Abstract
Background:
Osteosarcoma is the most common bone malignancy in children, adolescents, and young adults. Most study cohorts have 10% to 15% Hispanic patients that encompass many different Hispanic backgrounds. This study characterizes the effect of mainly Mexican American ethnicity on the outcome of children, adolescents, and young adults with osteosarcoma.
Methods:
A retrospective analysis of demographics, tumor characteristics, response to treatment, and survival outcome of all localized osteosarcoma of the extremity patients below 30 years of age was performed. A Kaplan-Meier estimates with log-rank tests and Cox proportional hazard regression models were used.
Results:
Fifty patients (median age, 15; range, 2 to 28 y) with localized high-grade osteosarcoma of the extremity were diagnosed between January 2000 and December 2010. The cohort was 70% Mexican Americans. With a median follow-up of 39 months (range, 5 to 142 mo), patients had a 5-year overall survival and event-free survival of 65% and 48%, respectively. We observed a significantly decreased 5-year event-free survival in patients diagnosed before age 12 relative to patients diagnosed between ages 12 and 29 (11% vs. 57%, P<0.001). We also found that tumor necrosis was not predictive of outcome in our patients.
Conclusions:
The preadolescent patients of predominately Mexican American ethnicity had an increased rate of relapse when compared with previous studies. Tumor necrosis is not directly predictive of outcome in this population.
Key Words: osteosarcoma, pediatrics, Hispanic, outcome, preadolescent
Osteosarcoma is the most common malignant bone tumor in children, adolescents, and young adults, with about 400 new cases diagnosed each year in the United States. It accounts for approximately 60% of all malignant bone tumors diagnosed in patients before the age of 20 with the peak incidence associated with puberty.1 Although osteosarcoma can occur in the axial skeleton, it typically presents in the metaphysis of long bones, a site of rapid bone growth during adolescence. The standard treatment for high-grade osteosarcoma requires both surgery and chemotherapy given preoperatively (neoadjuvant) and/or postoperatively. Despite improved surgical outcomes and efforts to intensify therapy, the 5-year event-free survival (EFS) remains 65% with no significant improvement in the past 20 years.2
In studies conducted pertaining to osteosarcoma outcome in pediatrics, only 10% to 15% of the cohorts are Hispanics. A study by Mirabello et al3 based on data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, indicates a higher incidence and slightly poorer outcome of osteosarcoma in Hispanics when compared with non-Hispanics. Hispanic patients that are reported in this studies come from a variety of locations including Mexico, South America, Cuba, and Latin America.
In this study we performed a retrospective analysis of the characteristics and outcomes in patients with localized high-grade osteosarcoma of the extremity diagnosed under the age of 30 treated at a single institution, University of Texas Health Science Center at San Antonio (UTHSCSA), over an 11-year period. The patients in this study were 70% Hispanic, homogenously of Mexican American ancestry, therefore giving us a unique cohort to study. To the best of our knowledge, this is the largest series composed of such patients. Interestingly, we found a decreased survival of preadolescent patients compared with the patients who were between 12 and 30 years old at diagnosis. Furthermore, our data suggests that tumor necrosis after neoadjuvant chemotherapy might not be directly predictive of outcome.
MATERIALS AND METHODS
Patient Selection and Data Elements
Our cohort consisted of 50 patients below 30 years of age diagnosed with localized high-grade osteosarcoma of the extremity between January 2000 and December 2010 that were treated by members of the UTHSCSA sarcoma team. Patients with axial primaries or metastatic disease at diagnosis were excluded from this analysis. During this time frame nearly uniform treatment was used for patients with osteosarcoma. Hospital and clinic records from University Hospital, CHRISTUS Santa Rosa Children’s Hospital, and the Cancer Therapy and Research Center were reviewed. A retrospective analysis of patient demographics (age at diagnosis, sex, date of diagnosis, race, and ethnicity), presence of predisposing factors, socioeconomic status (based on family income obtained from institutional survey), and tumor characteristics (location, histology, tumor volume, response to neoadjuvant chemotherapy, and type of primary surgery) was performed.
Ethnicity was assigned based on parental report, and the National Cancer Institute/Children’s Oncology Group (COG) definitions. According to this convention, the term Hispanic can include Mexican Americans, South Americans, or Cubans but our population of Hispanics was exclusively Mexican American. Tumor volume was defined as the absolute tumor volume (ATV) in cm3. ATV was defined as absolute tumor length (ATL)×absolute tumor width (ATW)×absolute tumor depth (ATD) (ATV in cm3=ATL×ATW×ATD×0.52). The diagnosis of osteosarcoma was confirmed by J.H.-H., a pediatric pathologist with expertise in bone tumors.
The project was conducted after approval was received from the institutional review board at the respective institution.
Treatment
Chemotherapy was utilized in the neoadjuvant and adjuvant settings. The chemotherapy regimens, specifically dosing, were based on body surface area and the 2 groups received equivalent treatment either on study or according to study protocol based on either AOST0331 or POG9754 depending on when the patient was diagnosed. The chemotherapy included combinations of methotrexate, cisplatin, adriamycin, ifosfamide, and etoposide; although not all agents were used in each patient. All 50 patients underwent surgery for local control and had negative surgical margins as confirmed by pathology. The type of surgery (limb salvage, amputation, rotationplasty) was determined by the extent of disease, involvement of neurovascular bundle, presence of pathologic fracture, and appraisal for best limb functionality after surgical resection. The resected specimens were examined for percentage of tumor necrosis in response to neoadjuvant chemotherapy and were assigned a grade of 1 to 6 as defined by Salzer-Kuntschik et al.4 In this grading system, grade 1 signifies no viable tumor; grade 2, solitary live cells or 1 islet of live cells <0.5 cm; grade 3, <10% viable cells; grade 4, 10% to 50% viable cells; grade 5, >50% alive tumor; and grade 6, 100% viable tumor. This system for tumor necrosis grading was chosen to standardize the 2 different grading systems that were used in the individual POG9754 and AOST0331 studies. Longitudinal tumor assessment (primary site and lungs) was performed according to POG9754 or AOST0331 recommendations.
Statistical Analysis
The primary focus of this analysis was EFS and overall survival (OS). EFS was calculated from the date of diagnosis until recurrence, secondary malignancy, death, or most recent follow-up examination showing absence of an event. OS was calculated from the date of diagnosis to death or most recent follow-up examination. The survival curves were calculated using the Kaplan-Meier method and the differences of survival curves were assessed using the log-rank test. Adjusted estimates were obtained from proportional hazards models with age, sex, ethnicity, family income, tumor volume, and tumor necrosis included as covariates. Power analysis was completed and all statistical testing was 2-sided with a significance level of 5%. SAS Version 9.2 (SAS Institute, Cary, NC) was used.
RESULTS
Demographic Data
Fifty patients with localized osteosarcoma of the extremity below 30 years of age were diagnosed between January 2000 and December 2010 at UTHSCSA. The complete demographic and clinicopathologic characteristics are shown in Table 1. The cohort was composed of 35 Hispanics (70%), 10 whites (20%), 4 blacks (8%), and 1 other (2%). Ethnicity was assigned based on parental report, and the National Cancer Institute/COG definitions. Therefore, although our cohort is comprised of mainly Hispanics, it is not exclusively Hispanics. However, as described in the Methods section our Hispanic population is uniquely composed of only Mexican Americans, whereas most of the studies that include Hispanics are more heterogenous.
TABLE 1.
Patient Demographics
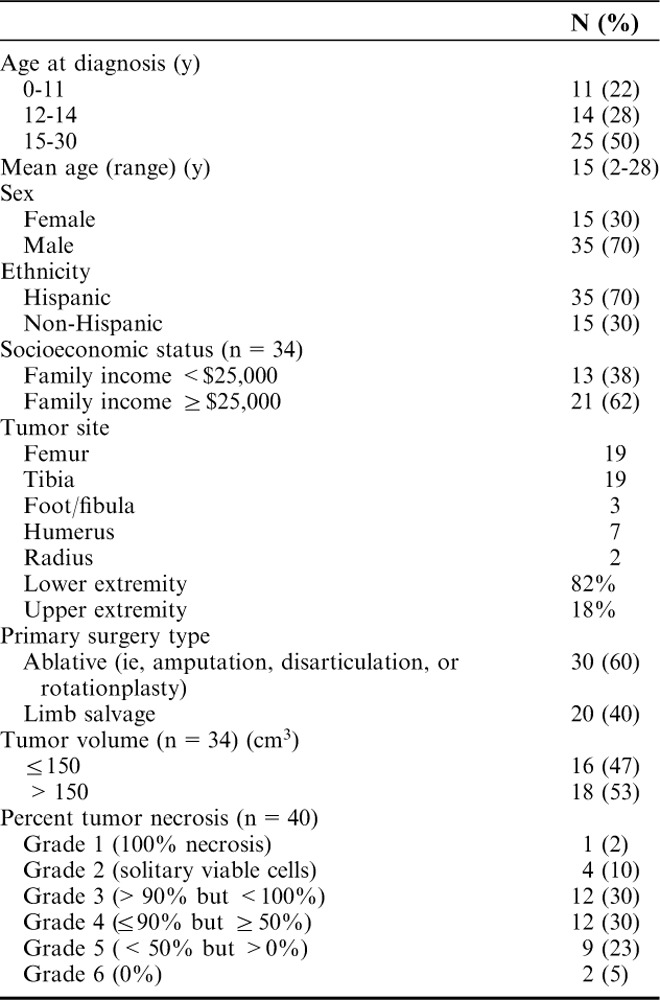
The mean age at diagnosis was 15 years (range, 2 to 28 y). Male to female ratio was approximately 3:1. Socioeconomic status based on family income was available for 68% of the cohort. Family income was <$25,000 for 13 patients and ≥$25,000 for 21 patients. There was no clinical or statistical correlation between socioeconomic status data and tumor size or extent of disease at presentation.
Clinicopathologic Characteristics
All patients presented with localized disease of an extremity. Tumor location was as follows: 41 (82%) in the lower extremity and 9 (18%) in the upper extremity. High-grade, conventional osteosarcoma was diagnosed in all patients. Tumor size was available for 34 (85%) patients. Eighteen (53%) patients had a tumor volume >150 cm3 and 16 (47%) patients had a tumor volume ≤150 cm3. No significant association could be found between Hispanic ethnicity and large tumor size (P=0.73). All patients had surgery for local control; 60% had ablative surgery (amputation, disarticulation, or rotationplasty), whereas 40% had limb salvage procedures. We acknowledge that this is an increased percentage of patients undergoing ablative procedures but this was due to the disproportionate number of patients with joint and neurovascular bundle involvement. All patients had a complete resection.
Histologic response to neoadjuvant chemotherapy was available for 40 (80%) patients. Seventeen (42%) patients were good responders with grades 1 to 3 necrosis (>90%). Twelve (30%) patients had grade 4 necrosis (between 50% and 90%) and 11 (28%) patients had a very poor response (<50% necrosis) including 2 patients with 100% viable tumor after neoadjuvant chemotherapy. Although the cohort of patients for which histologic response was available was 70% Hispanic, 10/11 (91%) of the patients with a very poor response were Hispanics.
Univariate Survival Analysis
The OS at 3 and 5 years was 73% and 65%, respectively, for the entire cohort. The 3- and 5-year EFS were 50% and 48%, respectively. Eleven patients diagnosed before the age of 12 experienced a statistically significant decreased 5-year EFS and OS relative to those diagnosed between the ages of 12 and 29 (11% vs. 57%, respectively, P=<0.001 for EFS and 25% vs. 76%, respectively, P=<0.001 for OS) (Table 2). There was no statistically significant difference in outcomes based on ethnicity, income, or tumor volume.
TABLE 2.
Patient Characteristics and Univariate Analysis
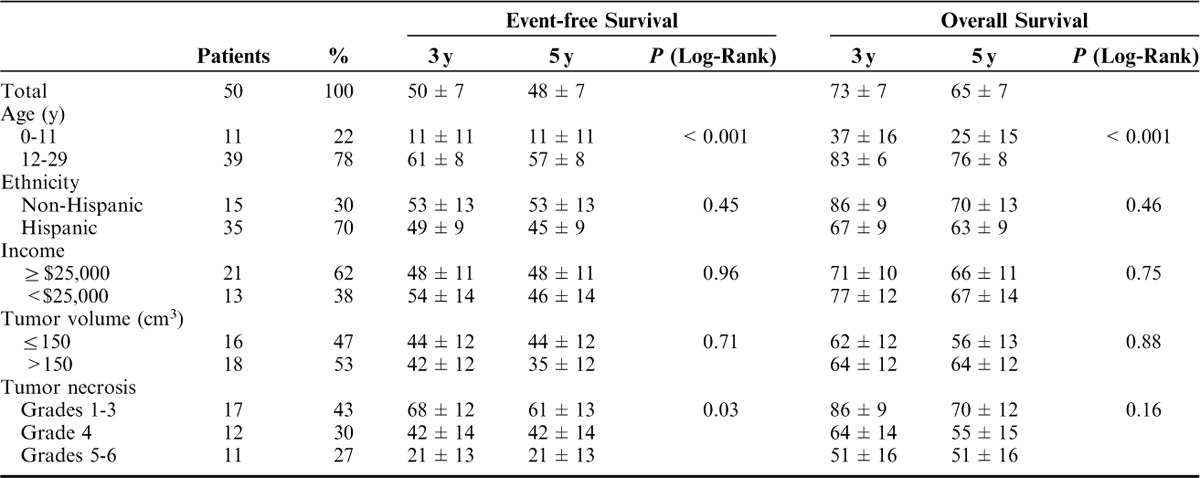
Although response to neoadjuvant chemotherapy has traditionally been used as a prognostic marker for both EFS and OS, it was of prognostic significance only for EFS (Table 2 and Figs. 1, 2) in our population. We observed an increased percentage of patients with <50% necrosis after neoadjuvant chemotherapy and chose to grade necrosis based on 6 categories as defined by Salzer-Kuntschik et al4 to further categorize the “poor responders.” The 5-year EFS when compared between groups categorized by grades 1 to 3, grade 4, and grades 5 to 6 necrosis showed a statistically significant decreased outcome in patients with grades 5 to 6 necrosis (61% vs. 42% vs. 21%, respectively, P=0.03). The 5-year OS for these patients was similar across the groups suggesting that our patient population was salvageable after initial relapse.
FIGURE 1.
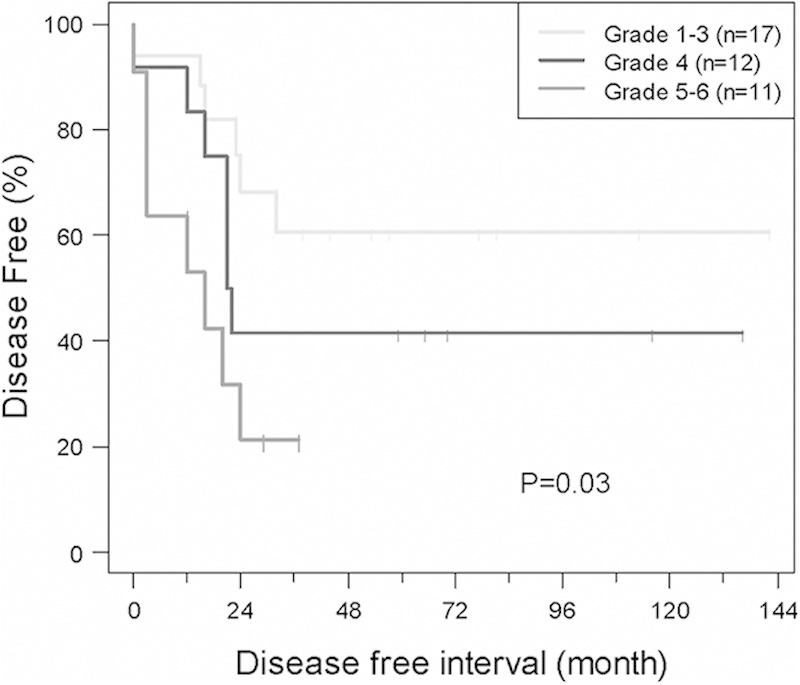
Event-free survival (EFS) of localized/extremity tumor patients based on tumor necrosis. The 5-year EFS for patients with grades 5 to 6 was significantly different than the 5-year EFS of patients with grades 1 to 3 and grade 4 tumor necrosis (P=0.03).
FIGURE 2.
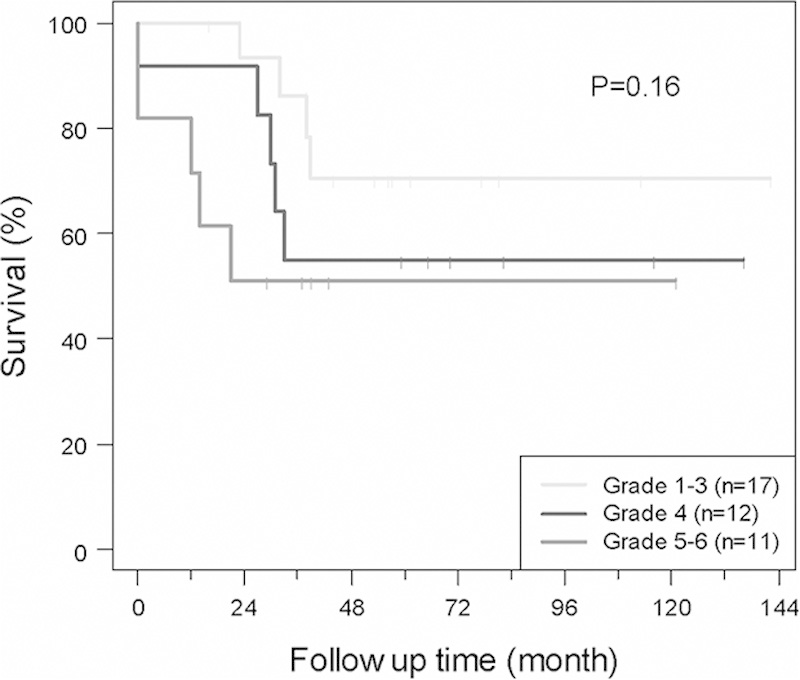
Overall survival (OS) of localized/extremity tumor patients based on tumor necrosis. There was no significant difference in OS based on tumor necrosis in this population.
Twenty-three of the 50 patients relapsed. There was no difference in the location of relapse when comparing the preadolescent patients to the older patients (young patients [n=9]: 22%, local; 55%, lung; 22%, lung/local; older patients [n=14]: 21%, local; 57%, lung; 21%, lung/local). For the complete cohort, isolated lung recurrence was observed in 13 patients. Overall, 4 patients experienced a local recurrence, 5 had combined local/lung recurrence, and 1 patient had progressive disease, meaning nonresponsive to neoadjuvant chemotherapy. The mean time to relapse was 19 months (range, 2 to 37 mo). Salvage treatment included surgical resection for the majority of patients, with many receiving postoperative chemotherapy. Eight of the 13 patients with isolated lung recurrences and 4 of the 10 with other types of recurrence/progression were successfully salvaged for an overall salvage rate of 52% for first relapses.
Multivariate Survival Analysis
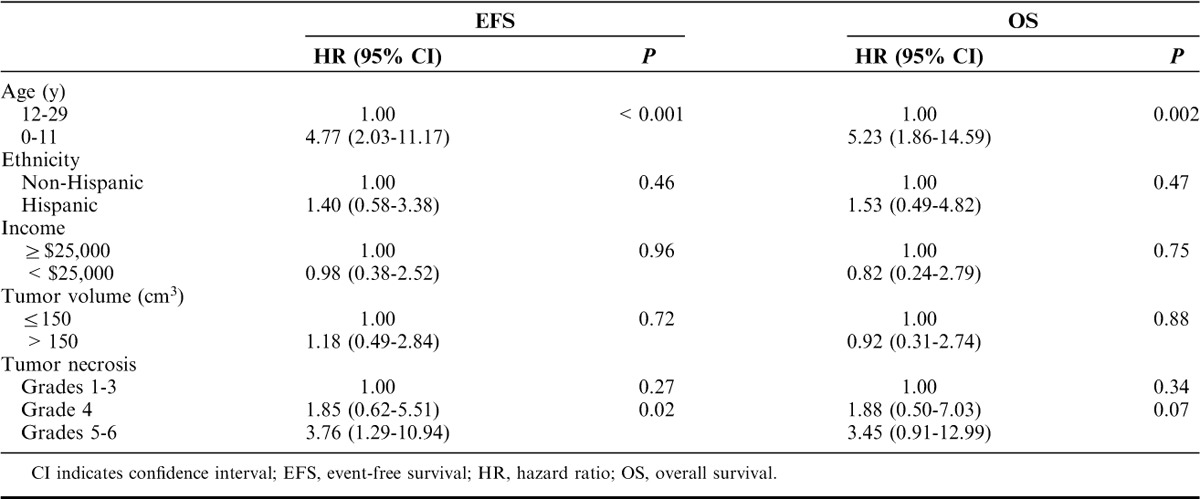
Multivariate Cox regression analysis was performed to assess the association between EFS or OS and predictors, such as age, ethnicity, income, tumor volume, and tumor necrosis. The results are summarized in Table 3. Patients below 12 years of age at diagnosis experienced a higher rate of relapse and death relative to those 12 to 29 years of age at diagnosis (hazard ratio [HR], 4.77; 95% CI, 2.03-11.17; P=<0.001 for EFS; HR, 5.23; 95% CI, 1.86-14.59; P=0.002 for OS). Grades 5 to 6 necrosis after neoadjuvant chemotherapy was significantly predictive of decreased EFS (HR, 3.76; 95% CI, 1.29-10.94; P=0.02) and there was a trend toward lower OS (HR, 3.45; 95% CI, 0.91-12.99; P=0.07). None of ethnicity, income, or tumor volume was significantly correlated with either EFS or OS.
TABLE 3.
Multivariate Analysis (Cox Models) of EFS and OS
DISCUSSION
The population of localized osteosarcoma of the extremity patients treated at UTHSCSA was 70% Hispanic (35/50), homogenously of Mexican American ancestry, giving us a unique cohort to study. Of the 50 patients in our cohort, 11 were categorized as preadolescent (below 12 y of age at diagnosis). Nine of the 35 Mexican American patients were preadolescents at diagnosis and to the best of our knowledge this is the largest series composed of such patients. In this study, the 5-year EFS of 48% was similar to results from the Brazilian Osteosarcoma Treatment Group who reported a 5-year EFS of only 53%.5 In this cohort, the 5-year EFS was inferior to a smaller study completed on patients in low-income countries in Latin America.6 These data suggest that there might be some similarities and differences in outcome among other Hispanic groups. Although the 5-year OS of 65% was comparable with that reported by large European groups,2,7 the EFS in this cohort was lower.2,8–12 In this regard, the data indicated that our patients had a higher risk of relapse after primary treatment, but were salvageable as denoted by OS rates.
Notably, our predominantly Mexican American preadolescent patients (below 12 y of age at diagnosis of which 9/11 are Hispanic) had an overall poor outcome with 5-year EFS of 11% and a 5-year OS of 25%. In stark contrast, many published studies report the outcome of preadolescent patients to be equivalent to those diagnosed after puberty.13–17 Bacci et al14 reported results on a large cohort of patients treated on protocol between 1972 and 1999, comparing the outcome of patients aged 12 years and below at diagnosis to that of patients between 13 and 40 years of age. The 2 groups have equivalent clinicopathologic features and similar histologic response to chemotherapy. The 5-year EFS is 60% versus 58% in favor of the preadolescents.14
In our analysis, the older patients (12 to 30 y of age at diagnosis) had a better 5-year EFS compared with the preadolescent patients. However, there was no difference in the response to neoadjuvant chemotherapy between the 2 age groups and thus would not explain the inferior outcome of the preadolescent patients. Moreover, our patients were very compliant and therefore received the recommended chemotherapy with no difference between preadolescents and older patients with regard to chemoreduction for toxicities. There is a recent report by Sharib et al18 that mentioned increased toxicity in young patients and in their Latino population with Ewing Sarcoma. We did not find an increase in toxicity in either of these groups within our cohort and thus could not use it as an explanation for the trend toward inferior outcome.
Traditionally, the histologic response to neoadjuvant chemotherapy has been used as a prognostic factor. In previous studies, good responders (>90% necrosis) had a 5-year EFS of 67% to 78%, whereas the poor responders had a 5-year EFS of 49% to 51%.8–10 In our cohort, tumor necrosis assessment was available for 40 patients. Of these patients 43% were “good responders.” Surprisingly, the 5-year EFS in the good responders was only 61%. The 5-year OS was 70% and this is comparable with that reported in larger studies8–10 as described above. The patients who were deemed “good responders” to neoadjuvant chemotherapy relapsed at a higher rate than expected but were salvageable. Eleven patients (91% Hispanic) in our cohort had a very poor response with <50% necrosis. Nine of the 11 patients relapsed, were lost to follow-up, or were censored within 3 years due to the time frame of the study. The patients that relapsed were salvageable and had a 5-year OS of 51%. Although our sample size is small (n=50), our analysis suggested that tumor necrosis in response to neoadjuvant chemotherapy regimens might not be a valid prognostic marker for EFS in our study.
In this study, the same orthopedic surgeon provided surgical management over the 11-year period. As well, the same pathologist confirmed diagnosis over the 11-year period. Chemotherapy was delivered according to standard protocols from the COG by pediatric or medical oncologists from different COG institutions. All patients were treated at institutions by medical teams trained in delivering treatment on osteosarcoma protocols, whether enrolled on study or not. Therefore, the inferior outcome in this population might not be the result of poor adherence to treatment or a decrease in treatment because of toxicity. In our cohort, the male to female ratio was much higher than expected and a disproportionate amount of patients received ablative surgical procedures. The observation of an increase in male to female ratio may in part be due to our small sample size. The higher proportion of patients receiving ablative surgical procedures was in part because our patients presented with more invasive disease at diagnosis.
In conclusion, we reviewed the clinicopathologic characteristics and disease outcome of 50 patients with localized high-grade osteosarcoma of the extremity treated by ≥1 members of the UTHSCSA sarcoma team over an 11-year period. Our cohort was comprised of 70% Hispanics of Mexican American descent and we found a decreased EFS but similar OS to what is reported. More importantly, we found a strikingly increased rate of relapse in young patients diagnosed before the age of 12. We also found that the percentage of tumor necrosis after neoadjuvant chemotherapy was not directly predictive of outcome in our population. The possibility exists that this difference in outcome is secondary to a difference in pharmacodynamics or pharmacogenomics leading to a difference in the metabolism of the various drugs used to treat osteosarcoma. It is also possible that a difference in tumor biology does exist and could be explored further in a larger study. A larger, multi-institutional study including patients with similar demographics to our study is warranted. More data related to outcomes in patients of Mexican American ancestry will potentially aid in future treatment decision making and management concerning this fast-growing population.
Footnotes
Supported in part by a National Institutes of Health NIH-NCATS UL1TR000149 CTSA grant through the Institute of Medicine and Science (IIMS) at the University of Texas Health Science Center at San Antonio to J.Y.H. and A.-M.L., and a Fellowship Training Grant through the Cancer Prevention & Research Institute of Texas (CPRIT) to A.J.S.
The authors declare no conflict of interest.
REFERENCES
- 1.Mascarenhas L, Siegel S, Spector L, et al. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age including SEER incidence and survival: 1975-2000 National Cancer Institute. NCI Report 2006.
- 2.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols.J Clin Oncol. 2002;20:776–790. [DOI] [PubMed] [Google Scholar]
- 3.Mirabello L, Troisi J, Savage S.Osteosarcoma incidence and survival rates from 1973 to 2004.Cancer. 2009;115:1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzer-Kuntschik M, Brand G, Delling G.Bestimmung des morphologischen regressionsgrades nach chemotherapie bei malignen knochentumoren.Pathologie. 1983;4:135–141. [PubMed] [Google Scholar]
- 5.Petrilli A, de Camargo B, Odone Filho V, et al. Fifteen years experience of the Brazilian Osteosarcoma Treatment Group (BOTC).J Clin Oncol. 2009;27:15s. [Google Scholar]
- 6.Howard S, Ortiz R, Baez L, et al. Protocol-Based treatment for children with cancer in low income countries in Latin America; A report on the recent meetings of the Monza International School of Pediatric Hematology/Oncology (MISPHO).Pediatr Blood Cancer. 2007;48:486–490. [DOI] [PubMed] [Google Scholar]
- 7.Picci P, Mercuri M, Ferrari S, et al. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution.Ann Oncol. 2010;21:1366–1373. [DOI] [PubMed] [Google Scholar]
- 8.Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution.Cancer. 2006;106:1154–1161. [DOI] [PubMed] [Google Scholar]
- 9.Smeland S, Muller C, Alvegard T, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders.Eur J Cancer. 2003;39:488–494. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs N, Bielack S, Epler D, et al. Long-term results of co-operative German-Austrian-Swiss osteosarcoma study group’s protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs.Ann Oncol. 1998;9:893–899. [DOI] [PubMed] [Google Scholar]
- 11.Goorin A, Schwartzentruber D, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651.J Clin Oncol. 2003;21:1574–1580. [DOI] [PubMed] [Google Scholar]
- 12.Meyers P, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol.J Clin Oncol. 1998;16:2452–2458. [DOI] [PubMed] [Google Scholar]
- 13.Rytting M, Pearson P, Raymond A, et al. Osteosarcoma in preadolescent patients.Clin Orthop Relat Res. 2000;373:39–50. [DOI] [PubMed] [Google Scholar]
- 14.Bacci G, Longhi A, Bertoni F, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients.J Pediatr Hematol Oncol. 2005;27:129–134. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Kim D, Lim J, et al. The survival of osteosarcoma patients 10 years old or younger is not worse than the survival of older patients: a retrospective analysis.Cancer Res Treat. 2007;39:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartford C, Wodowski K, Rao B.Osteosarcoma among children aged 5 years or younger: The St. Jude Children’s Research Hospital experience.J Pediatr Hematol Oncol. 2006;28:43–47. [PubMed] [Google Scholar]
- 17.Nagarajan R, Weigel B, Thompson R, et al. Osteosarcoma in the first decade of life.Pediatr Blood Cancer. 2003;41:480–483. [DOI] [PubMed] [Google Scholar]
- 18.Sharib J, Cyrus J, Horvai A, et al. Predictors of acute chemotherapy-associated toxicity in patients with Ewing sarcoma.Pediatr Blood Cancer. 2012;59:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]


