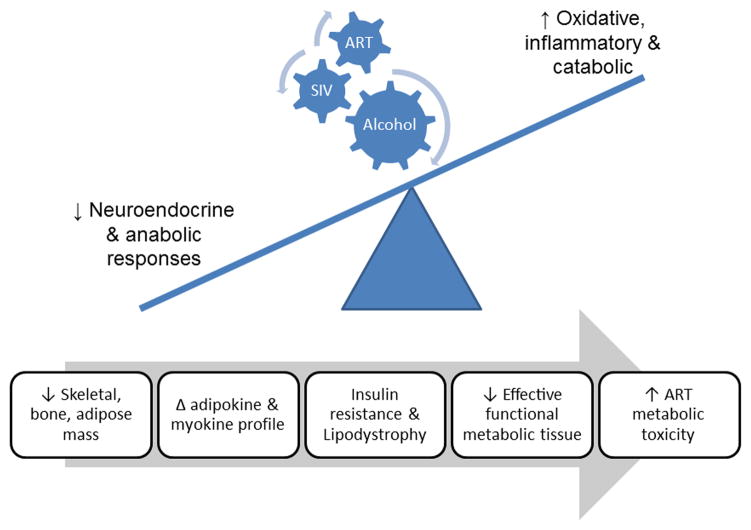
Results from others as well as those from our studies point to mechanisms involved in erosion of lean body mass and dysregulation of adipose mass phenotype in HIV patients (Fig. 3). Body mass constituted by both lean (muscle and bone) and fat is determined by the net balance between protein synthesis and breakdown in muscle, between mineralization and resorption in bone, and between lipogenesis and lipolysis in adipose tissue. The anabolic and catabolic arms of this balance are regulated by nutritional, endocrine, inflammatory, and oxidative mechanisms. Chronic alcohol consumption disrupts several of the above factors, leading to imbalance between anabolic and catabolic mechanisms [128–132]. Oxidative stress caused by either an excess production of reactive oxygen species or a reduction in antioxidant equivalents in tissue has been consistently shown to be an important mechanism of tissue injury caused by chronic alcohol abuse [133, 134]. Several mechanisms have been identified as contributors to alcohol-induced oxidative stress, including alcohol metabolism, mitochondrial toxicity, tissue inflammation, and glutathione depletion. Although the impact of oxidative stress on hepatic injury has been well established [135], conclusive evidence for its role in alcohol-induced metabolic derangements of muscle is lacking [136]. However, studies aimed at elucidating the mechanisms underlying catabolic and metabolic dysregulation processes in muscle [137, 138], bone [139, 140], and adipose tissue [141] suggest that oxidative stress may be a common mechanism through which alcohol can lead to metabolic dysregulation [142]. Similarly, chronic alcohol administration has been shown to result in enhanced proinflammatory cytokine expression, contributing to injury in several tissues [143, 144, 145, 146]. Upregulation of inflammatory cytokines has been found to aid in catabolic responses in skeletal muscle [147, 148] and bone [149, 150] and to dysregulate adipose tissue metabolism [151]. In addition, chronic alcohol administration is also associated with altered neuroendocrine regulation, in particular with decreased circulating levels of androgens [152, 153] and with impairments in the GH/IGF-I system [154, 155], both of which are critical factors mediating the anabolic and anticatabolic control of bone and muscle mass. HIV/AIDS is associated with dysregulation of these same neuroendocrine, oxidative, and inflammatory factors including the GH/IGF-I system [156, 157], androgens [158], proinflammatory cytokines [159, 160, 161, 162], and oxidative stress [163, 164].
Figure 3.
Chronic alcohol consumption exerts multisystemic effects on SIV-infected macaques that involve neuroendocrine, oxidative, and proinflammatory mechanisms. Chronic alcohol administration decreases musculoskeletal and adipose mass and favors tissue proinflammatory and pro-oxidative milieus that disrupt the balance between the synthetic and catabolic mechanisms leading to erosion of muscle, bone, and adipose tissue mass. The figure reflects the predicted alterations in neuroendocrine and anabolic processes that in the presence of a pro-oxidative, inflammatory, and catabolic milieu lead to decreased functional metabolic mass and possible augmentation of ART-induced metabolic burden and toxicity.
Alcohol Use Disorders Interfere with HIV Disease Management on Multiple Levels
The detrimental role of alcohol abuse in the HIV/AIDS epidemic is further substantiated by its correlation to lack of adherence to ART and decreased effectiveness of ART in control of the infection [165]. In addition to the impact of alcohol consumption on the natural progression of HIV infection [166], alcohol consumption may affect access, adherence, and responsiveness to ART [167, 168]. AUD interferes with disease management in HIV+ patients. AUD is a significant predictor of non-adherence to ART [169, 170, 171, 172]. PLWHA and AUD are consistently found to perform poorly at multiple levels of the HIV treatment cascade, resulting in a higher likelihood of virologic non-suppression [173, 174, 175]. Despite reduced morbidity and mortality from HIV with ART [176], many alcohol-using individuals fail to achieve the minimum 95% adherence and thus do not achieve complete viral suppression and the prevention of the development of a resistant virus [177, 178, 179, 180]. Moreover, alcohol abuse appears to accelerate disease progression, even if medications are taken correctly, by adversely impacting drug absorption and metabolism [167]. Some evidence suggests that lower CD4 counts and higher HIV RNA levels in HIV+ patients undergoing ART and intaking moderate to at-risk levels of alcohol, results in greater disease progression compared to abstainers [166]. Additional factors involved in alcohol’s exacerbation of the HIV epidemic are based on the positive correlations between alcohol use and high-risk sexual behaviors in individuals living with HIV/AIDS [181, 182, 183]. Excessive alcohol consumption increases the risk of HIV transmission and sexually-transmitted diseases, “super-/co-infections” (i.e. infection with a new and/or drug-resistant strain of the virus), and also increases resistance to drug treatments [184]. Successful HIV disease management can fail at any of the various aspects of the HIV treatment cascade, including failure to link, engage, and retain patients in care; medication non-adherence; and resistance to antiretroviral medications [185]. Thus, additional comorbid conditions that can potentially impact the risk of infection, transmission, viral control, and disease progression are prevalent in HIV-infected individuals with AUD.
Summary
Alcohol abuse in the HIV-infected population contributes to the burden of the disease, having implications that surpass the original issue of increased risky sexual behavior. The consequences of alcohol abuse alone before or during the course of HIV infection compound the damage inflicted by the virus or the resulting tissue inflammation. Moreover, it has now become increasingly evident that alcohol use disorders diminish adherence to therapy, may potentially aggravate unwanted drug-related side effects, and prevent effectiveness in viral control, further complicating the management of the disease. Additional studies are needed to understand the cellular mechanisms of alcohol-induced pathological effects and their interaction with those produced by the virus, its proteins, or the resulting inflammatory milieu associated with HIV infection. These will lead to improved understanding of potential targets for therapeutic or nutritional interventions aimed at reducing the burden of disease in this population.
The interaction of alcohol with the process of infectivity, viral replication, progression of disease, and mortality is of sufficient magnitude to warrant interventions to decrease or prevent alcohol abuse in HIV-infected individuals. Several measures have been found to be effective at minimizing HIV transmission associated with alcohol use, including early HIV testing and referral to treatment and behavioral interventions aimed at both HIV-infected and HIV-uninfected people with HIV risk behaviors. Moreover, in the alcohol-consuming population, behavioral and pharmacological treatment aimed at reduction of alcohol consumption enhances the effectiveness of HIV treatment [12]. In addition, indirect evidence strongly suggests that several of the comorbid biomedical consequences associated with alcohol use disorders significantly improve with reduction in alcohol intake. Thus, critical mechanisms that may impact disease progression, including tissue inflammation, loss of muscle and bone mass, and lipodystrophy provide important targets for behavioral or pharmacological interventions. Interventions specifically tailored to decrease alcohol use disorders in the HIV+ population are urgently needed to improve overall outcomes and reduce comorbid conditions in this vulnerable patient population. Clearly a systematic, integrated approach including clinicians, researchers, and public health officials is needed to deal with this ongoing epidemic.
Acknowledgments
The authors are grateful for editorial support from Betsy Giaimo and research support provided by NIAAA-07577, NIAAA-09803, UAA021995A, and AA-11290.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. HIV AIDS surveillance report. US HIV and AIDS cases reported through June 2000. 2000;12(1) [Google Scholar]
- 2.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64 (7):830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 3.Grant BF, Harford TC, Dawson DA, et al. Prevalence of DSM-IV alcohol abuse and dependence; United States. Alcohol Health and Research World. 1994;18(3):243–248. [PMC free article] [PubMed] [Google Scholar]
- 4.Lefevre F, O’Leary B, Moran M, et al. Alcohol consumption among HIV-infected patients. J Gen Intern Med. 1995;10(8):458–60. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- 5.Lee LM, Karon JM, Selik R, et al. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984–1997. JAMA. 2001;285(10):1308–15. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- 6.Samet JH, Phillips SJ, Horton NJ, et al. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20 (2):151–5. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- 7.Galvan FH, Bing EG, Fleishman JA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63(2):179–86. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 8.Kalichman SC, Amaral CM, White D, et al. Prevalence and clinical implications of interactive toxicity beliefs regarding mixing alcohol and antiretroviral therapies among people living with HIV/AIDS. AIDS Pt Care and STDs. 2009;23(6):449–54. doi: 10.1089/apc.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenfield TK, Midanik LT, Rogers JD. A 10-year national trend study of alcohol consumtion, 1984–1995: Is the period of declining drinking over? Am J Public Health. 2000;90(1):47–52. doi: 10.2105/ajph.90.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel L, Carrieri MP, Fugon L, et al. Harmful alcohol consumption and patterns of substance use in HIV-infected patients receiving antiretrovirals (ANRS-EN12-VESPA Study): Relevance for clinical management and intervention. AIDS Care. 2010;22(9):1136–45. doi: 10.1080/09540121003605039. [DOI] [PubMed] [Google Scholar]
- 11.Phillips SJ, Freedberg KA, et al. Screening for alcohol problems in HIV-infected primary care patients. J Gen Intern Med. 2001;16:165. [Google Scholar]
- 12.Samet JH, Cheng DM, Libman H, et al. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46(2):194–9. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina PE, Hoek JB, Nelson S, et al. Mechanisms of Alcohol-Induced Tissue Injury. Alcohol Clin Exp Res. 2003;27 (3):563–75. doi: 10.1097/01.ALC.0000057946.57330.F7. [DOI] [PubMed] [Google Scholar]
- 14.Gardner MB, Luciw PA. Simian immunodeficiency viruses and their relationship to the human immunodeficiency virus. AIDS. 1998;2:3–10. doi: 10.1097/00002030-198800001-00002. [DOI] [PubMed] [Google Scholar]
- 15.Arthur LO, Raymond VG, Marx P, Gardner MB. Simian acquired immunodeficiency syndrome. Prog Allergy. 1986;37:332–52. doi: 10.1159/000318452. [DOI] [PubMed] [Google Scholar]
- 16.Mohri H, Bonhoeffer S, Monard S, et al. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science. 1998;279:1223–27. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
- 17.McClure HM, Anderson DC, Ansari AA, et al. Nonhuman primate models for evaluation of AIDS therapy. Ann N Y Acad Sci. 1990;616:287–98. doi: 10.1111/j.1749-6632.1990.tb17849.x. [DOI] [PubMed] [Google Scholar]
- 18.Baskin GB, Murphey-Corb M, Watson EA, et al. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/Delta. Vet Pathol. 1988;25:456–67. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- 19.Bagby GJ, Zhang P, Purcell JE, et al. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006 Oct;30(10):1781–90. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 20.Molina PE, McNurlan M, Rathmacher J, et al. Chronic Alcohol Accentuates Nutritional, Metabolic, and Immune Alterations During Asymptomatic Simian Immunodeficiency Virus Infection. Alcohol Clin Exp Res. 2006;30(12):2065–78. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 21.Winsauer PJ, Moerschbaecher JM, Brauner IN, et al. Alcohol unmasks simian immunodeficiency virus-induced cognitive impairments in rhesus monkeys. Alcohol Clin Exp Res. 2002 Dec;26(12):1846–57. doi: 10.1097/01.ALC.0000042171.80435.F1. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Drug-associated HIV transmission continues in the United States. 2002. [Google Scholar]
- 23.Marcondes MC, Watry D, Zandonatti M, et al. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2008 Sep;32(9):1583–92. doi: 10.1111/j.1530-0277.2008.00730.x. Epub 2008 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Zha J, Nishitani J, et al. HIV-1 infection in peripheral blood lymphocytes (PBLs) exposed to alcohol. Virology. 2003;307(1):37–44. doi: 10.1016/s0042-6822(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 25.Zheng J, Yang OO, Xie Y, et al. Ethanol stimulation of HIV infection of oral epithelial cells. J Acquir Immune Defic Syndr. 2004;37(4):1445–53. doi: 10.1097/01.qai.0000129572.13008.db. [DOI] [PubMed] [Google Scholar]
- 26.Bagasra O, Kajdacsy-Balla A, Lischner HW, et al. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. J Infect Dis. 1993;167(4):789–97. doi: 10.1093/infdis/167.4.789. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R, Perez-Casanova AE, Tirado G, et al. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr. 2005;39(4):386–90. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- 28.Pahar B, Amedee AM, Thomas J, et al. Effects of alcohol consumption on antigen-specific cellular and humoral immune responses to SIV in rhesus macaques. J Acquir Immune Defic Syndr. 2013 Jun 24; doi: 10.1097/QAI.0b013e31829f6dca. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poonia B, Nelson S, Bagby GJ, et al. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res Hum Retroviruses. 2005 Oct;21(10):863–8. doi: 10.1089/aid.2005.21.863. Erratum in: AIDS Res Hum Retroviruses. 2006 Aug; 22(8): 824. Corrected and republished in: AIDS Res Hum Retroviruses. 2006 Jun; 22 (6): 589–94. [DOI] [PubMed] [Google Scholar]
- 30.Nelson S, Happel KI, Zhang P, et al. Effect of bacterial pneumonia on lung simian immunodeficiency virus (SIV) replication in alcohol consuming SIV-infected rhesus macaques. Alcohol Clin Exp Res. 2013 Jun;37(6):969–77. doi: 10.1111/acer.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller CL, Choi YK, Fallert BA, et al. Restricted SIV replication in rhesus macaque lung tissues during the acute phase of infection. Am J Pathol. 2002 Sep;161(3):969–78. doi: 10.1016/S0002-9440(10)64257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhillon NK, Pinson D, Dhillon S, et al. Bleomycin treatment causes enhancement of virus replication in the lungs of SHIV-infected macaques. Am J Physiol Lung Cell Mol Physiol. 2007 May;292(5):L1233–40. doi: 10.1152/ajplung.00293.2006. Epub 2007 Jan 12. [DOI] [PubMed] [Google Scholar]
- 33.Segal LN, Methé BA, Nolan A, et al. HIV-1 and bacterial pneumonia in the era of antiretroviral therapy. Proc Am Thorac Soc. 2011 Jun;8(3):282–7. doi: 10.1513/pats.201006-044WR. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boé DM, Nelson S, Zhang P, et al. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol Clin Exp Res. 2003 Nov;27(11):1838–45. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- 35.Siggins RW, Melvan JN, Welsh DA, et al. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumonia infection with enhancement of STAT3 signaling. J Immunol. 2011 Apr; doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theall KP, Amedee A, Clark RA, et al. Alcohol consumption and HIV-1 vaginal RNA shedding among women. J Stud Alcohol Drugs. 2008;69(3):454–8. doi: 10.15288/jsad.2008.69.454. [DOI] [PubMed] [Google Scholar]
- 37.Potula R, Haorah J, Knipe B, et al. Alcohol abuse enhances neuroinflammation and impairs immune responses in an animal model of human immunodeficiency virus-1 encephalitis. Am J Pathol. 2006;168(4):1335–44. doi: 10.2353/ajpath.2006.051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfefferbaum A, Rosenbloom MJ, Sassoon SA, et al. Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry. 2012 Sep 1;72(5):361–70. doi: 10.1016/j.biopsych.2012.02.018. Epub 2012 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fama R, Eisen JC, Rosenbloom MJ, et al. Upper and lower limb motor impairments in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31(6):1038–44. doi: 10.1111/j.1530-0277.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 40.Rothlind JC, Greenfield TM, Bruce AV, et al. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11(1):70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sassoon SA, Fama R, Rosenbloom MJ, et al. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31(8):1315–24. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 42.Fama R, Rosenbloom MJ, Sassoon SA, et al. Remote semantic memory for public figures in HIV infection, alcoholism, and their comorbidity. Alcoholism: Clinical and Experimental Research. 2011;35:265–276. doi: 10.1111/j.1530-0277.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dube B, Benton T, Cruess DG, et al. Neuropsychiatric manifestations of HIV infection and AID. Journal of Psychiatry and Neuroscience. 2005;30:237–246. [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan LE, Saitz R, Cheng DM, et al. The impact of alcohol use on depressive symptoms in human immunodeficiency virus-infected patients. Addiction. 2008;103:1461–146740. doi: 10.1111/j.1360-0443.2008.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fergusson DM, Boden JM, Horwood LJ. Tests of causal links bewteen alcohol abuse or dependence and major depression. Archives of General Psychiatry. 2009;66:260–266. doi: 10.1001/archgenpsychiatry.2008.543. [DOI] [PubMed] [Google Scholar]
- 46.Preedy VR, Salisbury JR, Peters TJ. Alcoholic muscle disease: features and mechanisms. J Pathol. 1994;173:309–315. doi: 10.1002/path.1711730405. [DOI] [PubMed] [Google Scholar]
- 47.Reilly ME, Mantle D, Richardson PJ, et al. Studies on the time course of ethanol’s acute effects on skeletal muscle protein synthesis: comparison with acute changes in proteolytic activity. Alcohol Clin Exp Res. 1997;21(5):792–798. [PubMed] [Google Scholar]
- 48.Pacy PJ, Preedy VR, Peters TJ, et al. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis-A stable isotope study. Alcohol & Alcoholism. 1991;26:505–513. doi: 10.1093/oxfordjournals.alcalc.a045152. [DOI] [PubMed] [Google Scholar]
- 49.Lang CH, Wu D, Frost RA, et al. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol. 1999;277:268–276. doi: 10.1152/ajpendo.1999.277.2.E268. [DOI] [PubMed] [Google Scholar]
- 50.Pruznak AM, Hong-Brown L, Lantry R, et al. Skeletal and cardiacmyopathy in HIV-1 transgenic rats. Am J Physiol Endocrinol Metab. 2008 Oct;295(4):E96473. doi: 10.1152/ajpendo.90482.2008. Epub 2008 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clary CR, Guidot DM, Bratina MA, et al. Chronic alcohol ingestion exacerbates skeletal muscle myopathy in HIV-1 transgenic rats. AIDS Res Ther. 2011 Aug 16;8:30. doi: 10.1186/1742-6405-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keusch GT, Farthing MJG. Nutritional aspects of AIDS. Ann Rev Nutr. 1990;10:475–501. doi: 10.1146/annurev.nu.10.070190.002355. [DOI] [PubMed] [Google Scholar]
- 53.Brennan MF. Uncomplicated starvation versus cancer cachexia. Cancer Res. 1997;37:2359–64. [PubMed] [Google Scholar]
- 54.Lang CH, Frost RA, Deshpande N, Kumar, et al. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab. 2003;285(6):E1205–15. doi: 10.1152/ajpendo.00177.2003. [DOI] [PubMed] [Google Scholar]
- 55.McNurlan MA, Garlick PJ, Steigbigel RT, et al. Responsiveness of muscle protein synthesis to growth hormone administration in HIV-infected individuals declines with severity of disease. J Clin Invest. 1997;100:2125–2132. doi: 10.1172/JCI119747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yarasheski K, Zachwieja JJ, Gischler J, et al. Increased plasma Gln and Leu Ra and inappropriate low muscle protein synthesis rate in AIDS wasting. Am J Physiol. 1998;275:E577–E583. doi: 10.1152/ajpendo.1998.275.4.E577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiao GM, Fagan J, Lieberman M, et al. Sepsis increases proteasome-dependent proteolysis and mRNA levels for the proteasome subunit RC3 in skeletal muscle. Surg Forum. 1995;46:10–12. [Google Scholar]
- 58.Yarasheski KE, Smith SR, Powderly WG. Reducing plasma HIV RNA improves muscle amino acid metabolism. Am J Physiol Endocrinol Metab. 2005;288:E278–E284. doi: 10.1152/ajpendo.00359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bailey JL, Wang X, England BK, et al. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent, ubiquitin-proteasome pathway. J Clin Invest. 1996;97:1447–1453. doi: 10.1172/JCI118566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molina PE, Tiao G, Meijerink J, et al. Sepsis-induced increase in brain endogenous morphine: regulator of proteolysis. Surg Forum XLVI. 1995:4–6. [Google Scholar]
- 61.Tiao G, Fagan JM, Samuels N, et al. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest. 1994;94:2255–2264. doi: 10.1172/JCI117588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang CH, Tiao G, James JH, et al. Burn injury stimulates multiple proteolytic pathways in skeletal muscle, including the ubiquitin-energy-dependent pathway. J Am Coll Surg. 1995;180:161–70. [PubMed] [Google Scholar]
- 63.Llovera M, Garcia-Martinez C, Agell N, et al. Ubiquitin gene ex expression is increased in skeletal muscle of tumor-bearing rats. FEBS Lett. 1994;338:311–18. doi: 10.1016/0014-5793(94)80290-4. [DOI] [PubMed] [Google Scholar]
- 64.Hasselgren PO. Role of the ubiquitin-proteasome pathway in sepsis-induced muscle catabolism. Mol Biol Rep. 1999;26(1–2):71–6. doi: 10.1023/a:1006916206260. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki K, Bond JS. Advances in Experimental Medicine and Biology. Vol. 389. Plenum Press; New York and London: 1996. Intracellular Protein Catabolism; pp. 1–230. [Google Scholar]
- 66.Tiao G, Fagan J, Roegner V, et al. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest. 1996;97:339–48. doi: 10.1172/JCI118421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Price SR, England BK, Bailey JL, et al. Acidosis and glucocorticoids concomitantly increase ubiquitin and proteasome subunit mRNAs in rat muscle. Am J Physiol. 1994;267:C955–C60. doi: 10.1152/ajpcell.1994.267.4.C955. [DOI] [PubMed] [Google Scholar]
- 68.Costelli P, Garzia-Martinez C, Llovera M, et al. Muscle protein waste in tumor-bearing rats is effectively antagonized by a 2-adrenergic agonist (Clenbuterol). Role of the ATP-ubiquitin-dependent proteolytic pathway. J Clin Invest. 1995;95:2367–72. doi: 10.1172/JCI117929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Llovera M, Garcia-Martinez C, Agell N, et al. Ubiquitin and proteasome gene expression is increased in skeletal muscle of slim AIDS patients. Int J Mol Med. 1998;2(1):69–73. [PubMed] [Google Scholar]
- 70.LeCapitaine NJ, Wang ZQ, Dufour JP, et al. Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. J Infect Dis. 2011 Oct 15;204(8):1246–55. doi: 10.1093/infdis/jir508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Molina PE, Lang CH, McNurlan M, et al. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res. 2008 Jan;32(1):138–47. doi: 10.1111/j.1530-0277.2007.00549.x. Epub 2007 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Attaix D, Ventadour S, Codran A, et al. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–86. doi: 10.1042/EB0410173. [DOI] [PubMed] [Google Scholar]
- 73.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1777–89. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franch HA, Price SR. Molecular signaling pathways regulating muscle proteolysis during atrophy. Curr Opin Clin Nutr Metab Care. 2005;8(3):271–5. doi: 10.1097/01.mco.0000165005.01331.45. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen BY, Clerici M, Venzon DJ, et al. Pilot study of the immunologic effects of recombinant human growth hormone and recombinant insulin-like growth factor in HIV-infected patients. AIDS. 1998;12(8):895–904. doi: 10.1097/00002030-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Otis JS, Guidot DM. Procysteine stimulates expression of keyanabolic factors and reduces plantaris atrophy in alcohol-fed rats. Alcohol Clin Exp Res. 2009 Aug;33(8):1450–9. doi: 10.1111/j.1530-0277.2009.00975.x. Epub 2009 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen VA, Le T, Tong M, et al. Impaired insulin/IGF signaling in experimental alcohol-related myopathy. Nutrients. 2012 Aug;4(8):1058–75. doi: 10.3390/nu4081058. Epub 2012 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Alvaro C, Teruel T, Hernandez R, et al. Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J Biol Chem. 2004;279(17):17070–8. doi: 10.1074/jbc.M312021200. [DOI] [PubMed] [Google Scholar]
- 79.Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, et al. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114(3):183–94. doi: 10.1080/13813450802181047. [DOI] [PubMed] [Google Scholar]
- 80.Thomas M, Langley B, Berry C, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 81.Gonzalez-Cadavid, Taylor WE, Yarasheski K, et al. Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. PNAS. 1998;95:14938–14943. doi: 10.1073/pnas.95.25.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bechet D, Tassa A, Taillandier D, et al. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol. 2005 Oct;37(10):2098–114. doi: 10.1016/j.biocel.2005.02.029. Epub 2005 Mar 23. Review. [DOI] [PubMed] [Google Scholar]
- 83.Gelato MC, Mynarcik D, McNurlan MA. Soluble tumour necrosis factor alpha receptor 2, a serum marker of resistance to the anabolic actions of growth hormone in subjects with HIV disease. Clin Sci (Lond) 102(1):85–90. 02002. [PubMed] [Google Scholar]
- 84.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8(3):255–63. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 85.Späte U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(3):265–9. doi: 10.1097/00075197-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 86.Fernández-Solà J, Nicolás JM, Sacanella E, et al. Low-dose ethanol consumption allows strength recovery in chronic alcoholic myopathy. QJM. 2000 Jan;93(1):35–40. doi: 10.1093/qjmed/93.1.35. [DOI] [PubMed] [Google Scholar]
- 87.Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121(5):406–18. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bikle DD. Alcohol-induced bone disease. World Rev Nutr Diet. 1973;73:53–73. doi: 10.1159/000422460. [DOI] [PubMed] [Google Scholar]
- 89.Saville PD. Alcohol-related skeletal disorders. Ann N Y Acad Sci. 1975;252:287–91. doi: 10.1111/j.1749-6632.1975.tb19169.x. [DOI] [PubMed] [Google Scholar]
- 90.Kim MJ, Shim MS, Kim MK, et al. Effect of chronic alcohol ingestion on bone mineral density in males without liver cirrhosis. Korean J Intern Med. 2003;18(3):174–80. doi: 10.3904/kjim.2003.18.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spencer H, Rubio N, Rubio E, et al. Chronic alcoholism. Frequently overlooked cause of osteoporosis in men. Am J Med. 1986;80(3):393–7. doi: 10.1016/0002-9343(86)90712-6. [DOI] [PubMed] [Google Scholar]
- 92.Malik P, Gasser RW, Kemmler G, et al. Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: a cross-sectional study. Alcohol Clin Exp Res. 2009;33(2):375–81. doi: 10.1111/j.1530-0277.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 93.Santori C, Ceccanti M, Diacinti D, et al. Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest. 2008;31(4):321–6. doi: 10.1007/BF03346365. [DOI] [PubMed] [Google Scholar]
- 94.Laitinen K, Välimäki M. Alcohol and bone. Calcif Tissue Int. 1991;49 (Suppl):S70–3. doi: 10.1007/BF02555094. [DOI] [PubMed] [Google Scholar]
- 95.Santori C, Ceccanti M, Diacinti D, et al. Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest. 2008;31(4):321–6. doi: 10.1007/BF03346365. [DOI] [PubMed] [Google Scholar]
- 96.Laitinen K, Lamberg-Allardt C, Tunninen R, et al. Bone mineral density and abstention-induced changes in bone and mineral metabolism in noncirrhotic male alcoholics. Am J Med. 1992 Dec;93(6):642–50. doi: 10.1016/0002-9343(92)90197-j. [DOI] [PubMed] [Google Scholar]
- 97.Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121(5):406–18. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cazanave C, Dupon M, Lavignolle-Aurillac V, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008;22(3):395–402. doi: 10.1097/QAD.0b013e3282f423dd. [DOI] [PubMed] [Google Scholar]
- 99.Thomas J, Doherty SM. HIV infection--a risk factor for osteoporosis. J Acquir Immune Defic Syndr. 2003;33(3):281–91. doi: 10.1097/00126334-200307010-00001. [DOI] [PubMed] [Google Scholar]
- 100.Bongiovanni M, Tincati C. Bone diseases associated with human immunodeficiency virus infection: pathogenesis, risk factors and clinical management. Curr Mol Med. 2006;6(4):395–400. doi: 10.2174/156652406777435435. [DOI] [PubMed] [Google Scholar]
- 101.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–74. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 102.Vanderschueren D, Vandenput L, Boonen S, et al. Androgens and bone. Endocr Rev. 2004;25(3):389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 103.Hofbauer LC, Khosla S, Dunstan CR, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15(1):2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 104.Michael H, Härkönen PL, Väänänen HK, et al. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res. 2005;20(12):2224–32. doi: 10.1359/JBMR.050803. [DOI] [PubMed] [Google Scholar]
- 105.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30(2):340–6. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 106.Kimble RB. Alcohol, cytokines, and estrogen in the control of bone remodeling. Alcohol Clin Exp Res. 1997;21(3):385–91. doi: 10.1111/j.1530-0277.1997.tb03780.x. [DOI] [PubMed] [Google Scholar]
- 107.Späte U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(3):265–9. doi: 10.1097/00075197-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 108.Bellido T, Jilka RL, Boyce BF, et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest. 1995;95(6):2886–95. doi: 10.1172/JCI117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manolagas SC. Role of cytokines in bone resorption. Bone. 1995;17(2 Suppl):63S–67S. doi: 10.1016/8756-3282(95)00180-l. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Nishida S, Elalieh HZ, et al. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21(9):1350–8. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Emanuele MA, Emanuele N. Alcohol and the male reproductive system. Alcohol Res Health. 2001;25(4):282–7. [PMC free article] [PubMed] [Google Scholar]
- 112.Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril. 2005;84(4):919–24. doi: 10.1016/j.fertnstert.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 113.Van Thiel DH, Lester R. Alcoholism: its effect on hypothalamic pituitary gonadal function. Gastroenterology. 1976;71(2):318–27. [PubMed] [Google Scholar]
- 114.Barbaro G. Highly active antiretroviral therapy-associated metabolic syndrome: pathogenesis and cardiovascular risk. Am J Ther. 2006;13(3):248–60. doi: 10.1097/01.mjt.0000162013.66614.16. [DOI] [PubMed] [Google Scholar]
- 115.Kosmiski L, Kuritzkes D, Lichtenstein K, et al. Adipocyte-derived hormone levels in HIV lipodystrophy. Antivir Ther. 2003;8:9–15. [PubMed] [Google Scholar]
- 116.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 117.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 118.Jan V, Cervera P, Maachi M, et al. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther. 2004;9(4):555–64. [PubMed] [Google Scholar]
- 119.Haugaard SB, Andersen O, Dela F, et al. Defective glucose and lipid metabolism in human immunodeficiency virus-infected patients with lipodystrophy involve liver, muscle tissue and pancreatic beta-cells. Eur J Endocrinol. 2005 Jan;152(1):103–12. doi: 10.1530/eje.1.01835. [DOI] [PubMed] [Google Scholar]
- 120.Sevastianova K, Sutinen J, Kannisto K, et al. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2008;295(1):E85–91. doi: 10.1152/ajpendo.90224.2008. [DOI] [PubMed] [Google Scholar]
- 121.Addolorato G, Capristo E, Marini M, et al. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol. 2000;95(9):2323–7. doi: 10.1111/j.1572-0241.2000.02320.x. [DOI] [PubMed] [Google Scholar]
- 122.Leggio L, Malandrino N, Ferrulli A, et al. Is cortisol involved in the alcohol-related fat mass impairment? A longitudinal clinical study. Alcohol Alcohol. 2009;44(2):211–5. doi: 10.1093/alcalc/agn116. [DOI] [PubMed] [Google Scholar]
- 123.Jarvis CM, Hayman LL, Braun LT, et al. Cardiovascular risk factors and metabolic syndrome in alcohol-and nicotine-dependent men and women. J Cardiovasc Nurs. 2007;22(6):429–35. doi: 10.1097/01.JCN.0000297387.21626.88. [DOI] [PubMed] [Google Scholar]
- 124.Nicolás JM, Fernández-Solà J, Fatjó F, et al. Increased circulating leptin levels in chronic alcoholism. Alcohol Clin Exp Res. 2001;25(1):83–8. [PubMed] [Google Scholar]
- 125.Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60(12):790–7. doi: 10.1002/iub.124. [DOI] [PubMed] [Google Scholar]
- 126.Tang H, Sebastian BM, Axhemi A, et al. Ethanol-induced oxidative stress via the CYP2E1 pathway disrupts adiponectin secretion from adipocytes. Alcohol Clin Exp Res. 2012 Feb;36(2):214–22. doi: 10.1111/j.1530-0277.2011.01607.x. Epub 2011 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kang L, Sebastian BM, Pritchard MT, et al. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res. 2007 Sep;31(9):1581–8. doi: 10.1111/j.1530-0277.2007.00452.x. Epub 2007 Jul 11. [DOI] [PubMed] [Google Scholar]
- 128.Molina PE. Alcohol--intoxicating roadblocks and bottlenecks in hepatic protein and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295(1):E1–2. doi: 10.1152/ajpendo.90412.2008. [DOI] [PubMed] [Google Scholar]
- 129.Worrall S, Wilce PA. The effect of chronic ethanol feeding on cytokines in a rat model of alcoholic liver disease. Alcohol Suppl. 1994;2:447–51. [PubMed] [Google Scholar]
- 130.Sonntag WE, Boyd RI. Diminished insulin-like growth factor-1 levels after chronic ethanol: relationship to pulsatile growth hormone release. Alcohol Clin Exp Res. 1989;13:3–7. doi: 10.1111/j.1530-0277.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 131.Saito T, Kobatake K, Ozawa H, et al. Aromatic and branched-chain amino acid levels in alcoholics. Alcohol. 1994;29:133–5. [PubMed] [Google Scholar]
- 132.Shaw S, Lieber CS. Plasma amino acids in the alcoholic: nutritional aspects. Alcohol Clin Exp Res. 1983;7(1):22–27. doi: 10.1111/j.1530-0277.1983.tb05405.x. [DOI] [PubMed] [Google Scholar]
- 133.Fernandez-Checa JC, Kaplowitz N, Colell A, et al. Oxidative stress and alcoholic liver disease. Alcohol Health & Research World. 1997;21:321–4. [PMC free article] [PubMed] [Google Scholar]
- 134.Cederbaum AI. Introduction—Serial review: Alcohol, oxidative stress, and cell injury. Free Radical Biology & Medicine. 2001;31:1524–6. doi: 10.1016/s0891-5849(01)00741-9. [DOI] [PubMed] [Google Scholar]
- 135.Nanji AA, Zhao S, Sadrzadeh SM, et al. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-treated ethanol-fed rats. Alcoholism: Clinical and Experimental Research. 1994;8:1280–5. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 136.Preedy VR, Adachi J, Asano M, et al. Free radicals in alcoholic myopathy: indices of damage and preventive studies. Free Radic Biol Med. 2002;32(8):683–7. doi: 10.1016/s0891-5849(01)00794-8. [DOI] [PubMed] [Google Scholar]
- 137.Russell ST, Eley H, Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal. 2007;19(8):1797–806. doi: 10.1016/j.cellsig.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 138.Otis JS, Brown LA, Guidot DM. Oxidant-induced atrogin-1 and transforming growth factor-beta1 precede alcohol-related myopathy in rats. Muscle Nerve. 2007;36(6):842–8. doi: 10.1002/mus.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bai XC, Lu D, Liu AL, et al. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280(17):17497–506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 140.Grassi F, Tell G, Robbie-Ryan M, et al. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc Natl Acad Sci U S A. 2007;104(38):15087–92. doi: 10.1073/pnas.0703610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Russell ST, Eley H, Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal. 2007;19(8):1797–806. doi: 10.1016/j.cellsig.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 142.Eriksson JW. Metabolic stress in insulin’s target cells leads to ROS accumulation - a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581(19):3734–42. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 143.McClain CJ, Song Z, Barve SS, et al. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G497–502. doi: 10.1152/ajpgi.00171.2004. [DOI] [PubMed] [Google Scholar]
- 144.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 145.Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33(2):191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szabo G, Mandrekar P, Oak S, et al. Effect of ethanol on inflammatory responses. Implications for pancreatitis. Pancreatology. 2007;7(2–3):115–23. doi: 10.1159/000104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lang CH, Krawiec BJ, Huber D, et al. Sepsis and inflammatory insults downregulate IGFBP-5, but not IGFBP-4, in skeletal muscle via a TNF-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R963–72. doi: 10.1152/ajpregu.00684.2005. [DOI] [PubMed] [Google Scholar]
- 148.Frost RA, Lang CH. Alteration of somatotropic function by proinflammatory cytokines. J Anim Sci. 2004;82(E-Suppl):E100–109. doi: 10.2527/2004.8213_supplE100x. [DOI] [PubMed] [Google Scholar]
- 149.Manolagas SC. Role of cytokines in bone resorption. Bone. 1995;17(2 Suppl):63S–67S. doi: 10.1016/8756-3282(95)00180-l. [DOI] [PubMed] [Google Scholar]
- 150.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65(12 Pt 2):S147–51. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 151.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–71. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 152.Lester R, Van Thiel DH. Gonadal function in chronic alcoholic men. Adv Exp Med Biol. 1977;85A:399–413. doi: 10.1007/978-1-4899-5181-6_24. [DOI] [PubMed] [Google Scholar]
- 153.Isidori AM, Lenzi A. Risk factors for androgen decline in older males: lifestyle, chronic diseases and drugs. J Endocrinol Invest. 2005;28(3 Suppl):14–22. [PubMed] [Google Scholar]
- 154.Ronis MJ, Wands JR, Badger TM, et al. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31(8):1269–85. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 155.Röjdmark S, Brismar K. Decreased IGF-I bioavailability after ethanol abuse in alcoholics: partial restitution after short-term abstinence. J Endocrinol Invest. 2001;24(7):476–82. doi: 10.1007/BF03343879. [DOI] [PubMed] [Google Scholar]
- 156.Frost RA, Fuhrer J, Steigbigel R, et al. Wasting in the acquired immune deficiency syndrome is associated with multiple defects in the serum insulin-like growth factor system. Clin Endocrinol. 1996;44:501–14. doi: 10.1046/j.1365-2265.1996.705526.x. [DOI] [PubMed] [Google Scholar]
- 157.Nguyen BY, Clerici M, Venzon DJ, et al. Pilot study of the immunologic effects of recombinant human growth hormone and recombinant insulin-like growth factor in HIV-infected patients. AIDS. 1998;12(8):895–904. doi: 10.1097/00002030-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 158.Poretsky L, Can S, Zumoff B. Testicular dysfunction in human immunodeficiency virus-infected men. Metabolism. 1995;44(7):946–53. doi: 10.1016/0026-0495(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 159.Alonso K, Pontiggia P, Mendenica R, et al. Cytokine patterns in adults with AIDS. Immunol Invest. 1997;26:341–350. doi: 10.3109/08820139709022691. [DOI] [PubMed] [Google Scholar]
- 160.Rimaniol AC, Zylberberg H, Zavala F, et al. Inflammatory cytokines and inhibitors in HIV infection: correlation between interleukin-1 receptor antagonist and weight loss. AIDS. 1996;10:1349–56. doi: 10.1097/00002030-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 161.Alonso K, Pontiggia P, Mendenica R, et al. Cytokine patterns in adults with AIDS. Immunol Invest. 1997;26:341–50. doi: 10.3109/08820139709022691. [DOI] [PubMed] [Google Scholar]
- 162.Rimaniol AC, Zylberberg H, Zavala F, et al. Inflammatory cytokines and inhibitors in HIV infection: correlation between interleukin-1 receptor antagonist and weight loss. AIDS. 1996;10:1349–56. doi: 10.1097/00002030-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 163.Treitinger A, Spada C, Verdi JC, et al. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur J Clin Invest. 2000;30(5):454–9. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- 164.Israël N, Gougerot-Pocidalo MA. Oxidative stress in human immunodeficiency virus infection. Cell Mol Life Sci. 1977;53(11–12):864–70. doi: 10.1007/s000180050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Centers for Disease Control and Prevention. Trends in the HIV and AIDS epidemic. 1998. [Google Scholar]
- 166.Samet JH, Cheng DM, Libman H, et al. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46(2):194–9. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Miguez MJ, Shor-Posner G, Morales G, et al. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8(1):33–7. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- 168.Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43(4):411–7. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–23. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lucas GM, Gebo KA, Chaisson RE, et al. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–74. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 171.Parsons JT, Rosof E, Mustanski B. The temporal relationship between alcohol consumption and HIV-medication adherence: a multilevel model of direct and moderating effects. Health Psychol. 2008;27(5):628–37. doi: 10.1037/a0012664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Samet JH, Horton NJ, Meli S, et al. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28(4):572–7. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 173.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chander G, Himelhoch S, Moore RD. Substance abuse and Psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–89. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 175.Palepu A, Tyndall MW, Li K, et al. Alcohol use and incarceration adversely affect HIV-1 RNA suppression among injection drug users starting antiretroviral therapy. J Urban Health. 2003;80(4):667–75. doi: 10.1093/jurban/jtg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–97. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 177.Hecht FM, Grant RM, Petropoulos CJ, Dillon B, Chesney MA, Tian H, et al. Sexual transmission of an HIV-1 variant resistant to multiple reverse-transcriptase and protease inhibitors. N Engl J Med. 1998;339(5):307–11. doi: 10.1056/NEJM199807303390504. [DOI] [PubMed] [Google Scholar]
- 178.McNabb J, Ross JW, Abriola K, Turley C, Nightingale CH, Nicolau DP. Adherence to highly active antiretroviral therapy predicts virologic outcome at an inner-city human immunodeficiency virus clinic. Clin Infect Dis. 2001;33(5):700–5. doi: 10.1086/322590. [DOI] [PubMed] [Google Scholar]
- 179.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 180.Race E, Dam E, Obry V, Paulous S, Clavel F. Analysis of HIV cross-resistance to protease inhibitors using a rapid single-cycle recombinant virus assay for patients failing on combination therapies. AIDS. 1999;13(15):2061–8. doi: 10.1097/00002030-199910220-00008. [DOI] [PubMed] [Google Scholar]
- 181.Raj A, Reed E, Santana MC, Walley AY, Welles SL, Horsburgh CR, et al. The associations of binge alcohol use with HIV/STI risk and diagnosis among heterosexual African American men. Drug Alcohol Depend. 2009;101(1–2):101–6. doi: 10.1016/j.drugalcdep.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 182.Shuper PA, Joharchi N, Irving H, Rehm J. Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: review and meta-analysis. AIDS Behav. 2009;13(6):1021–36. doi: 10.1007/s10461-009-9589-z. [DOI] [PubMed] [Google Scholar]
- 183.Seth P, Wingood GM, DiClemente RJ, Robinson LS. Alcohol use as a marker for risky sexual behaviors and biologically confirmed sexually transmitted infections among young adult African-American women. Womens Health Issues. 2011;21(2):130–5. doi: 10.1016/j.whi.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Henrich TJ, Lauder N, Desai MM, Sofair AN. Association of alcohol abuse and injection drug use with immunologic and virologic responses to HAART in HIV-positive patients from urban community health clinics. J Community Health. 2008;33(2):69–77. doi: 10.1007/s10900-007-9069-1. [DOI] [PubMed] [Google Scholar]
- 185.Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC, Koup RA, Mellors JW, Connick E, Conway B, Kilby M, Wang L, Whitcomb JM, Hellmann NS, Richman DD. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002 Aug 8;347(6):385–94. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]