Abstract
The present study addressed whether developmental improvement in working memory span task performance relies upon a growing ability to proactively plan response sequences during childhood. 213 children completed a working memory span task in which they used a touchscreen to reproduce orally presented sequences of animal names. Children were assessed longitudinally at seven time points between 3 and 10 years of age, and 21 young adults completed the same task. Proactive response sequence planning was assessed by comparing recall durations for the first item (preparatory interval) and subsequent items. At preschool age, the preparatory interval was generally shorter than subsequent item recall durations, whereas it was systematically longer during elementary school and in adults. Although children mostly approached the task reactively at preschool, they proactively planned response sequences with increasing efficiency from age 7 on, like adults. These findings clarify the nature of the changes in executive control that support working memory performance with age.
Keywords: working memory, reactive and proactive control, recall duration, response sequence planning, children
Many daily activities require children to actively process and maintain information over short periods of time. For instance, understanding a bedtime story requires remembering information about the characters and the plot and integrating new information as the story unfolds. Working memory, which is devoted to such temporary maintenance and processing of information, develops steadily during childhood (Gathercole, Pickering, Ambridge, & Wearing, 2004; McAuley & White, 2011). The present study explores to what extent proactive planning contributes to working memory development during childhood.
In most models of working memory, executive control is responsible for maintaining, processing and actively retrieving information. According to Baddeley's model (Baddeley, 2003), the central executive controls information maintainance in the phonological loop and the visuospatial sketchpad, and processing in the episodic buffer. The latter components correspond to the activated portion of long-term memory in Cowan's model (e.g., Cowan, 2010). However, this model distingusihes between two levels of activation; only the most activated information is directly accessible to consciousness, maintained in the focus of attention and operated upon by executive control. Similarly, Unsworth and Engle (2007) distinguish between information maintained in primary memory, which is readily accessible to the conscious mind, and information in secondary memory, which is no longer attended but can be easily retrieved. In this model, executive control serves both information maintenance in primary memory and information retrieval from secondary memory.
Given the prominent position of executive control in working memory models, age-related changes in executive control likely affect, perhaps even drive, working memory development during childhood. Such changes are often thought to result from a quantitative increase in processing speed (Case, 1985; Fry & Hale, 2000; Towse, Hitch, & Hutton, 1998). For instance, according to the time-switching model (Towse et al., 1998), attention is switched between maintenance and processing episodes, with faster processing speed with advancing age shortening processing episodes and freeing up attention for longer maintenance episodes. Recent findings suggest that developing executive control allows children to alternate more strategically between processing and maintenance, with attention quickly returning to maintenance within processing episodes from 7 years on (Camos & Barrouillet, 2011). Such an age-related strategy shift points out qualitative changes in working memory during childhood, which is also consistent with the development of rehearsal strategies (e.g., Flavell, Beach, & Chinsky, 1966).
A major source of qualitative variability in executive control, which may affect working memory performance, relates to temporal dynamics. According to the “dual mechanisms of control” theory (Braver, 2012; Braver, Gray, & Burgess, 2007), executive control can be engaged proactively or reactively. Proactive control, which relies on sustained activity in the lateral prefrontal cortex (PFC), is engaged in anticipation of future cognitive demands (e.g., looking up driving directions before going to a new place), hence preventing interference with the current task before it even arises, when upcoming interference can be reliably predicted. In contrast, reactive control is transiently recruited on an as-needed basis as a function of on-the-moment demands (e.g., figuring out how to get to a new place when one is already driving). It is associated with transient lateral PFC actitivity and serves to overcome interference after it occurred, in particular when it could not be predicted (e.g., Marklund & Persson, 2012). Although young adults engage flexibly the most adaptive control mode as a function of the context, as evidenced by changes in lateral PFC activity and pupil dilation in response to experimental manipulations that encourage a specific mode (Braver, Paxton, Locke, & Barch, 2009; Chiew & Braver, 2013), they also show individual differences. Adults with higher working memory capacity engage proactive control more often than low-working memory individuals who engage reactive control preferentially (Braver et al., 2007). Critically, control mode selection also varies developmentally (Chatham, Frank, & Munakata, 2009; Killikelly & Szűcs, 2013; Vallesi & Shallice, 2007). For instance, in a task requiring to respond to specific prime-probe combinations, more mental effort (as shown by greater pupil dilation) is observed after probe onset at 3 years of age, hence showing no anticipation of the target, whereas it is observed before probe onset at 8 years (Chatham et al., 2009); suggesting that preschoolers rely mostly on reactive control, whereas proactive control is more frequent during middle childhood.
Response planning is a critical feature of proactive control (Andrews-Hanna et al., 2011; Killikelly & Szűcs, 2013; West, Bailey, Tiernan, Boonsuk, & Gilbert, 2012). Its contribution to working memory can be measured through recall item duration, that is, the time that elapses between the recall of two successive items. Unlike span length (i.e., the highest amount of information that children can recall accurately), recall durations offer direct insight on the temporal dynamic of memory search and recall processes and they correlate with academic achievement over and beyond span length (Cowan, 1992; Cowan et al., 1994, 1998, 2003; Towse, Cowan, Horton, & Whytock, 2008; Towse et al., 2008). Critically, the recall duration for the first item in the memorandum, that is, the preparatory interval, is longer than subsequent item recall durations in working memory span tasks during middle childhood and adulthood. At that age, individuals proactively retrieve and sequentially organize the to-be-recalled items before initiating their response (Cowan et al., 2003; Tehan & Lalor, 2000; Towse et al., 2008a, 2008b; Towse, Hitch, Horton, & Harvey, 2010). In contrast, it is unknown whether preschool-age children proactively plan response sequences. As preschoolers tend to exert control reactively (Chatham et al., 2009), they may not plan response sequences, but instead immediately initiate their responses and retrieve each item separately. If so, preschoolers should not show longer preparatory intervals relative to subsequent item recall durations. In contrast, if working memory development is entirely driven by quantitative changes in processing speed or storage capacity and/or changes in executive control unrelated to response sequence planning, preschoolers should show similar preparatory intervals as school-age children and adults.
To examine whether proactive planning of the response sequence increases with age, children were assessed longitudinally on a working memory span task at seven time points between 3 and 10 years of age. In this task, children had to reproduce sequences of auditorily presented animal names by pressing buttons on a touchscreen, which required maintaining actively and processing the names to translate the auditory items into their corresponding visual items. Confirmatory factor analysis has shown that performance on this task loads onto a latent factor common to other measures of early childhood executive control, including tasks tapping working memory, resistance to distractor interference, and response inhibition tasks (Wiebe et al., 2011). Because this task departs from those used in previous reports of the preparatory interval in adults, the present study also included a group of young adults to check that adults proactively plan response sequences on this task.
We hypothesized that, as preschoolers, children would approach the task reactively, whereas by elementary school they would show proactive response sequence planning. If so, the preparatory interval should differ from subsequent item recall durations only after preschool. Further, as planning the response sequence should be more demanding for longer sequences (due to more items having to be retrieved and organized sequentially), the preparatory interval should increase across sequences at ages where response sequence planning is observed. In contrast, if working memory development is entirely driven by quantitative changes in processing speed or executive control changes unrelated to response sequence planning, the preparatory interval should be longer than subsequent item recall durations even at preschool.
Method
Participants
Study participants were 213 children (104 girls and 109 boys; 149 White non-Hispanic, 5 African American, 23 Hispanic, 1 Asian and 35 multiple race) assessed longitudinally in the preschool and elementary periods. The exact N varied across time points due to some children dropping out of the study and others being recruited. Children were recruited through birth announcements, local preschools, the local health department, and by word of mouth from a Midwestern small city. Parents completed a telephone screening before study enrollment. Children with diagnosed developmental or language delays or behavioral disorders or whose families planned to move out of the area within the study timeline were deemed ineligible and not enrolled. Children were enrolled initially in a project for which they were administered a battery of executive tasks every 9 months between the ages of 3 years 0 months and 5 years 3 months in a lagged cohort sequential design. Data from three time points were included in the present study: 3 years 9 months, 4 years 6 months, and 5 years 3 months. The data at age 3;0 were not used because most children had a maximal span length of only 1 (59%) or 2 (33%), hence strongly limiting the comparison between the preparatory interval and subsequent item recall durations. Children were tested within two weeks of the exact targeted age (mean age 3.75, SD = .04 and age range = 3.67-3.83; mean age 4.50, SD = .04 and age range = 4.42-4.58; mean age 5.24, SD = .04 and age range = 5.16-5.33). The same children were later enrolled in a follow-up study in which they completed another battery of executive tasks every year from grade 1 through grade 4 (Grade 1: mean age 7.22, SD = .32 and age range = 6.50-8.00; Grade 2: mean age 8.11, SD = .36 and age range = 7.33-8.99; Grade 3: mean age 9.09, SD = .38 and age range = 8.25-10.00; Grade 4: mean age 9.93, SD = .36 and age range = 9.25-10.67). Stratified sampling on social risk was used to ensure a balanced sample (36.15% were eligible for public medical assistance). The majority of participants’ mothers had completed at least some college education: 2% had less than a high school diploma/GED equivalent, 10% had a high school diploma/GED equivalent, 38% had some college education, 51% had a 4-year college degree or beyond. Parental informed consent was obtained for all children prior to participation.
A group of 21 adults (10 women and 11 men; 20 were White and one was African American, mean age = 20.21 years, SD = .97 year) also participated. They were undergraduate students from the major university in the same geographic area. They completed informed consent before beginning the session and received course credit in exchange for participation.
Materials and Procedure
Children were administered a battery of executive tasks at each time point (for further details, see Wiebe et al., 2011) by a trained examiner in one session (first 3 age points) or two sessions (later age points) of about 120 minutes each (including other tasks not reported here). Short breaks were used when necessary to maintain cooperation and interest. Parents were compensated for study participation, and the children received developmentally appropriate toys, stickers, and other small items. Adult participants were tested at the laboratory by a trained experimenter in a 15-min. session in which they only completed Nebraska Barnyard.
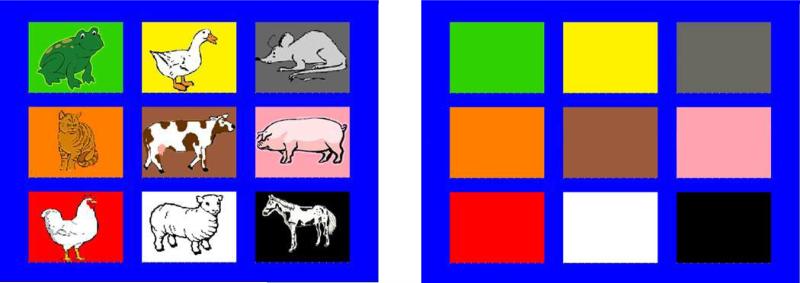
Working memory was assessed using Nebraska Barnyard (adapted from the Noisy Book, Hughes, Dunn, & White, 1998). The task required actively maintaining animal names and matching them with their corresponding colored squares on the touchscreen before recalling them by pressing the colored squares in the correct order. The version administered at ages 3;9, 4;6 and 5;3 was presented using Perl v5.8.8 (ActiveState Software, Vancouver, BC), whereas the version administered at later ages was presented using E-prime 2.0 (Psychology Software Tools, Pittsburgh, PA, USA). Children were introduced to a set of 9 pictures, each representing a different animal on a differently colored background and arranged in a 3 × 3 grid (Figure 1). Children were asked to get their “pointy finger ready” by positioning it below the grid of pictures. In the familiarization phase, children pressed each animal picture and the computer produced the corresponding sound. Then, the animal pictures were removed (but box colors remained the same) and children completed a set of 9 practice trials during which the examiner named each animal individually, and the child was required to press the colored square corresponding to that animal. Finally, trials with sequences of animals were administered, beginning with sequences of 2 animals and increasing progressively until the child's performance met the discontinuation criterion. Items were presented at a pace of one per second. Voice inflection on the last animal name in each sequence signalled sequence end and served as a cue for participants to start recalling. Up to 3 trials were administered at each span length: if the first 2 trials for a span were correct, participants were automatically given credit for the third trial, which was omitted, and if all 3 trials for a span were incorrect, the task was discontinued. For the version of the task presented in Perl, accuracy and recall duration for each item were coded from videos by trained undergraduate students using Noldus Observer 5.12 (Noldus Information Technology, Wageningen, Netherlands). Two cameras with different angles were used so as to capture precisely the time frame when children pressed each button. 20% of the videos were double coded to assess inter-rater agreement (M = 94.6%). Children who were enrolled in the first year of the follow-up study completed this version of the task, using Eprime, for the first year only. Assessments completed in any of the other 4 years of the follow-up study and among the adults included an Eprime version in which animal names sequences were not read by the experimenter but pre-recorded and presented through the E-Prime interface.
Figure 1.
Illustration of the grid of colored squares with the animals (as used during the familiarization phase) and without the animals (as used during the practice and test trials). Participants had to reproduce sequences of animal names by pressing the colored squares on a touchscreen. Colors from top to bottom, left to right: green, yellow, gray, orange, brown, pink, red, white, and black. The background color is blue.
Three measures were computed: preparatory interval, item recall duration, and span length. Preparatory interval was the time that elapsed between the end of the auditory item sequence and the first picture press. Item recall duration was scored as the time that elapsed between the prior picture press and the subsequent picture press for a given item. Item recall durations were computed for correct trials only (i.e., trials for which all items were pressed in the correct order) and averaged across items (excluding the first one). Span length was scored as the highest sequence of animals that the participant correctly reproduced in the right order.
The data were analyzed separately for adults and children because of the substantial difference in sample size and the longitudinal nature of the child data. The longitudinal analysis for the child data was achieved with multilevel modeling (MLM), which allows modeling the dependency over time and levels (e.g. participants and button presses nested within sequence) (see Hoffman & Rovine, 2007; Quene, 2004), hence capitalizing in the longitudinal and repeated measures design of the present study. The temporal position of a given item within a sequence was referred as the “item temporal order”. Given that our hypothesis focused on response sequence planning, we contrasted the recall duration of the first item (i.e., preparatory interval) with the mean recall duration of subsequent items within each sequence. Recall times were log-transformed to correct for non-normal distributions and minimize the influence of age-related differences in baseline recall durations. Because the maximal sequence length reached at each age varied, sequence length could not be entered as a predictor along with age. Instead, separate models were computed for each sequence length in order to examine the effect of age. A specific age point was entered for a sequence length if at least 15% of the participants contributed data. All age points were included in the analyses of 2- and 3-item sequences. For 4-items sequences, 4;6 and later age points were included. The analysis for 5-item sequences included ages 7 through 10, and finally the one for 6-item sequences included ages 8 through 10. Item temporal order, age and their interaction were used as predictors. Importantly, recall durations in Nebraska Barnyard necessarily vary as a function of both cognitive processes and spatial distance among buttons because children responded with one finger of one hand and had to move across space as they pressed buttons. Response execution time necessarily varied as a function of the spatial distance between buttons. For instance, going from the left bottom button to right top button necessitates a bigger finger move and thus more time than going from the left bottom button to the middle bottom button. Therefore, the spatial distance in cm in between buttons, or between the start position below the grid and the first correct button, was entered as a predictor in the models. Its main effect was estimated to allow us to control for it while examining the effects of the other predictors. Similarly, we entered the method of administration (i.e., sequences read by the examiner vs. pre-recorded sequences) as a predictor so as to control for its potential effect. To probe whether sequence significantly affected the difference between the preparatory intervals and subsequent item recall durations at each age point, we ran a second series of models for each age point separately, including the sequence length as a predictor.
For the adult sample, a single model allowed us to examine both whether the preparatory interval was longer than subsequent item recall durations and whether this difference increased with the sequence length. Therefore, the multilevel model was comprised of buttons nested within sequence.
All study analyses were run using the PROC MIXED component of the SAS 9.3 statistical package (SAS Institute, Cary, NC, USA).
Results
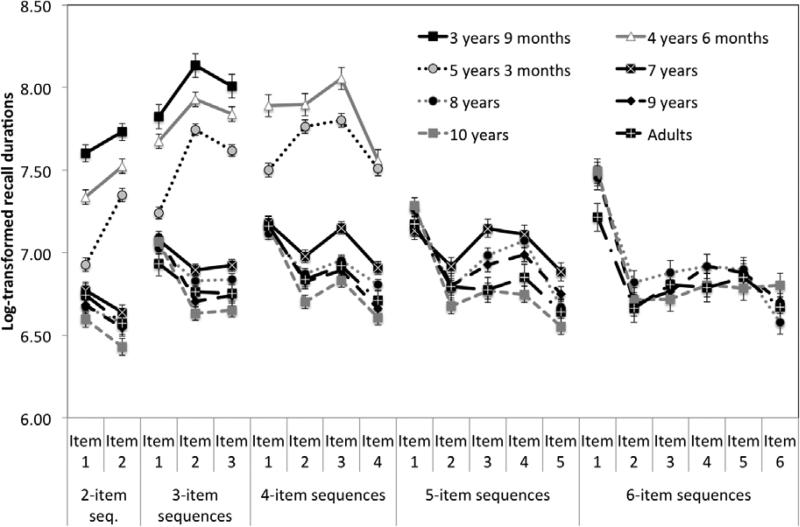
Table 1 shows the maximal span length and the proportion of correct trials at each time point and for each sequence length. The maximal span length significantly increased with age, F(6, 899) = 430.91, p < .0001, ηp2= .74. Mean item recall durations were computed based on the correct trials and are shown in Figure 2.
Table 1.
Proportion of correct trials for each sequence length and age point, and mean maximal span length (and standard deviations).
| Proportion of correct trials |
Mean maximal span length | |||||
|---|---|---|---|---|---|---|
| 2 items | 3 items | 4 items | 5 items | 6 items | ||
| Age 3;9 | 52.2% (N=146) | 17.8% (N=117) | 6.0% (N=44) | 0% (N=7) | -- | 2.4 (.6) |
| Age 4;6 | 74.9% (N=176) | 41.9% (N=169) | 23.2% (N=107) | 3.4% (N=49) | 0% (N=5) | 2.9 (.9) |
| Age 5;3 | 85.4% (N=207) | 65.1% (N=207) | 39.5% (N=182) | 4.5% (N=133) | 2.1% (N=16) | 3.6 (.8) |
| Age 7 | 98.0% (N=125) | 95.0% (N=124) | 79.7% (N=125) | 29.6% (N=124) | 9.7% (N=70) | 4.7 (.7) |
| Age 8 | 98.5% (N=168) | 94.9% (N=168) | 83.3% (N=168) | 37.9% (N=165) | 14.3% (N=116) | 4.9 (.8) |
| Age 9 | 98.6% (N=178) | 67.7% (N=178) | 87.6% (N=178) | 46.8% (N=176) | 18.8% (N=134) | 5.1 (.9) |
| Age 10 | 99.6% (N=114) | 97.0% (N=114) | 88.3% (N=114) | 58.5% (N=113) | 21.6% (N=100) | 5.4 (.8) |
| Adults | 100% (N=21) | 93.3% (N=21) | 92.5% (N=21) | 66.7% (N=20) | 43.6% (N=15) | 6.1 (1.11) |
Figure 2.
Mean log-transformed preparatory interval and item recall durations in seconds for each sequence length as a function of the button serial position and age, controlling for button spatial distance. Vertical bars indicate standard errors. At preschool age, children adopted a reactive approach whereas they proactively planned their response sequence during elementary school.
Adults
The effects of item temporal order1, sequence length, and button spatial distance on recall durations were significant, F(1, 651) = 102.33, p < .001, ηp2= .14; F(4, 653) = 22.86, p < .001, ηp2= .12; F(1, 651) = 10.77, p = .001, ηp2=.02. Critically, item temporal order and sequence length interacted, F(4, 651) = 4.45, p = .001, ηp2= .03 (Figure 2). The preparatory interval was longer than the mean recall times for subsequent items for all sequence lengths (Table 2). Further, the preparatory intervals significantly increased from 2- and 3-item sequences to 5-item sequences, t (651) = −2.32, p = .020, d = −.18 and t (651) = −2.95, p = .003, d = −.23, respectively, and 6-item sequences, t (651) = −2.84, p = .004, d = −.22 and t (651) = −3.42, p < .001, d = −.27. It also significantly increased from 4- to 6-item sequences, t (651) = −2.36, p = .018, d = −.18. These findings confirm that the preparatory interval reflects response sequence planning and that adults proactively planned their response sequence on the Nebraska Barnyard, consistent with previous studies (e.g., Towse et al., 2008a, 2008b).
Table 2.
Mean log-transformed recall durations for the first item (preparatory interval) and subsequent items (averaged across items) as a function of item sequence length and age. Standard errors appear in parentheses. Significant pairwise comparisons and the longer duration are shown in bold.
| Sequence Length | Age | Preparatory interval | Subsequent Item Recall duration (average) | Comparison |
|---|---|---|---|---|
| 2 items | 3;9 | 7.60 (0.05) | 7.73 (0.05) | t (3881) = −2.05, p = .040, d = −.07 |
| 4;6 | 7.34 (0.04) | 7.52 (0.04) | t (3881) = −3.72, p < .001, d = −.12 | |
| 5;3 | 6.93 (0.04) | 7.35 (0.04) | t (3881) = −9.42, p < .001, d = −.30 | |
| 7 | 6.77 (0.04) | 6.64 (0.04) | t (3881) = 2.32, p = .020, d = .07 | |
| 8 | 6.67 (0.04) | 6.57 (0.04) | t (3881) = 1.97, p = .048, d = .06 | |
| 9 | 6.68 (0.04) | 6.55 (0.04) | t (3881) = 2.89, p = .003, d = .09 | |
| 10 | 6.60 (0.05) | 6.43 (0.05) | t (3881) = 2.86, p = .004, d = .09 | |
| Adults | 6.74 (0.08) | 6.57 (0.08) | t (651) = 2.86, p = .004, d = .22 | |
| 3 items | 3;9 | 7.82 (0.07) | 8.07 (0.05) | t (4957) = −2.96, p = .003, d = −.08 |
| 4;6 | 7.67 (0.04) | 7.88 (0.04) | t (4958) = −4.39, p < .001, d = −.12 | |
| 5;3 | 7.24 (0.04) | 7.68 (0.03) | t (4958) = −12.32, p < .001, d = −.35 | |
| 7 | 7.08 (0.04) | 6.91 (0.03) | t (4957) = 4.06, p < .001, d = .12 | |
| 8 | 7.03 (0.03) | 6.83 (0.03) | t (4958) = 5.40, p < .001, d = .15 | |
| 9 | 7.10 (0.03) | 6.72 (0.03) | t (4958) = 10.75, p < .001, d = .31 | |
| 10 | 7.07 (0.04) | 6.64 (0.03) | t (4957) = 9.92, p < .001, d = .28 | |
| Adults | 6.93 (0.07) | 6.76 (0.06) | t (651) = 2.70, p = .007, d = .21 | |
| 4 items | 4;6 | 7.87 (0.07) | 7.86 (0.04) | t (5256) = 0.25, p = .799, d = .01 |
| 5;3 | 7.48 (0.04) | 7.71 (0.03) | t (5256) = −5.22, p < .001, d = −.14 | |
| 7 | 7.14 (0.04) | 7.02 (0.02) | t (5258) = 2.86, p = .004, d = .08 | |
| 8 | 7.07 (0.03) | 6.89 (0.02) | t (5259) = 5.12, p < .001, d = .14 | |
| 9 | 7.12 (0.04) | 6.80 (0.02) | t (5259) = 9.05, p < .001, d = .25 | |
| 10 | 7.12 (0.04) | 6.72 (0.03) | t (5258) = 9.07, p < .001, d = .25 | |
| Adults | 7.14 (0.06) | 6.82 (0.04) | t (651) = 4.10, p < .001, d = .32 | |
| 5 items | 7 | 7.13 (0.06) | 7.01 (0.03) | t (3116) = 2.05, p = .040, d = .07 |
| 8 | 7.26 (0.05) | 6.88 (0.03) | t (3116) = 8.71, p < .001, d = .31 | |
| 9 | 7.26 (0.04) | 6.87 (0.03) | t (3116) = 9.97, p < .001, d = .36 | |
| 10 | 7.28 (0.05) | 6.69 (0.04) | t (3116) = 13.59, p < .001, d = .49 | |
| Adults | 7.17 (0.08) | 6.77 (0.06) | t (651) = 6.91, p < .001, d = .54 | |
| 6 items | 8 | 7.46 (0.07) | 6.82 (0.04) | t (1003) = 8.91, p < .001, d = .56 |
| 9 | 7.48 (0.07) | 6.79 (0.05) | t (1003) = 11.77, p < .001, d = .74 | |
| 10 | 7.50 (0.07) | 6.76 (0.05) | t (1003) = 11.54, p < .001, d = .73 | |
| Adults | 7.22 (0.08) | 6.76 (0.06) | t (651) = 6.73, p < .001, d = .53 | |
Children
For the 2-item sequence length, age had a significant effect on recall durations, F(1, 3914) = 93.29, p < .001, ηp2= .02, which was qualified by a significant interaction with item temporal order, F(6, 3879) = 21.40, p < .001, ηp2= .03. Table 2 shows the pairwise comparisons between the preparatory interval and the average of subsequent item recall durations. The preparatory interval was shorter than the recall duration of the second item from ages 3;9 to 5;3, whereas it was longer than the recall duration of the second item at later age points. As shown in Figure 3, the reactive pattern observed at preschool surprisingly was more pronounced at age 5;3 than 4;6, t (3881) = 3.49, p < .001, d = .11. The switch from reactive to proactive patterns between 5;3 and 7 was significant, t (3881) = −7.73, p < .001, d = −.25, whereas the proactive pattern did not change later on, all ps > .342. There was also significant main effects of age, F(6, 3914) = 93.29, p < .001, ηp2= .13, and button spatial distance, F(1, 3916) = 33.14, p < .001, ηp2= .01, indicating that recall durations increased as a function of the button spatial distance between two presses. The effect of method was not significant, p = .330.
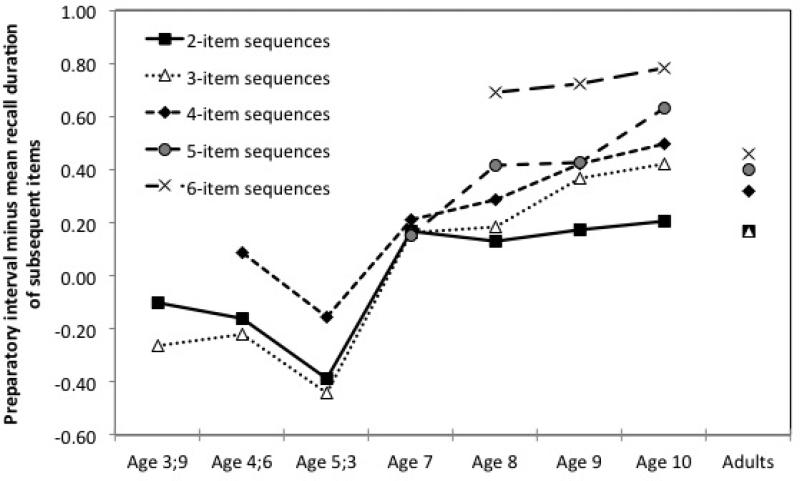
Figure 3.
Differences between log-transformed preparatory intervals and the mean of log-transformed recall durations of subsequent items as a function of the sequence length and age. Proactive response sequence planning increased with age, especially for the longer sequences.
For the 3-item sequence length, the main effects of item temporal order, F(1, 4957) = 4.00, p = .045, ηp2= .001, and age, F(6, 4999) = 123.48, p < .001, ηp2= .13, significantly interacted, F(6, 4956) = 69.46, p < .001, ηp2= .08. The preparatory interval was shorter than the average recall duration of subsequent items at all three preschool age points, whereas the reverse pattern was observed between ages 7 and 10. Between ages 4;6 and 5;3, recall durations on subsequent items became even longer relative to the first items, t (4957) = 3.90, p < .001, d = .11. In addition to the significant difference between ages 5;3 and 7, t (4957) = 3.90, p < .001, d = .11, the proactive pattern increased in magnitude between ages 8 and 9, t (4957) = −3.66, p < .001, d = −.10 (Figure 3). Both button spatial distance and method were significant, F(1, 5014) = 10.37, p < .001, ηp2= .002, and F(1, 4800) = 8.31, p < .001, ηp2= .002, respectively.
For the 4-item sequence length, the effect of age, item temporal order, and their interaction were again significant, F(5, 5165) = 102.38, p < .001, ηp2= .09, F(1, 5263) = 41.96, p < .001, ηp2= .01, and F(5, 5254) = 27.80, p < .001, ηp2= .03, respectively. Surprisingly, there was no difference between the preparatory interval and subsequent item recall durations at age 4;6, whereas children took longer to recall subsequent items than the first item at age 5;3. During elementary school, children took longer to recall the first item, suggesting that they planned their response sequence. The difference between the preparatory interval and subsequent item recall durations became more pronounced between 4;6 and 5;3, t (5255) = 2.91, p = .003, d = .08, changed in direction between 5;3 and 7, t (5255) = −5.85, p < .001, d = −.16, and the magnitude of the proactive pattern increased between 7 and 8, t (5255) = −2.74, p = .006, d = −.08. Both button spatial distance and method were significant, F(1, 5302) = 105.70, p < .001, ηp2= .02 and F(1, 4409) = 7.05, p = .008, ηp2= .002, respectively.
For 5-item sequence length, the main effect of age fell short of significant, F(3, 3111) = 2.54, p = .054, ηp2= .002, while item temporal order had a significant effect, F(1, 3116) = 246.58, p < .001, ηp2= .07, that interacted with age, F(3, 3115) = 15.22, p < .001, ηp2= .01. From ages 7 through 10, children showed longer preparatory intervals than subsequent item recall durations. The proactive pattern increased in magnitude from ages 7 to 8, t (3116) = −3.74, p < .001, d = −.13, and 9 to 10, t (3116) = −3.54, p < .001, d = −.13. The effect of button spatial distance was significant, F(1, 3137) = 47.72, p < .001, ηp2= .01, whereas the effect of method was not, p = .840
For 6-item sequence length, there was a significant effect of item temporal order, F(1, 1001) = 321.50, p < .001, ηp2= .24, whereas its interaction with age was not significant, p = .609. Children showed longer preparatory intervals than subsequent item recall durations from ages 8 through 10. There was a significant effect of button spatial distance, F(1, 1013) = 18.43, p < .001, ηp2= .02, whereas the effects of age and method were not significant, ps > .405. Taken together, these findings suggest a change from a reactive approach to Nebraska Barnyard at preschool age to proactive response sequence planning during elementary school.
Finally, we examined whether the time difference between the preparatory intervals and subsequent item recall durations was influenced by sequence length at each age point. At age 3;9, there was no interaction between item temporal order and sequence, p = .184, further suggesting that children that young did not plan their response sequences. At age 4;6, there was a significant Item temporal order × Sequence interaction, F(2, 1278) = 3.79, p = .022, ηp2 = .01, due to a shorter difference for 4-item sequences than 2- and 3-item sequences, t (1278) = −2.27, p = .023, d = −.13 and t (1278) = −2.72, p = .006, d = −.15 respectively. The exact same pattern was observed at age 5;3, F(2, 2378) = 8.60, p < .001, ηp2= .01, with a smaller difference for 4-item sequences than 2- and 3-item sequences, t (2377) = −3.20, p = .001, d = −.13 and t (2378) = −4.00, p < .001, d = −.16, respectively. These findings suggest that the reactive pattern became less pronounced as the sequence to be recalled was more challenging at ages 4;6 and 5;3. Surprisingly, there was no interaction between item temporal order and sequence at age 7, p = .983. In contrast, Item temporal order and Sequence significantly interacted at ages 8, 9 and 10, F(4, 3865) = 12.64, p < .001, ηp2= .01, F(4, 4420) = 16.82, p < .001, ηp2= .01, and F(4, 3098) = 18.39, p < .001, ηp2= .02, respectively. At 8, the difference between the preparatory interval and recall durations of subsequent items significantly increased from 2- to 4-item sequences, t (3665) = −2.05, p = .040, d = −.07, 4- to 5-item sequences, t (3665) = −3.18, p = .001, d = −.11, and 5- to 6-item sequences, t (3665) = −2.58, p = .010, d = −.09. At 9, the difference increased significantly between 2- and 3-item sequences, t (4420) = −4.50, p < .001, d = −.14, and between 5- and 6-item sequences, t (4420) = −4.08, p < .001, d = −.12. At 10, the pairwise comparisons were significant between 2- and 3-item, and 4- and 5-item sequences, t (3098) = −4.00, p < .001, d = −.14, t (3098) = −3.19, p < .001, d = −.11. As expected, once children have switched to a proactive profile (except at 7 years), response sequence planning takes more time as the number of items increase.
Discussion
The present study used item recall durations to examine whether the temporal dynamic of working memory processes shows a reactive to proactive shift during childhood. At ages 3;9, 4;6 and 5;3, preschoolers generally approached the working memory span task reactively, not planning the response sequence, as suggested by shorter preparatory intervals than subsequent item recall durations. Preschoolers likely encoded items passively and retrieved and translated into a specific button each item only after recalling the previous one in an “as-needed” fashion. In contrast, children from 7 through 10 years of age and adults proactively planned their response sequences, as suggested by longer preparatory intervals than subsequent item recall durations. During elementary school, children, like adults, delayed responding in order to proactively plan the response sequence, which likely required retrieving and translating most items before starting to respond, although additional retrieval likely took place in between presses as well. Further, proactive sequence planning changed during elementary school, becoming more sensitive to the number of items to be organized. These findings reveal that children shift from reactive to proactive control with age in the context of a working memory span task and show that this shift in control mode affects response sequence planning.
Working memory development during childhood cannot be fully explained by quantitative changes in processing speed and executive control efficiency. Our findings clearly point out qualitative changes in the control strategies that children use over time (see also Camos et al., 2011; Chevalier, Huber, Wiebe, & Espy, 2013). They clarify the nature of the executive changes that drive growing working memory during childhood, by revealing that a shift in the temporal dynamic of control helps children proactively plan response sequences. These findings are consistent with previous evidence for a reactive to proactive transition in executive control during childhood (Andrews-Hanna et al., 2011; Chatham et al., 2009; Killikelly & Szűcs, 2013). Furthermore, the observed transition between 5 and 7 years of age converges with prior findings showing important changes in children's working memory performance around that time. Specifically, children start switching attention between maintenance and processing in a finer way around 7 years of age (Camos et al., 2011), and the structural components of working memory (central executive, phonological loop, visuospatial sketchpad) can be observed from 6 years of age onward (Gathercole et al., 2004).
Such a change in proactive response sequence planning may shed light on the interplay between active maintenance in primary memory and active retrieval in secondary memory, as defined by Unsworth and Engle (2007). Because preschoolers do not plan the response sequence, they may maintain actively in primary memory the first item only whereas subsequent items have to be retrieved from secondary memory while responding. If true, it would explain why the preparatory interval was not just equivalent to subsequent item recall durations, but actually shorter at preschool. Consistently, unlike adults, young children have recently been found to rely mostly on primary memory and not to use secondary memory to support primary memory when it is saturated (Roome & Towse, 2013). One open question is whether school-age children and adults maintained all animal names in primary memory during encoding and then started planning the response sequence right after the last item was encoded, or if they started planning the response sequence during item encoding by translating each item into its corresponding button and virtually constructing the spatial trajectory as each new item was heard. If the latter is true, then perhaps younger children could be more likely to adopt a similar strategy if animal names were easier to associate with their corresponding buttons (e.g., by displaying the animal pictures on the buttons during the test phase), encouraging them to construct the spatial trajectory during encoding. Indeed, recent findings suggest that preschoolers can be encouraged to engage proactive control through environmental manipulations (Chevalier, Martis, Curran, & Munakata, submitted).
Interestingly, the reactive pattern observed early in childhood, with preparatory intervals shorter than subsequent item recall durations, became more pronounced over the preschool period. This surprising tendency may reflect strengthening or more consistent use of the strategy consisting in prioritizing (i.e., maintaining in primary memory) the first item in the series over time. More consistent use of this strategy may lead children to build a better representation of its advantages and limitations, which helps them to search for or select alternative strategies, hence potentially explaining why the reactive pattern became more pronounced before the switch to the proactive pattern. Indeed, such meta-cognitive representations have been hypothesized to drive the development of executive control (Zelazo, 2004) and influence children's use of proactive and reactive control (Chevalier et al., submitted). Nevertheless, the reactive pattern was less marked for 4-item sequences, especially at age 4;6. This attenuation of the observed reactive pattern may be due to a subsample of preschoolers (potentially the most cognitively advanced) starting to plan their response sequence when the task is sufficiently challenging. This is all the more plausible since 4-item sequences are more challenging at age 4;6 than 5;3 and 4-year-olds passing this sequence length represent a more selected sample (44 out of 146 at 4;6 and 107 out of 176 at 5;3) of potentially more cognitively advanced children.
During elementary school, children more systematically planned their response sequences. Consistently, the difference between the preparatory intervals and subsequent item recall durations increased with the sequence length from ages 8 through 10 and during adulthood, hence confirming that response sequence planning took more time with more items to organize sequentially. Interestingly, at 7 years of age, the sequence length did not affect this difference, suggesting that children that age did not plan their response sequence as effectively as they did later in childhood. Response sequence planning continued to develop after 7 as shown by increasing differences between the preparatory intervals and subsequent item recall durations with advancing age, especially for the longest sequences. Consistent with these findings, although children start to engage proactive control from about 6 years of age, proactive control continues to increase through early adulthood on other executive control measures (Andrews-Hanna et al., 2011; Waxer & Morton, 2011).
Although the difference between the preparatory intervals and subsequent item recall durations changed with advancing age, these differences seem to be driven in part by shorter recall durations of subsequent items, hence raising the possibility that children improved at retrieving later items in the sequence, perhaps in spite of similar response planning across ages. However, this interpretation cannot account straightforwardly for increasing differences between preparatory intervals and subsequent item recall durations as a function of sequence length. Most importantly, it holds only if one assumes that response sequence planning and retrieval are independent processes. Yet, they are more likely to be intrinsically related because better initial planning should yield faster recall durations for subsequent items.
In conclusion, the present study clarified the nature of executive control changes that drive changes in working memory performance during childhood. Specifically, they showed that children mostly adopt a reactive approach until 5 years of age whereas response sequence planning emerges around 7 years and increases in efficiency through age 10. Of course, it remains possible that processes other than proactive response planning may also contribute to the present results; therefore this question should be further investigated through experimental manipulations in future studies. Of particular interest will be whether variables that affect the developmental trajectory of executive control, such as sex or socio-economic status (e.g., Clark et al., 2013), also influence the developmental course of response sequence planning. Finally, the two-year gap between ages 5 and 7 did not allow us to examine precisely how this shift occurs during that period; therefore research is needed to determine whether it changes sharply or steadily and the extent to which this trajectory varies across children.
Acknowledgments
This work was supported by NIH grants MH065668, DA023653, DA024769, HD038051, and HD050309. We are grateful to the participating families and everyone at the Developmental Cognitive Neuroscience Laboratory.
Footnotes
We also ran the same analyses separating all items in each sequence. These analyses revealed the same significant effects.
References
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PloS one. 2011;6(6):e21598. doi: 10.1371/journal.pone.0021598. doi:10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature reviews. Neuroscience. 2003;4(10):829–39. doi: 10.1038/nrn1201. doi:10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Barrouillet P, Gavens N, Vergauwe E, Gaillard V, Camos V. Working memory span development: a time-based resource-sharing model account. Developmental psychology. 2009;45(2):477–90. doi: 10.1037/a0014615. doi:10.1037/a0014615. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012;16(2):106–13. doi: 10.1016/j.tics.2011.12.010. doi:10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. Oxford University Press; Oxford, UK: 2007. pp. 76–106. [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7351–6. doi: 10.1073/pnas.0808187106. doi:10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camos V, Barrouillet P. Developmental change in working memory strategies: from passive maintenance to active refreshing. Developmental Psychology. 2011;47(3):898–904. doi: 10.1037/a0023193. doi:10.1037/a0023193. [DOI] [PubMed] [Google Scholar]
- Case R. Intellectual development: Birth to adulthood. Academic Press; New York: 1985. [Google Scholar]
- Chatham CH, Frank MJ, Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):5529–33. doi: 10.1073/pnas.0810002106. doi:10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Huber KL, Wiebe SA, Espy KA. Qualitative change in executive control during childhood and adulthood. Cognition. 2013;128(1):1–12. doi: 10.1016/j.cognition.2013.02.012. doi:10.1016/j.cognition.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Martis SB, Curran T, Munakata Y. Meta-cognitive processes in the development of executive function: The case of reactive and proactive control. 2013 doi: 10.1162/jocn_a_00782. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Frontiers in psychology. 2013;15 doi: 10.3389/fpsyg.2013.00015. doi:10.3389/fpsyg.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Sheffield TD, Chevalier N, Nelson JM, Wiebe S. a, Espy KA. Charting early trajectories of executive control with the shape school. Developmental Psychology. 2013;49(8):1481–93. doi: 10.1037/a0030578. doi:10.1037/a0030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. Verbal memory span and the timing of spoken recall. Journal of Memory and Language. 1992;31:668–684. [Google Scholar]
- Cowan N, Keller TA, Hulme C, Roodenrys S, McDougall S, Rack J. Verbal memory span in children: Speech timing clues to the mechanisms underlying age and word length effects. Journal of Memory and Language. 1994;33:234–250. [Google Scholar]
- Cowan N, Wood NL, Wood PK, Keller TA, Nugent LD, Keller CV. Two separate verbal processing rates contributing to short-term memory span. Journal of Experimental Psychology: General. 1998;127(2):141–60. doi: 10.1037//0096-3445.127.2.141. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9622911. [DOI] [PubMed] [Google Scholar]
- Cowan Nelson. Multiple concurrent thoughts: The meaning and developmental neuropsychology of working memory. Developmental neuropsychology. 2010;35(5):447–474. doi: 10.1080/87565641.2010.494985. doi:10.1080/875656412010494985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan Nelson, Towse JN, Hamilton Z, Saults JS, Elliott EM, Lacey JF, Hitch GJ. Children's working-memory processes: A response-timing analysis. Journal of Experimental Psychology: General. 2003;132(1):113–132. doi: 10.1037/0096-3445.132.1.113. doi:10.1037/0096-3445.132.1.113. [DOI] [PubMed] [Google Scholar]
- Flavell JH, Beach DR, Chinsky JM. Spontaneous verbal rehearsal in a memory task as a function of age. Child Development. 1966;37:283–299. [PubMed] [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biological Psychology. 2000;54(1-3):1–34. doi: 10.1016/s0301-0511(00)00051-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11035218. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40(2):177–90. doi: 10.1037/0012-1649.40.2.177. doi:10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Rovine MJ. Multilevel models for the experimental psychologist: foundations and illustrative examples. Behavior Research Methods. 2007;39(1):101–17. doi: 10.3758/bf03192848. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17552476. [DOI] [PubMed] [Google Scholar]
- Hughes C, Dunn J, White A. Trick or treat? Uneven understanding of mind and emotion and executive dysfunction in “hard-to-manage” preschoolers. Journal of Child Psychology and Psychiatry. 1998;39:981–994. [PubMed] [Google Scholar]
- Killikelly C, Szűcs D. Delayed development of proactive response preparation in adolescents: ERP and EMG evidence. Developmental cognitive neuroscience. 2013;3:33–43. doi: 10.1016/j.dcn.2012.08.002. doi:10.1016/j.dcn.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund P, Persson J. Context-dependent switching between proactive and reactive working memory control mechanisms in the right inferior frontal gyrus. NeuroImage. 2012;63(3):1552–60. doi: 10.1016/j.neuroimage.2012.08.016. doi:10.1016/j.neuroimage.2012.08.016. [DOI] [PubMed] [Google Scholar]
- McAuley T, White DA. A latent variables examination of processing speed, response inhibition, and working memory during typical development. Journal of Experimental Child Psychology. 2011;108(4):453–468. doi: 10.1016/j.jecp.2010.08.009. doi:10.1016/j.jecp.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quene H. On multi-level modeling of data from repeated measures designs: a tutorial. Speech Communication. 2004;43(1-2):103–121. doi:10.1016/j.specom.2004.02.004. [Google Scholar]
- Roome H, Towse JN. The re-invention of primary and secondary memory: How does it contribute to our understanding of immediate free recall?; Poster presented at the Joint Annual Conference of the British Psychological Society Developmental and Cognitive Sections; Reading, UK. 2013, September. [Google Scholar]
- Tehan G, Lalor DM. Individual differences in memory span: The contribution of reahearsal, access to lexical memory, and output speed. The Quarterly Journal of Experimental Psychology. 2000;53A:1012–1038. doi: 10.1080/713755933. [DOI] [PubMed] [Google Scholar]
- Towse JN, Cowan N, Hitch GJ, Horton NJ. The recall of information from working memory. Insights from behavioural and chronometric perspectives. Experimental Psychology. 2008;55:371–383. doi: 10.1027/1618-3169.55.6.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towse JN, Cowan N, Horton NJ, Whytock S. Task experience and children's working memory performance: a perspective from recall timing. Developmental Psychology. 2008;44(3):695–706. doi: 10.1037/0012-1649.44.3.695. doi:10.1037/0012-1649.44.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towse JN, Hitch GJ, Hutton U. A reevaluation of working memory capacity in children. Journal of Memory and Language. 1998;39(2):195–217. doi:10.1006/jmla.1998.2574. [Google Scholar]
- Towse JN, Hitch GJ, Horton N, Harvey K. Synergies between processing and memory in children's reading span. Developmental Science. 2010;13:779–789. doi: 10.1111/j.1467-7687.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007;114(1):104–32. doi: 10.1037/0033-295X.114.1.104. doi:10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Shallice T. Developmental dissociations of preparation over time: deconstructing the variable foreperiod phenomena. Journal of Experimental Psychology: Human Perception and Performance. 2007;33(6):1377–88. doi: 10.1037/0096-1523.33.6.1377. doi:10.1037/0096-1523.33.6.1377. [DOI] [PubMed] [Google Scholar]
- Waxer M, Morton JB. The development of future-oriented control: an electrophysiological investigation. NeuroImage. 2011;56(3):1648–54. doi: 10.1016/j.neuroimage.2011.02.001. doi:10.1016/j.neuroimage.2011.02.001. [DOI] [PubMed] [Google Scholar]
- West R, Bailey K, Tiernan BN, Boonsuk W, Gilbert S. The temporal dynamics of medial and lateral frontal neural activity related to proactive cognitive control. Neuropsychologia. 2012;50(14):3450–60. doi: 10.1016/j.neuropsychologia.2012.10.011. doi:10.1016/j.neuropsychologia.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Sheffield T, Nelson JM, Clark CAC, Chevalier N, Espy KA. The structure of executive function in 3-year-olds. Journal of Experimental Child Psychology. 2011;108(3):436–452. doi: 10.1016/j.jecp.2010.08.008. doi:10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD. The development of conscious control in childhood. Trends in Cognitive Sciences. 2004;8(1):12–17. doi: 10.1016/j.tics.2003.11.001. doi:10.1016/j.tics.2003.11.001. [DOI] [PubMed] [Google Scholar]