Abstract
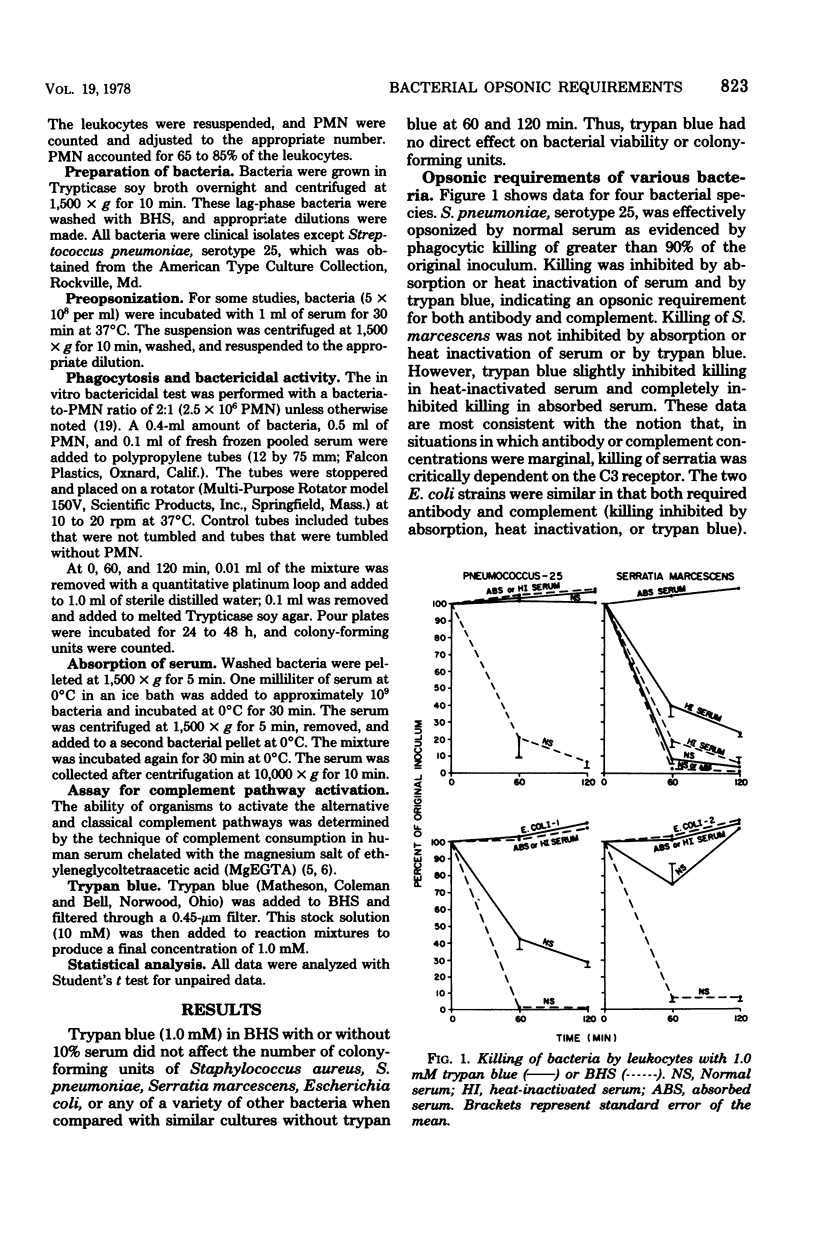
We studied human serum opsonins by using combinations of heat inactivation and chelation to inhibit complement, adsorption to remove antibody, and trypan blue to inactivate the C3 receptor of human polymorphonuclear leukocytes. Streptococcus pneumoniae, serotype 25, required both complement and immunoglobulin for opsonization, even though that strain activated the alternative complement pathway. Both strains of Escherichia coli required antibody and complement, but varied in the degree of dependence on the C3 opsonin, since trypan blue moderately inhibited the killing of E. coli-1 and markedly inhibited the killing of E. coli-2. Serratia marcescens was opsonized in heat-inactivated serum (limited complement) or serum absorbed at 0°C with S. marcescens (limited antibody), but depended on the C3 receptor in absorbed serum. S. marcescens activated the alternative pathway. Thus, opsonic requirements varied with the availability of opsonins. Requirements for bacterial opsonization vary with species and strains within species, perhaps reflecting quantitative relationships among alternative and classical pathway activation of C3, efficiency of adsorption of C3 or immunoglobulin G to bacterial surfaces, and efficiency of attachment of these ligands to polymorphonuclear leukocyte receptors. Furthermore, although not always sufficient for opsonization, the C3 opsonin (activated through either the classical or alternative pathway) appears necessary for effective phagocytosis and killing of all strains studied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R. Oxidative bactericidal mechanisms of polymorphonuclear leukocytes. J Infect Dis. 1975 Mar;131(3):295–303. doi: 10.1093/infdis/131.3.295. [DOI] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P. Comparison of ethyleneglycoltetraacetic acid and its magnesium salt as reagents for studying alternative complement pathway function. Infect Immun. 1977 Apr;16(1):124–128. doi: 10.1128/iai.16.1.124-128.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Fine D. P. Pneumococcal type-associated variability in alternate complement pathway activation. Infect Immun. 1975 Oct;12(4):772–778. doi: 10.1128/iai.12.4.772-778.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Effects of staphylococcal protein A on heat labile opsonins. J Immunol. 1974 Mar;112(3):1177–1180. [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974 Aug;10(2):402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Opsonic activity in human serum chelated with ethylene glycoltetra-acetic acid. Immunology. 1974 Jun;26(6):1251–1256. [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Bianco C., Silverstein S. C. Characterization of the macrophage receptro for complement and demonstration of its functional independence from the receptor for the Fc portion of immunoglobulin G. J Exp Med. 1975 Jun 1;141(6):1269–1277. doi: 10.1084/jem.141.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The adherence of leucocytes and platelets induced by fixed IgG antibody or complement. Immunology. 1969 Jan;16(1):107–121. [PMC free article] [PubMed] [Google Scholar]
- Humphreys D. W., Wheat L. J., White A. Staphylococcal heat-stable opsonins. J Lab Clin Med. 1974 Jul;84(1):122–128. [PubMed] [Google Scholar]
- Jasin H. E. Human heat labile opsonins: evidence for their mediation via the alternate pathway of complement activation. J Immunol. 1972 Jul;109(1):26–31. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani B. Different roles of IgG and complement receptors in phagocytosis by polymorphonuclear leukocytes. J Immunol. 1975 Jul;115(1):15–17. [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Simberkoff M. S., Ricupero I., Rahal J. J., Jr Host resistance to Serratia marcescens infection: serum bactericidal activity and phagocytosis by normal blood leukocytes. J Lab Clin Med. 1976 Feb;87(2):206–217. [PubMed] [Google Scholar]
- Wheat L. J., Humphreys D. W., White A. Opsonization of staphylococci by normal human sera: the role of antibody and heat-labile factors. J Lab Clin Med. 1974 Jan;83(1):73–78. [PubMed] [Google Scholar]



