Abstract
Objectives:
Identification of people with lower (white-coat effect) or higher (masked effect) blood pressure at home compared to the clinic usually requires ambulatory or home monitoring. This study assessed whether changes in SBP with repeated measurement at a single clinic predict subsequent differences between clinic and home measurements.
Methods:
This study used an observational cohort design and included 220 individuals aged 35–84 years, receiving treatment for hypertension, but whose SBP was not controlled. The characteristics of change in SBP over six clinic readings were defined as the SBP drop, the slope and the quadratic coefficient using polynomial regression modelling. The predictive abilities of these characteristics for lower or higher home SBP readings were investigated with logistic regression and repeated operating characteristic analysis.
Results:
The single clinic SBP drop was predictive of the white-coat effect with a sensitivity of 90%, specificity of 50%, positive predictive value of 56% and negative predictive value of 88%. Predictive values for the masked effect and those of the slope and quadratic coefficient were slightly lower, but when the slope and quadratic variables were combined, the sensitivity, specificity, positive and negative predictive values for the masked effect were improved to 91, 48, 24 and 97%, respectively.
Conclusion:
Characteristics obtainable from multiple SBP measurements in a single clinic in patients with treated hypertension appear to reasonably predict those unlikely to have a large white-coat or masked effect, potentially allowing better targeting of out-of-office monitoring in routine clinical practice.
Keywords: home–clinic blood pressure difference, hypertension, masked effect, sensitivity, specificity, white-coat effect
INTRODUCTION
Hypertension is an important risk factor for cardiovascular disease [1], which is the major cause of morbidity and mortality worldwide [2]. In those with established hypertension, effective management depends on accurate measurement of blood pressure in order to target antihypertensive treatment appropriately and avoid unnecessary treatment and healthcare costs [3]. This measurement usually takes place in the physician's office (or clinic) in a primary care setting. However, clinic blood pressure measurements frequently under/overestimate true blood pressure which may result in incorrect classification and hence subsequent management [4,5].
Depending on the direction of the error, such deviations can be defined as ‘white-coat’ or ‘masked’ effects [6,7]. Patients with a significant white-coat effect have higher clinic blood pressure than would be expected for the corresponding ambulatory or home monitoring and are therefore at risk of over-treatment [6]. Conversely, patients with a significant masked effect have higher blood pressures with home or ambulatory monitoring than would be expected for the clinic blood pressure and therefore can be under-treated [7], thereby potentially leading to increased target organ damage [8,9] and cardiovascular mortality [10,11].
Recent research suggests that the white-coat effect may be reduced by taking a mean of multiple clinic blood pressure readings over a short space of time [12–15]. This can be done manually or systematically, using an automated blood pressure monitor such as the BpTRU device used here, which measures blood pressure six times [14]. For many patients, such multiple measurements result in a reduction in blood pressure, up to 15/6 mmHg [14,15], reducing any white-coat effect. Recently, this method has also been shown to reduce the proportion of patients displaying a masked effect in the clinic [16].
We hypothesized that the characteristics of multiple measurements of clinic blood pressure are related to the home–clinic blood pressure difference, thus potentially allowing targeting of out-of-office blood pressure monitoring in patients with white-coat or masked effects significant enough to influence management. We examined this hypothesis using data from a trial in which both multiple clinic and home blood pressure measurements were taken [17].
METHODS
An extended description of the methods used in this study can be found in the supplemental digital content.
Population
This was an observational cohort study. Participants comprised individuals screened for inclusion in the Telemonitoring and Self-management in the Control of Hypertension 2 (TASMINH2) trial who were subsequently randomized to undertake self-monitoring of blood pressure at home [17]. Participants were recruited from 24 general practices in the West Midlands, UK, between March 2007 and May 2008, following written informed consent. Inclusion criteria for the trial were age 35–84 years, receiving treatment for hypertension and blood pressure not controlled below 140/90 mmHg at baseline. Patients randomized to the intervention arm of the trial, with at least 4 days of home blood pressure readings in the first month after randomization, were included in the analysis.
Data collection
At baseline, and 6 and 12 months of follow-up, clinic blood pressure was measured in a standardized fashion using a validated [18] BpTRU BPM-100 blood pressure monitor (BpTRU Medical Devices Inc., Coquitlam, British Columbia, Canada). After participants had been seated for 5 min of rest, six blood pressure measurements were taken automatically at 1-min intervals (choice of 1–5-min intervals), all of which were recorded (i.e. including the first reading which is usually discarded) [19,20] by a researcher who was present throughout the measurement period. Patient demographics (age, sex and ethnicity) and details of previous history of cardiovascular disease were obtained from the patient or extracted from their medical records.
Home blood pressure was measured by patients themselves, using the Omron705IT (Omron Healthcare Europe, Hoofddorp, the Netherlands). Patients were asked to measure their blood pressure whilst seated, twice in the morning at 5-min intervals, during the first week of each month and over a 12-month period. All blood pressure data were transmitted to the research team via an automated modem device (i-modem; Netmedical, De Meern, the Netherlands).
No attempt was made to impute missing data. Those patients with missing self-monitoring data were excluded from the analysis. All data collection was approved by the Sandwell and West Birmingham Local Research Ethics Committee (reference; 05/Q2709/103).
Analysis
The focus of the analysis was on the characteristics of SBP, rather than DBP, as this is considered to be more closely associated with underlying cardiovascular disease risk [21,22]. Descriptive statistics were used to describe the mean [±95% confidence intervals (CIs)] clinic SBP for each of the six measurements taken using the BpTRU device. Summary data are presented as means ± SD (or 95% CIs) and percentages of the trial population [in the intervention (self-monitoring) arm, unless otherwise stated].
Home blood pressure was measured over a period of up to 7 days and mean home blood pressure was calculated having discarded the first day's readings in accordance with recommendations (up to 12 readings) [23]. To calculate the home–clinic difference, the first clinic blood pressure reading (measured at baseline using BpTRU) was subtracted from the mean home blood pressure (measured in the first month after baseline measurement, prior to any changes in antihypertensive medication). The first clinic reading was used in order to maximize the white-coat or masked effects under investigation.
We estimated the drop in SBP (sixth minus the first reading) in each individual patient at baseline and generated coefficients to represent the slope (linear) and quadratic (curve) components of this drop using polynomial regression modelling. These ‘characteristics’ were chosen because they represent a simple and straightforward approach (BP drop) which would be easy to implement in clinical practice and also a more complex model which more accurately represents the trends observed previously when blood pressure is measured repeatedly in a clinic setting [15]. These clinic blood pressure characteristics were plotted against the corresponding home–clinic difference for each individual and the relationship between the two was investigated using Pearson's correlation coefficient.
The relationship between clinic SBP and white-coat and masked effects was also examined. It was not possible to study white-coat and masked hypertension because this terminology conventionally refers to treatment-naive patients and all patients enrolled into the TASMINH2 trial had uncontrolled treated hypertension, and therefore their blood pressures did not straddle the diagnostic threshold. Because there are no standard definitions of the white-coat or masked effect, the sample population was divided into three groups on the basis of the degree of difference between home and clinic blood pressure. An arbitrary range of group boundaries were examined and one-way analysis of variance (ANOVA) was used to identify a boundary combination for the final analysis groups which provided the largest between-group variation (different home–clinic differences between white-coat, normal and masked effect groups) relative to the smallest within-group variation (patients within each group with similar home–clinic differences).
White-coat effect – any patient with a home–clinic blood pressure difference of greater than or equal to 0.2 SDs below the mean home–clinic difference for the total population (−17.7 mmHg or below).
Masked effect – any patient with a home–clinic blood pressure difference of greater than or equal to 1.1 SDs above the mean home–clinic difference for the total population (5.0 mmHg or above).
No white-coat or masked effect (normal) – any patient with a home–clinic blood pressure difference between 0.2 SDs below the mean and 1.1 SDs above the mean home–clinic difference for the total population (−17.7 to 5.0 mmHg).
The predictive abilities of the blood pressure drop, the slope and the quadratic coefficients for patients displaying white-coat and masked blood pressure characteristics were investigated using binary logistic regression. This generated probabilities that a given drop, slope or quadratic coefficient would predict a white-coat or masked effect which was used in a receiver operating characteristic analysis to estimate the coefficient thresholds with the highest sensitivity and specificity combination (where sensitivity was >90%) and positive predictive value (PPV)/negative predictive value (NPV) for a given effect.
Sensitivity analyses
To test the impact of the assumptions made in the main analysis on the relationship between clinic blood pressure characteristics and the home–clinic difference, a series of sensitivity analyses were undertaken using Pearson's correlation coefficient. A second set of sensitivity analyses examined the reliability of the definition of the white-coat and masked effect by studying whether the use of alternative boundary combinations for both conditions altered the results. These sensitivity analyses are detailed in the ‘expanded methods’ in the supplemental digital content.
RESULTS
Of the 480 patients who enrolled in the trial and attended follow-up, 234 (49%) were randomized to the intervention arm (self-monitoring). Sufficient home monitoring blood pressure data were available from 220 patients (94%) (at least 4 days of readings within a week in the first month after randomization) who were subsequently included, as pre-specified for the primary analysis.
Demographic characteristics of those included in the analysis were similar to those in the original trial (Table 1). Mean age was 67 ± 9 years and just over half were female (53%). Most were of white ethnic origin (95%), baseline clinic blood pressure was raised (150/85 mmHg) and comorbidities were uncommon.
TABLE 1.
Characteristics of those enrolled in the TASMINH2 trial and those who completed home monitoring (who were included in the present analysis)
| Characteristic | TASMINH2 trial population | Home-monitoring population |
| Number of patients | 480 | 220 |
| Age (years) | 67 ± 9 | 67 ± 9 |
| Sex (% female) | 260 (53%) | 117 (53%) |
| Mean ± SD of baseline SBP (mmHg) | 151 ± 17 | 150 ± 12 |
| Mean ± SD of baseline DBP (mmHg) | 85 ± 15 | 85 ± 8 |
| Mean ± SD of readings 2–6 baseline SBP (mmHg) | 148 ± 7 | 148 ± 7 |
| Mean ± SD of readings 2–6 baseline DBP (mmHg) | 84 ± 3 | 84 ± 3 |
| Ethnicity | ||
| White British | 461 (96.0%) | 209 (95.0%) |
| Black African-Caribbean | 7 (1.5%) | 5 (2.2%) |
| South Asian | 10 (2.1%) | 4 (1.8%) |
| Mixed race | 1 (0.2%) | 1 (0.5%) |
| Other/unknown | 1 (0.2%) | 1 (0.5%) |
| Cardiovascular disease comorbidity | ||
| Angina | 30 (6.3%) | 14 (6.4%) |
| Myocardial infarction | 14 (2.9%) | 7 (3.2%) |
| Coronary artery bypass graft | 22 (4.7%) | 11 (5.0%) |
| Stroke | 21 (4.6%) | 12 (5.5%) |
| Peripheral vascular disease | 11 (2.3%) | 4 (1.8%) |
| Heart failure | 2 (0.4%) | 2 (0.9%) |
| Chronic kidney disease | 39 (8.1%) | 13 (5.9%) |
| Diabetes | 35 (7.3%) | 18 (8.2%) |
TASMINH2, Telemonitoring and Self-management in the Control of Hypertension 2.
The overall mean difference in SBP between the first clinic reading taken and the mean home blood pressure in the first month was −14 ± 17 mmHg (home–clinic difference). The clinic blood pressure drop, slope and quadratic coefficients were all significantly associated with the home–clinic difference (Fig. 1). In the sensitivity analyses, this effect remained significant regardless of the population studied, the estimate of home–clinic difference used or the time point in the trial at which variables were compared (supplemental digital content, Table S1).
FIGURE 1.
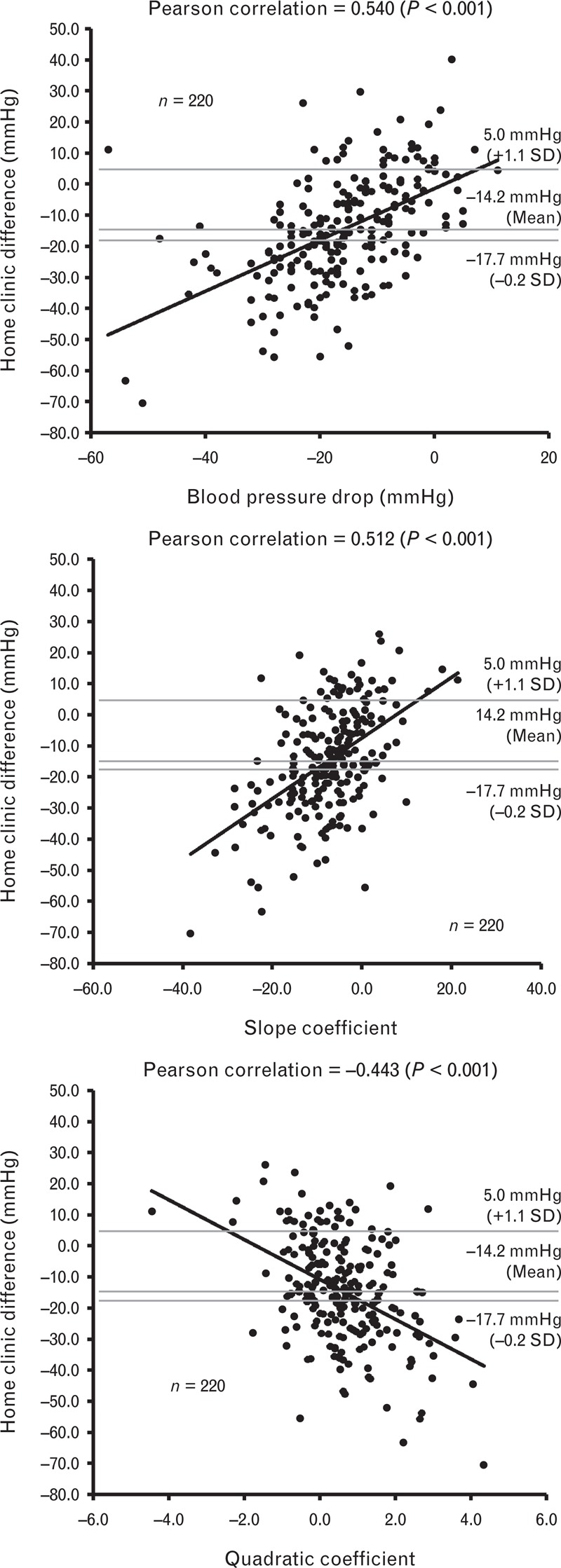
Scatter plots showing the correlation between home–clinic SBP difference and the blood pressure drop, slope and quadratic coefficients of SBP measurements in the clinic.
The mean blood pressure, home–clinic difference, clinic blood pressure drop, slope and quadratic coefficients for the ‘normal’, white-coat and masked effect groups are detailed in the supplemental digital content (Table S2). As expected, initial clinic blood pressure readings were higher in the white-coat effect group and overall clinic blood pressure decreased across the entire six readings (Fig. 2). However, this decrease was more pronounced in the white-coat effect group, and blunted in the masked effect group. The drop, slope and quadratic coefficients of clinic SBP were predictive of white-coat and masked effects with similar degrees of accuracy (Table 2). A drop in clinic blood pressure of more than 11 mmHg was predictive of the white-coat effect with a sensitivity of 90% (95% CI 82–95%), specificity of 50% (95% CI 41–59%), PPV of 56% (95% CI 48–65%) and NPV of 88% (95% CI 78–94%) [area under the curve (AUC) of 0.78, 95% CI 0.72–0.84]. Both the slope and quadratic coefficients were less predictive, but when combined, the predictive accuracy of these coefficients was comparable to that of the blood pressure drop (Table 2). Comparable predictive accuracy was identified for all predictors using a range of different definitions for white-coat and masked effects in the sensitivity analyses (Table 2).
FIGURE 2.
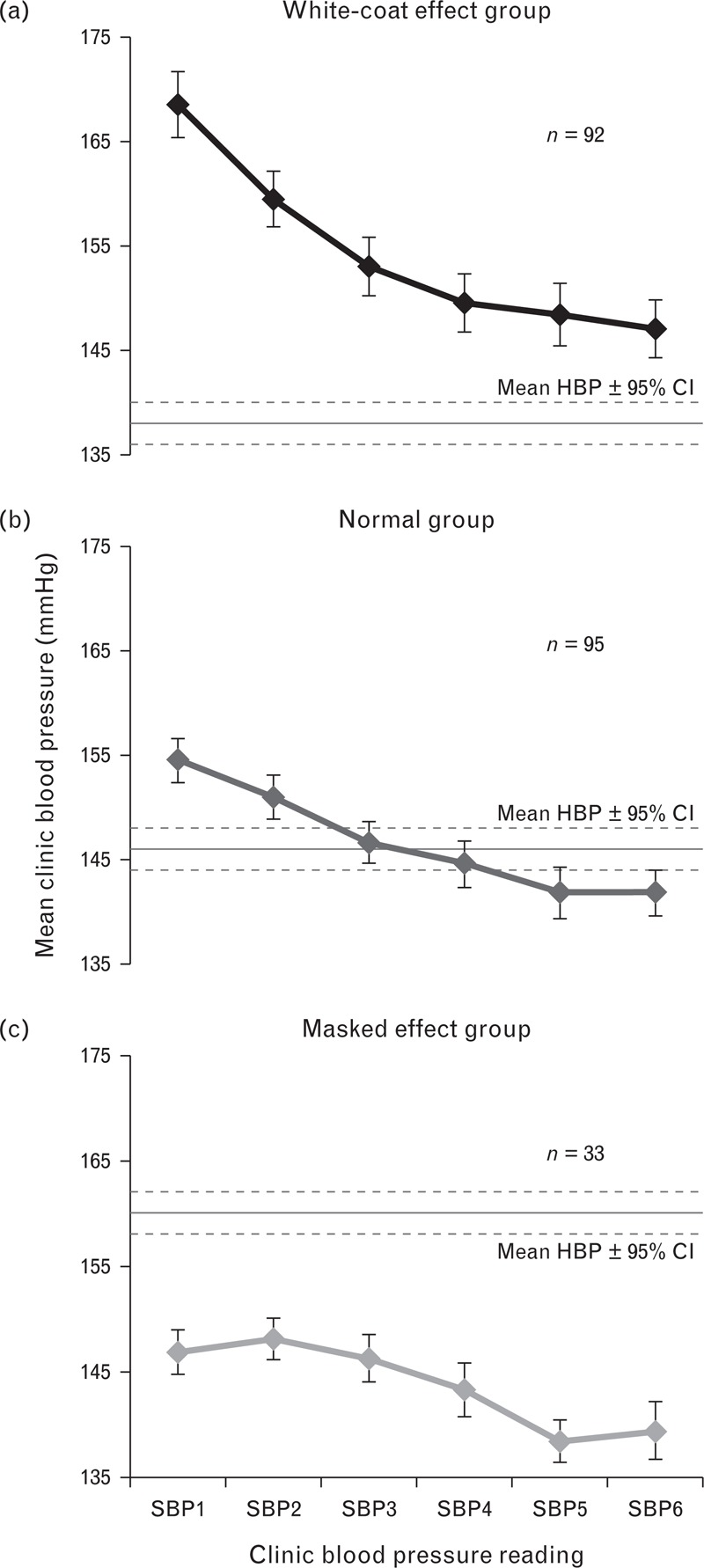
Mean (± 95% confidence intervals) SBP for each clinic reading in white-coat, normal and masked effect groups. SBP1-6, clinic SBP readings 1–6. HBP, mean home blood pressure ± 95% confidence intervals; this differed between each group (white-coat group: 138 mmHg, 95% CI 137, 140 mmHg; normal group: 146 mmHg, 95% CI 145, 147 mmHg; masked group: 160 mmHg, 95% CI 158, 161 mmHg) because each was defined on the basis of the home–clinic blood pressure difference.
TABLE 2.
Sensitivity, specificity, positive and negative predictor values of the blood pressure drop, slope and quadratic coefficients for ‘white-coat’ and ‘masked’ effects
| Condition | Predictor | Predictor thresholda | AUC (95% CI) | Sensitivity (95% CI) | Sensitivity rangeb | Specificity (95% CI) | Specificity (range)b | PPV (95% CI) | PPV (range)b | NPV (95% CI) | NPV (range)b |
| White-coat effectc | BP drop | More than −11 mmHg | 0.78 (0.72–0.84) | 90% (82–95%) | 90–93% | 50% (41–59%) | 42–53% | 56% (48–65%) | 22–62% | 88% (78–94%) | 86–96% |
| Slope | Below –0.60 | 0.74 (0.67–0.80) | 92% (85–97%) | 91–93% | 29% (21–38%) | 28–40% | 48% (41–56%) | 27–52% | 84% (70–93%) | 80–95% | |
| Quadratic | Above –0.27 | 0.69 (0.62–0.77) | 90% (82–95%) | 90–91% | 27% (20–36%) | 22–37% | 47% (40–55%) | 26–45% | 80% (65–90%) | 82–94% | |
| Slope + quadratic | Above –0.96 | 0.78 (0.72–0.84) | 90% (82–95%) | 90–93% | 49% (40–57%) | 39–50% | 56% (47–64%) | 21–60% | 87% (77–94%) | 86–96% | |
| Masked effectd | BP drop | Less than –19 mmHg | 0.77 (0.68–0.86) | 91% (76–98%) | 90–100% | 40% (33–47%) | 22–53% | 21% (15–29%) | 3–27% | 96% (89–99%) | 93–100% |
| Slope | Above –9.22 | 0.76 (0.67–0.85) | 94% (80–99%) | 90–100% | 42% (35–49%) | 20–42% | 22% (16–30%) | 7–25% | 98% (91–100%) | 98–100% | |
| Quadratic | Below 0.95 | 0.72 (0.62–0.82) | 91% (76–98%) | 90–100% | 36% (29–44%) | 13–39% | 20% (14–28%) | 7–23% | 96% (88–99%) | 96–99% | |
| Slope + quadratic | Above –2.31 | 0.79 (0.71–0.87) | 91% (76–98%) | 83–92% | 48% (41–56%) | 41–73% | 24% (17–32%) | 6–31% | 97% (91–99%) | 97–99% |
AUC, area under the curve; BP, blood pressure; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
aPredictor threshold refers to the thresholds for white-coat/masked effect on the predictor (BP drop, slope and/or quadratic) scale.
bThe range of values from sensitivity analysis (using alternative boundaries for white-coat, normal and masked effects).
cThe white-coat effect group was defined as any patient with a home–clinic difference of less than −0.2 SDs (−17.7 mmHg) below the mean.
dThe masked effect group was defined as any patient with a home–clinic difference of greater than +1.1 SDs (5.0 mmHg) above the mean.
The clinic blood pressure change (‘drop’) was also predictive of the masked effect (a decrease in clinic blood pressure of less than 19 mmHg was predictive of a masked effect with a sensitivity of 91% (95% CI 76–98%), specificity of 40% (95% CI 33–47%), PPV of 21% (95% CI 15–29%) and NPV of 96% (95% CI 89–99%); AUC 0.77 (95% CI 0.68–0.86). Studied as individual coefficients, both the slope and quadratic coefficients had comparable predictive values to that of the clinic blood pressure drop, but combined, the specificity and PPVs of these coefficients were improved (Table 2). These values were again robust to the results in the sensitivity analyses.
DISCUSSION
Main findings
The study has found a significant relationship between the characteristics of the change in repeated blood pressure measurements during a single clinic visit and the difference between clinic and repeated home blood pressure measurement. It was possible to rule out a white-coat or masked effect in a group of treated patients, thus allowing appropriate targeting of treatment intensification on the basis of clinic measurements in some patients, and the potential need for out-of-office measurement in others. Such estimations were possible using a simple calculation (blood pressure drop between the first and sixth readings in a single clinic visit) which could easily be incorporated into routine clinical practice (see, for example, Box 1). The more complex analyses incorporating the slope and shape of the blood pressure curves had no additional benefit for predicting the white-coat effect, but when combined, resulted in a more specific test for the masked effect. Was this to be considered a clinically useful improvement, the methodology needed to estimate these variables in clinical practice could be developed into an algorithm and made available online (see, for example, Box 2) or incorporated into automated blood pressure monitors in a similar way to that used in atrial fibrillation detection [24].
Box 1.

no caption available.
Box 2.

no caption available.
Strengths and limitations
This was a post-hoc analysis of data from the TASMINH2 trial [17] and so the results need to be interpreted with caution. Patients were drawn from the primary care, but all had previously poorly controlled blood pressure and had agreed to participate in a trial which required them to measure their own blood pressure which might limit generalizability. Despite this, data were robustly collected using validated equipment and trained staff, hence it is unlikely that the relationships found here are due to measurement errors. Blood pressure was measured in the clinic using a standardized protocol, which, in contrast to previous studies [12–15], included a researcher being present throughout the six blood pressure readings whilst the patient sat silently. This contrast in procedures had no obvious impact on the blood pressure measurements taken: the mean drop in blood pressure in the present study (13/4 mmHg) was comparable with that seen in previous work (15/6 mmHg) [14,15].
White-coat and masked hypertension are defined on the basis of the effect of out-of-office pressure differences on the diagnostic threshold [6,25]. Rather than studying the predictive abilities of clinic blood pressure characteristics for these arbitrarily defined conditions, patients were assessed for a white-coat or masked effect. This both improved the power to detect an effect and was necessary because the cohort were all receiving treatment at baseline and did not contain an adequate range of patients with blood pressure above and below the clinic threshold for hypertension (140/90 mmHg) [26]. It is therefore possible that patients displaying a masked effect may have been under-represented. Both the white-coat and masked effects are important issues in the management of hypertension, even in patients with a diagnosis of hypertension who are already receiving treatment. Patients with a significant white-coat effect may appear to be uncontrolled, thus receiving additional unnecessary medication if treatment decisions are based on clinic blood pressure measurements alone. Those with a significant masked effect may not have treatment optimized, despite having underlying uncontrolled blood pressure, due to clinic blood pressure appearing to be normal or close to the hypertension threshold.
There is no widely accepted definition of white-coat or masked effects. Patients were therefore defined as displaying normal blood pressure characteristics, white-coat or masked effects objectively using statistically rigorous yet necessarily arbitrary thresholds. The sensitivity analyses conducted here demonstrate that altering these thresholds did not materially affect the predictive abilities of clinic blood pressure characteristics. Approximately, 42% of patients were observed to display a white-coat effect and 15% a masked effect. These proportions are similar to the previously reported estimations of the prevalence of white-coat (36–47%) [27,28] and masked hypertension (9–21%) [16,28–30] in treated patients with the caveat that such proportions vary depending on the population studied, how blood pressure is measured and how each condition is defined [31,32].
The study used data from the TASMINH2 trial [17] and hence home blood pressures were used to define out-of-office blood pressure. Arguably, this is less robust than ambulatory blood pressure monitoring, which is considered the ‘gold standard’ measure of out-of-office blood pressure [5], largely due to a greater evidence base linking it with end-organ damage and cardiovascular outcomes [33–38]. Despite this, home monitoring is accepted as a reliable alternative when diagnosing white-coat and masked hypertension [39], although it is possible that masked effects due to nocturnal hypertension could have been missed in the present study. Future research should therefore test the hypotheses proposed here using both home and ambulatory blood pressure monitoring.
Relationship to other literature
Other groups have compared multiple clinic blood pressure measurements with out-of-clinic blood pressure monitoring, but the current study is novel in its consideration of the drop, the slope and the shape of the change in blood pressure with repeated measurement. Previous work has shown that clinic blood pressure measurements taken with the BpTRU device are comparable with ambulatory blood pressure measurements in treated hypertensive patients [12,13,15]. In one study, Godwin et al.[13] showed that, in a treated and poorly controlled population, the mean of multiple clinic blood pressures predicts true controlled SBP (on the basis of ambulatory blood pressure monitoring) with a PPV of 80–87% and NPV of 49–59% (depending on the threshold used). However, this population had better blood pressure control than in the present study with many more results above and below the threshold for hypertension and so is not directly comparable. Further work should compare the two approaches in an untreated population being considered for diagnosis of hypertension and assess whether they are complementary.
Implications for clinical practice and future research
The study is the first to consider how more detailed phenotyping of clinic blood pressure (measured using a simple method routinely available in clinical practice) might be used to reduce the need for additional more complicated blood pressure investigations such as ambulatory blood pressure monitoring. The ‘clinic blood pressure drop’ or the more complex algorithm based on slope and curve of multiple readings could be incorporated into a triaging tool for more targeted use of out-of-office blood pressure monitoring (see, for example, Boxes 1 and Boxes 2). This could be utilized (as it has been here) in treated hypertensive patients to optimize management. Future research should consider whether the same algorithm could be applied to an untreated or controlled group in order to assess the need for ambulatory blood pressure monitoring. The current diagnostic algorithm recommended in the UK guidelines [26] suggests that all patients with raised clinic blood pressure should be referred for out-of-office monitoring, whilst the European Society of Hypertension recommends out-of-office monitoring when white-coat or masked hypertension is ‘suspected’, although it is not clear how one would suspect them [40]. Whilst the UK guidelines should improve the targeting of treatment to those without a white-coat effect, there is currently no reliable method to identify the masked effect [3,15]. Multiple clinic readings might fill this gap, although with the caveat that they may not identify all features from ambulatory monitoring such as nocturnal hypertension.
More work is needed to establish the appropriate thresholds for white-coat and masked hypertension using this new method in untreated patients with raised clinic pressure, those with normotension and those with apparently controlled clinic blood pressure. Further research could include evaluating the predictive abilities of multiple clinic readings for white-coat and masked effects when less than six readings are taken; whether the methodology requires a specific device (the BpTRU); and whether referring patients for out-of-office blood pressure monitoring on the basis of clinic blood pressure characteristics results in more or less unnecessary referrals than those approaches detailed in the new UK guidelines [26] and algorithms [41].
In conclusion, these data suggest, for the first time, that the characteristics of SBP measured repeatedly in a single clinic visit are predictive of clinical differences in blood pressure between repeated home and clinic measurements.
ACKNOWLEDGEMENTS
The authors would like to thank the patients and practices that took part in the original TASMINH2 study without whom this work would not have been possible.
Contributors: J.S., R.H., F.D.R.H. and R.J.Mc.M. had the original idea. J.S. undertook the analyses and wrote the first draft with R.J.Mc.M. and R.H. All authors subsequently refined the manuscript and approved the final version. R.J.Mc.M. is the guarantor.
Sources of funding: This study presents independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding scheme (RP-PG-1209–10051). R.J.Mc.M. holds an NIHR Professorship. J.S. was funded by the NIHR Birmingham and Black Country Collaboration for Leadership in Applied Health Research and Care during part of this work, but now holds a Medical Research Council Strategic Skills Postdoctoral Fellowship. B.W. is a NIHR Senior Investigator and is supported by the NIHR UCL Hospitals Biomedical Research Centre. The TASMINH2 trial was funded by the UK Department of Health Policy Research Programme and the National Coordinating Centre for Research Capacity Development. The views and opinions expressed are those of the authors and do not necessarily reflect those of the NHS, NIHR, or the Department of Health. All equipment used in the study was purchased commercially.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Supplementary Material
Reviewer's Summary Evaluation
Reviewer 1
Analysis of multiple clinic pressures device is compared with home blood pressure monitoring to arrive at predictors for deciding whether home monitoring will be helpful. Participants were part of the TASMINH2 trial and already on treatment. The question is whether six clinic pressures can accurately predict either the White Coat Effect or Masked Hypertension, so that home monitoring can be restricted to a targeted population. Clearly, additional studies of this issue are needed, but this report has important implications for management (i.e. home monitoring, yes or no) and especially for environments where home monitoring is impractical, yet the estimating White Coat effect or Masked Hypertension effect is needed.
Footnotes
Abbreviations: AUC, area under the curve; BP, blood pressure; HBP, home blood pressure; NPV, negative predictive value; PPV, positive predictive value; TASMINH2, Telemonitoring and Self-management in the Control of Hypertension 2
REFERENCES
- 1.Prospective Studies Collaboration. Age-specific relevance of usual BP to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360:1347–1360. [DOI] [PubMed] [Google Scholar]
- 3.Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD, et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet 2011; 378:1219–1230. [DOI] [PubMed] [Google Scholar]
- 4.Jin Y, Bies R, Gastonguay MR, Stockbridge N, Gobburu J, Madabushi R. Misclassification and discordance of measured blood pressure from patient's true blood pressure in current clinical practice: a clinical trial simulation case study. J Pharmacokinet Pharmacodyn 2012; 39:283–294. [DOI] [PubMed] [Google Scholar]
- 5.Hodgkinson J, Mant J, Martin U, Guo B, Hobbs FD, Deeks JJ, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ 2011; 342:d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickering TG, Coats A, Mallion JM, Mancia G, Verdecchia P. Blood Pressure Monitoring. Task force V: white-coat hypertension. Blood Press Monit 1999; 4:333–341. [DOI] [PubMed] [Google Scholar]
- 7.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension 2002; 40:795–796. [DOI] [PubMed] [Google Scholar]
- 8.Liu JE, Roman MJ, Pini R, Schwartz JE, Pickering TG, Devereux RB. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med 1999; 131:564–572. [DOI] [PubMed] [Google Scholar]
- 9.Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation 2001; 104:1385–1392. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension 2006; 47:846–853. [DOI] [PubMed] [Google Scholar]
- 11.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, et al. Prognosis of ‘masked’ hypertension and ‘white-coat’ hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol 2005; 46:508–515. [DOI] [PubMed] [Google Scholar]
- 12.Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 h ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord 2005; 5:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godwin M, Birtwhistle R, Delva D, Lam M, Casson I, MacDonald S, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract 2011; 28:110–117. [DOI] [PubMed] [Google Scholar]
- 14.Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62. [DOI] [PubMed] [Google Scholar]
- 15.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Grant FC, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. Br Med J 2011; 342:d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. The conventional versus automated measurement of blood pressure in the office (CAMBO) trial: masked hypertension substudy. J Hypertens 2012; 30:1937–1941. [DOI] [PubMed] [Google Scholar]
- 17.McManus R, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet 2010; 376:163–172. [DOI] [PubMed] [Google Scholar]
- 18.Mattu GS, Heran BS, Wright JM. Overall accuracy of the BpTRU: an automated electronic blood pressure device. Blood Press Monit 2004; 9:47–52. [DOI] [PubMed] [Google Scholar]
- 19.Mattu GS, Perry TL, Wright JM. Comparison of the oscillometric blood pressure monitor (BPM-100beta) with the auscultatory mercury sphygmomanometer. Blood Press Monit 2001; 6:153–159. [DOI] [PubMed] [Google Scholar]
- 20.Wright JM, Mattu GS, Perry TL, Gelfer ME, Strange KD, Zorn A, et al. Validation of a new algorithm for the BPM-100 electronic oscillometric office blood pressure monitor. Blood Press Monit 2001; 6:161–165. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. Am J Cardiol 1971; 27:335–346. [DOI] [PubMed] [Google Scholar]
- 22.Rutan GH, Kuller LH, Neaton JD, Wentworth DN, McDonald RH, Smith WM. Mortality associated with diastolic hypertension and isolated systolic hypertension among men screened for the Multiple Risk Factor Intervention Trial. Circulation 1988; 77:504–514. [DOI] [PubMed] [Google Scholar]
- 23.Stergiou GS, Parati G. The optimal schedule for self-monitoring of blood pressure by patients at home. J Hypertens 2007; 25:1992–1997. [DOI] [PubMed] [Google Scholar]
- 24.Wiesel J, Fitzig L, Herschman Y, Messineo FC. Detection of atrial fibrillation using a modified microlife blood pressure monitor. Am J Hypertens 2009; 22:848–852. [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Eguchi K, Kario K. Masked hypertension: a review. Hypertens Res 2007; 30:479–488. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Clinical Excellence. Hypertension: clinical management of primary hypertension in adults. Nice clinical guideline 127. http://www.nice.org.uk/CG127 2011. [Google Scholar]
- 27.MacDonald MB, Laing GP, Wilson MP, Wilson TW. Prevalence and predictors of white-coat response in patients with treated hypertension. CMAJ 1999; 161:265–269. [PMC free article] [PubMed] [Google Scholar]
- 28.Park SJ, Park JB, Choi DJ, Youn HJ, Park CG, Ahn YK, et al. Detection of masked hypertension and the ‘mask effect’ in patients with well controlled office blood pressure. Circ J 2011; 75:357–365. [DOI] [PubMed] [Google Scholar]
- 29.Bobrie G, Chatellier G, Genes N, Clerson P, Vaur L, Vaisse B, et al. Cardiovascular prognosis of ‘masked hypertension’ detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA 2004; 291:1342–1349. [DOI] [PubMed] [Google Scholar]
- 30.Obara T, Ohkubo T, Kikuya M, Asayama K, Metoki H, Inoue R, et al. Prevalence of masked uncontrolled and treated white-coat hypertension defined according to the average of morning and evening home blood pressure value: from the Japan Home versus Office Measurement Evaluation Study. Blood Press Monit 2005; 10:311–316. [DOI] [PubMed] [Google Scholar]
- 31.Verdecchia P, Schillaci G, Boldrini F, Zampi I, Porcellati C. Variability between current definitions of ‘normal’ ambulatory blood pressure. Implications in the assessment of white coat hypertension. Hypertension 1992; 20:555–562. [DOI] [PubMed] [Google Scholar]
- 32.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med 2011; 154:781–788. [DOI] [PubMed] [Google Scholar]
- 33.Fagard RH, Staessen JA, Thijs L. Prediction of cardiac structure and function by repeated clinic and ambulatory blood pressure. Hypertension 1997; 29:22–29. [DOI] [PubMed] [Google Scholar]
- 34.Imai Y, Ohkubo T, Sakuma M, Tsuji I, Satoh H, Nagai K. Predictive power of screening blood pressure, ambulatory blood pressure and blood pressure measured at home for overall and cardiovascular mortality: a prospective observation in a cohort from Ohasama, northern Japan. Blood Press Monit 1996; 1:251–254. [PubMed] [Google Scholar]
- 35.Mancia G, Zanchetti A, Gabiti-Rosei E, Benemio G, De GR, Forgari R. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation 1997; 95:1464–1470. [DOI] [PubMed] [Google Scholar]
- 36.Ohkubo T, Hozawa A, Nagai K, Kikuya M, Tsuji I, Ito S. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama study. J Hypertens 2000; 18:847–854. [DOI] [PubMed] [Google Scholar]
- 37.Staessen JA, Thijs L, Fagard R, O’Brien E, Clement D, de Leeuw P. Predicting cardiovascular risk using conventional vs. ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999; 282:539–546. [DOI] [PubMed] [Google Scholar]
- 38.Verdecchia P, O’Brien E, Pickering T, Staessen JA, Parati G, Myers M, et al. When can the practicing physician suspect white coat hypertension? Statement from the Working Group on Blood Pressure Monitoring of the European Society of Hypertension. Am J Hypertens 2003; 16:87–91. [DOI] [PubMed] [Google Scholar]
- 39.Nasothimiou EG, Tzamouranis D, Rarra V, Roussias LG, Stergiou GS, Nasothimiou EG, et al. Diagnostic accuracy of home vs. ambulatory blood pressure monitoring in untreated and treated hypertension. Hypertens Res 2012; 35:750–755. [DOI] [PubMed] [Google Scholar]
- 40.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 41.Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens 2010; 28:703–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


