Abstract
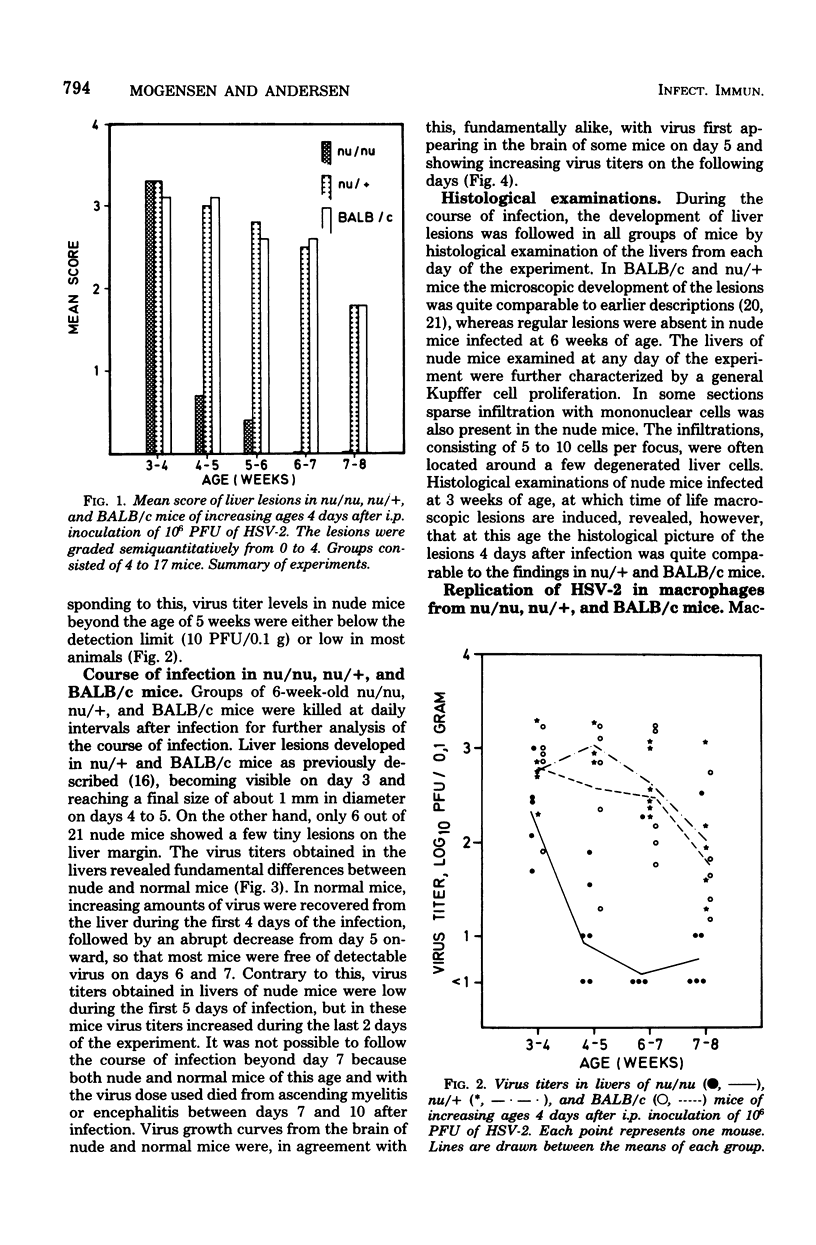
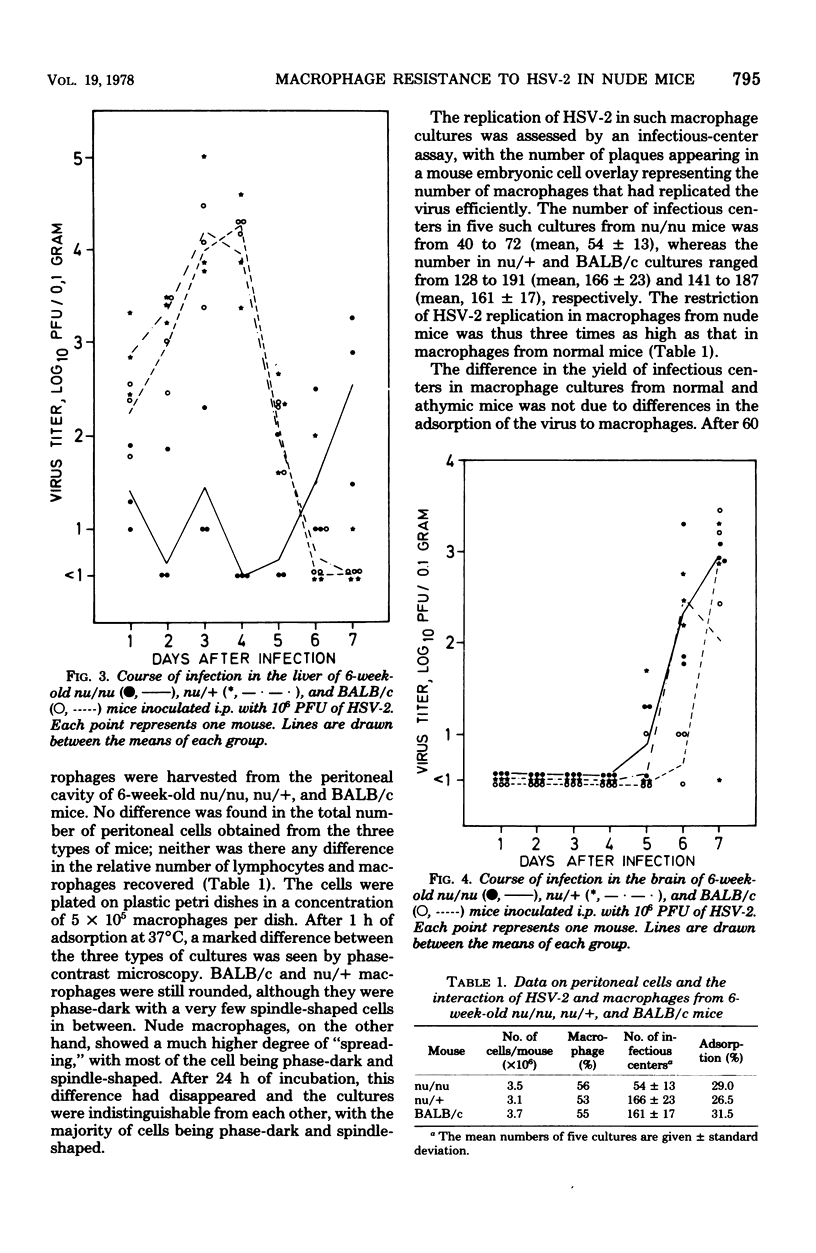
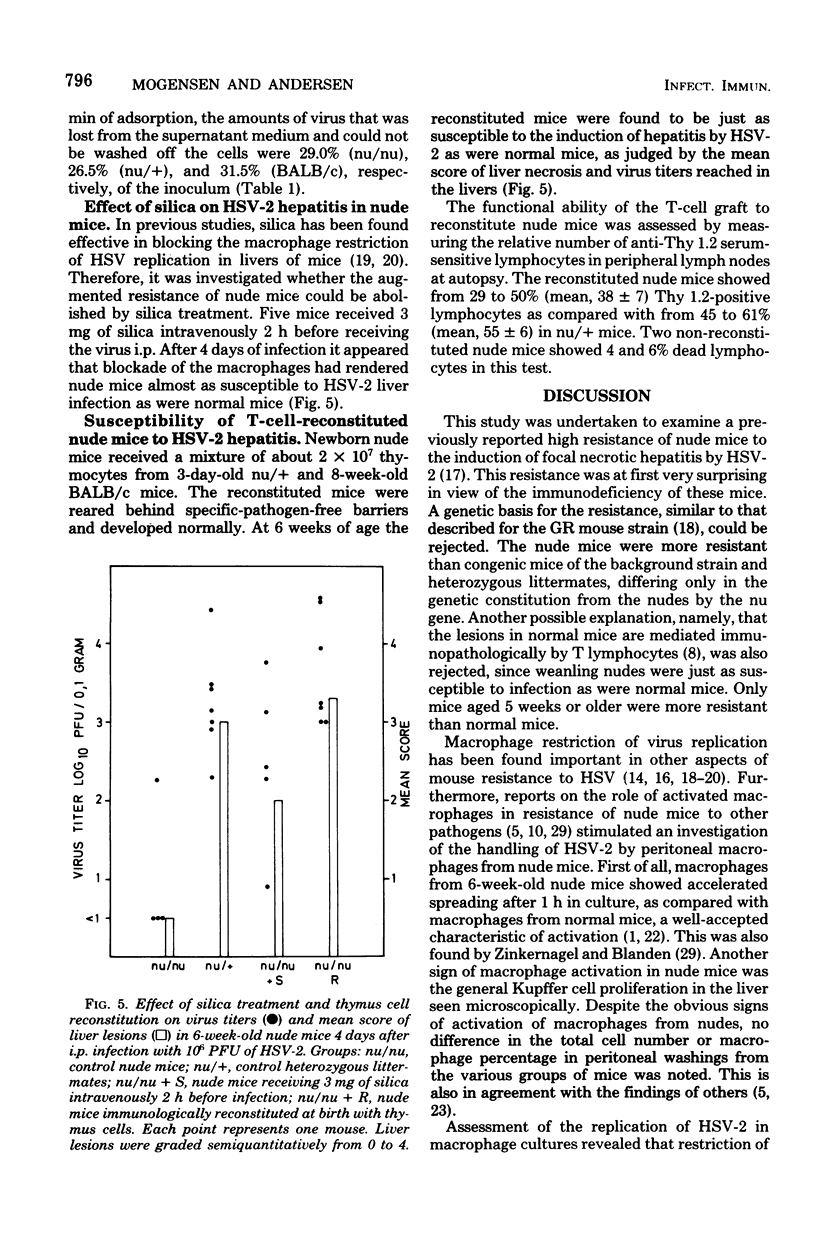
Congenitally athymic nude (nu/nu) mice of a BALB/c genetic background were found considerably more resistant to the induction of focal necrotic hepatitis by herpes simplex virus type 2 (HSV-2) tha, were phenotypically normal littermates (nu/+) or BALB/c mice. The augmented resistance was age dependent, as it was only manifested in mice from 4 to 5 weeks of age. Studies of the course of infection showed that nude mice were able to restrain virus multiplication in the liver far better than normal mice in the early phase of infection. However, they seemed inferior to normal mice in eliminating the infectious process. In vitro investigation of peritoneal macrophages revealed that macrophages from 6-week-old nude mice exhibited accelerated spreading and were three times as restrictive in the replication of HSV-2 as macrophages from normal mice. However, no difference was found in the efficiency of adsorption/phagocytosis between macrophages from nude and normal mice. The increased resistance of nude mice could be abolished by blockade of the microphage function of the mice by silica. Nude mice reconstituted at birth with thymus cells were just as susceptible to infection as normal mice. These data suggest that the increased resistance of nude mice to HSV-2 hepatitis is due to the presence of nonspecifically activated macrophages before infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. 3. Regression infectious foci. J Exp Med. 1971 May 1;133(5):1090–1104. doi: 10.1084/jem.133.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Crewther P., Warner N. L. Serum immunoglobulins and antibodies in congenitally athymic (nude) mice. Aust J Exp Biol Med Sci. 1972 Oct;50(5):625–635. doi: 10.1038/icb.1972.55. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Hof H. Cell-mediated resistance to infection with Listeria monocytogenes in nude mice. Infect Immun. 1977 Feb;15(2):382–385. doi: 10.1128/iai.15.2.382-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B. Immunostimulation with bacterial phospholipid extracts. Proc Natl Acad Sci U S A. 1974 Feb;71(2):573–577. doi: 10.1073/pnas.71.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. Peyer's patches, gut IgA plasma cells and thymic function: study in nude mice bearing thymic grafts. J Immunol. 1975 Aug;115(2):361–364. [PubMed] [Google Scholar]
- HOWARD J. G., ROWLEY D., WARDLAW A. C. Investigations on the mechanism of stimulation of non-specific immunity by bacterial lipopolysaccharides. Immunology. 1958 Jul;1(3):181–203. [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Effect of silica on the pathogenic distinction between herpes simplex virus type 1 and 2 hepatitis in mice. Infect Immun. 1977 Aug;17(2):274–277. doi: 10.1128/iai.17.2.274-277.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Biological conditions influencing the focal necrotic hepatitis test for differentiation between herpes simplex virus types 1 and 2. Acta Pathol Microbiol Scand B. 1976 Jun;84(3):154–158. [PubMed] [Google Scholar]
- Mogensen S. C. Genetics of macrophage-controlled resistance to hepatitis induced by herpes simplex virus type 2 in mice. Infect Immun. 1977 Aug;17(2):268–273. doi: 10.1128/iai.17.2.268-273.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Teisner B., Andersen H. K. Focal necrotic hepatitis in mice as a biological marker for differentiation of Herpesvirus hominis type 1 and type 2. J Gen Virol. 1974 Oct;25(1):151–155. doi: 10.1099/0022-1317-25-1-151. [DOI] [PubMed] [Google Scholar]
- Mogensen S. Role of macrophages in hepatitis induced by Herpes simplex virus types 1 and 2 in mice. Infect Immun. 1977 Mar;15(3):686–691. doi: 10.1128/iai.15.3.686-691.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. R., Rawls W. E., Perey D. Y., Tompkins W. A. Macrophage activation in congenitally athymic mice raised under conventional or germ-free conditions. J Reticuloendothel Soc. 1977 Jan;21(1):13–20. [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- Takeya K., Mori R., Imaizumi N. Suppressed multiplication of Listeria monocytogenes within macrophages derived from thymectomized mice. Nature. 1968 Jun 22;218(5147):1174–1174. doi: 10.1038/2181174a0. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Collins M. J., Jr, Parker J. C. Naturally occurring mouse hepatitis virus infection in the nude mouse. Lab Anim Sci. 1977 Jun;27(3):372–376. [PubMed] [Google Scholar]
- Wortis H. H. Immunological studies of nude mice. Contemp Top Immunobiol. 1974;3:243–263. doi: 10.1007/978-1-4684-3045-5_10. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]



