Abstract
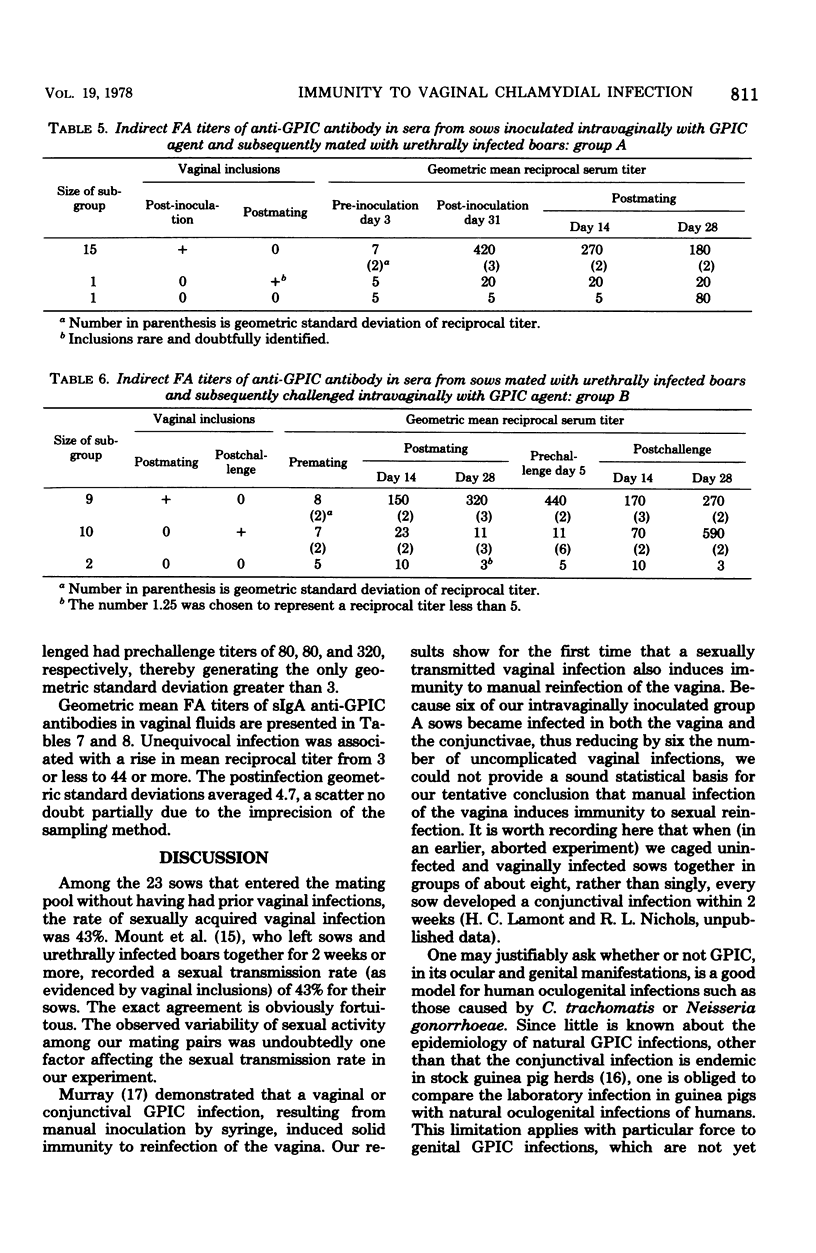
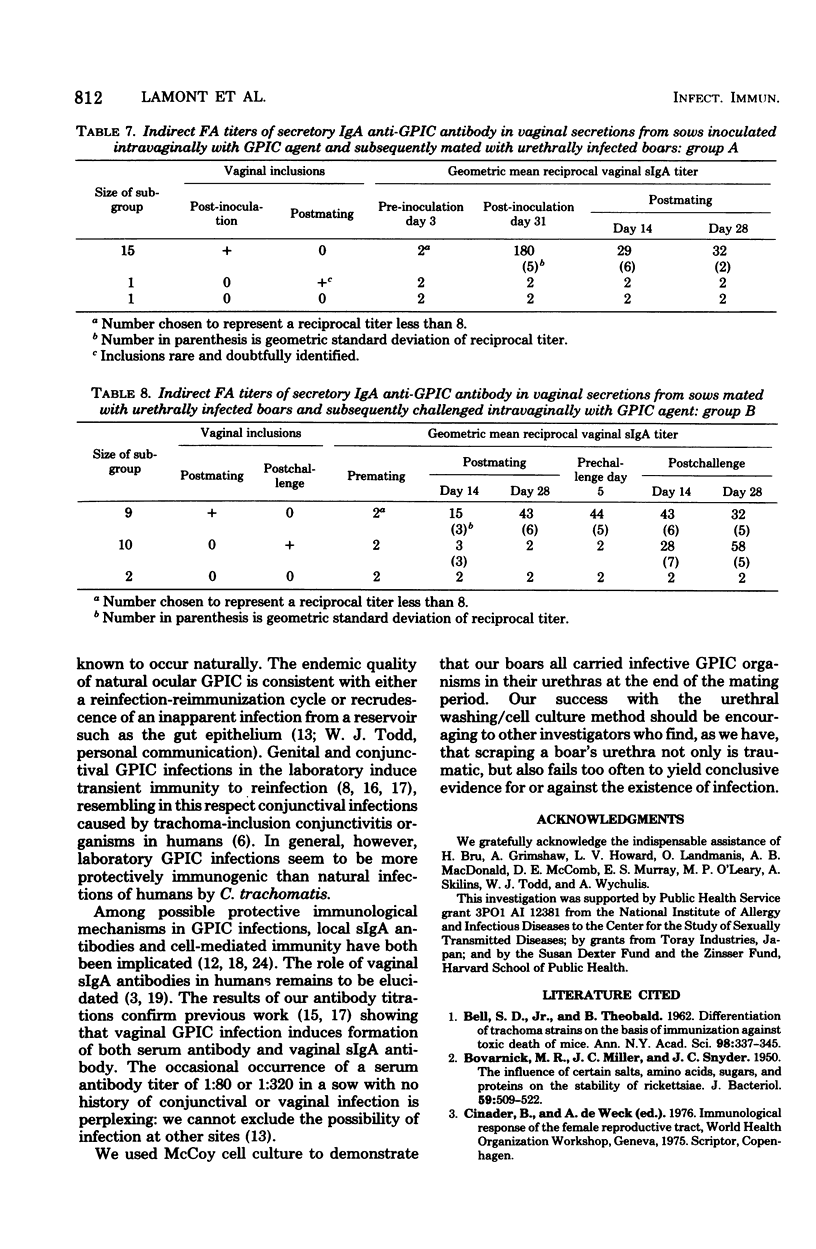
Guinea pig boars were inoculated intraurethrally with the chlamydial agent of guinea pig inclusion conjunctivitis (GPIC). At the heights of their urethral infections, they were caged with sows in estrus. Whereas some of the sows had not been previously exposed to GPIC agent, others had received an intravaginal inoculation 5 to 8 weeks earlier. Those sows for which infected boars provided the first exposure were challenged by intravaginal inoculation 5 to 8 weeks later. Vaginal and conjunctival scrapings were taken regularly and stained for chlamydial inclusions. Titers of serum anti-GPIC antibodies and of vaginal secretory IgA anti-GPIC antibodies were determined by immunofluorescence. Our results show for the first time that a sexually acquired vaginal GPIC infection induces immunity to manual reinfection of the vagina. Because of the high incidence of secondary conjunctival infections among the vaginally infected sows, we could not provide a sound statistical basis for our tentative conclusion that manual infection of the vagina induces immunity to sexual reinfection. The results of our antibody titrations confirm previous work showing that vaginal GPIC infection induces formation of both serum antibody and vaginal secretory immunoglobulin A antibody.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL S. D., Jr, THEOBALD B. Differentiation of trachoma strains on the basis of immunization against toxic death of mice. Ann N Y Acad Sci. 1962 Mar 5;98:337–346. doi: 10.1111/j.1749-6632.1962.tb30556.x. [DOI] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Weiss E., Quan A. L., Dressler H. R. Observations on guinea pig inclusion conjunctivitis agent. J Infect Dis. 1966 Apr;116(2):203–207. doi: 10.1093/infdis/116.2.203. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Howard L. V., O'Leary M. P., Nichols R. L. Animal model studies of genital chlamydial infections. Immunity to re-infection with guinea-pig inclusion conjunctivitis agent in the urethra and eye of male guinea-pigs. Br J Vener Dis. 1976 Aug;52(4):261–265. doi: 10.1136/sti.52.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES B. R. OCULAR SYNDROMES OF TRIC VIRUS INFECTION AND THEIR POSSIBLE GENITAL SIGNIFICANCE. Br J Vener Dis. 1964 Mar;40:3–18. doi: 10.1136/sti.40.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdan J. J., Schachter J., Okumoto M. Inclusion conjunctivitis in the guinea pig. Am J Ophthalmol. 1967 Jul;64(1):116–124. doi: 10.1016/0002-9394(67)93351-x. [DOI] [PubMed] [Google Scholar]
- MURRAY E. S. GUINEA PIG INCLUSION CONJUNCTIVITIS VIRUS. I. ISOLATION AND IDENTIFICATION AS A MEMBER OF THE PSITTACOSIS-LYMPHOGRANULOMA-TRACHOMA GROUP. J Infect Dis. 1964 Feb;114:1–12. doi: 10.1093/infdis/114.1.1. [DOI] [PubMed] [Google Scholar]
- McComb D. E., Puzniak C. I. Micro cell culture method for isolation of Chlamydia trachomatis. Appl Microbiol. 1974 Oct;28(4):727–729. doi: 10.1128/am.28.4.727-729.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabber F., Bear S. E., Cerny J. The effect of cyclophosphamide on the recovery from a local chlamydial infection. Guinea-pig inclusion conjunctivitis (GPIC). Immunology. 1976 Jun;30(6):929–933. [PMC free article] [PubMed] [Google Scholar]
- Mount D. T., Barron A. L. Intrarectal infection of guinea pigs with the agent of guinea pig inclusion conjunctivitis. Proc Soc Exp Biol Med. 1976 Dec;153(3):388–391. doi: 10.3181/00379727-153-39552. [DOI] [PubMed] [Google Scholar]
- Mount D. T., Bigazzi P. E., Barron A. L. Experimental genital infection of male guinea pigs with the agent of guinea pig inclusion conjunctivitis and transmission to females. Infect Immun. 1973 Dec;8(6):925–930. doi: 10.1128/iai.8.6.925-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. T., Bigazzi P. E., Barron A. L. Infection of genital tract and transmission of ocular infection to newborns by the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1972 Jun;5(6):921–926. doi: 10.1128/iai.5.6.921-926.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E. S., Charbonnet L. T., MacDonald A. B. Immunity to chlamydial infections of the eye. I. The role of circulatory and secretory antibodies in resistance to reinfection with guinea pig inclusion conjunctivitis. J Immunol. 1973 Jun;110(6):1518–1525. [PubMed] [Google Scholar]
- O'Reilly R. J., Lee L., Welch B. G. Secretory IgA antibody responses to Neisseria gonorrhoeae in the genital secretions of infected females. J Infect Dis. 1976 Feb;133(2):113–125. doi: 10.1093/infdis/133.2.113. [DOI] [PubMed] [Google Scholar]
- REEVE P., TAVERNE J. A simple method for total particle counts of trachoma and inclusion blennorrhoea viruses. Nature. 1962 Sep 1;195:923–924. doi: 10.1038/195923a0. [DOI] [PubMed] [Google Scholar]
- Schachter J., Causse G., Tarizzo M. L. Chlamydiae as agents of sexually transmitted diseases. Bull World Health Organ. 1976;54(3):245–254. [PMC free article] [PubMed] [Google Scholar]
- Watson R. R., MacDonald A. B., Murray E. S., Modabber F. Z. Immunity to chlamydial infections of the eye. 3. Presence and duration of delayed hypersensitivity to guinea pig inclusion conjuctivitis. J Immunol. 1973 Aug;111(2):618–623. [PubMed] [Google Scholar]


