Abstract
Endophthalmitis following pars plana vitrectomy is a very uncommon cause of endophthalmitis. Cases reported over the last decade show a decrease in incidence over time. To optimize visual outcome, early diagnosis and treatment are essential. In this review we report a summary of the incidence of endophthalmitis following vitrectomy, various risk factors for their occurrence, the microbiological profile and the visual outcomes post treatment.
Keywords: endophthalmitis, pars plana vitrectomy, visual outcome, prophylaxis, risk factors, sutureless vitrectomy
Introduction
Endophthalmitis is characterized by severe inflammation of the ocular tissues and fluids. Acute-onset postoperative infectious endophthalmitis is the most frequent category and may be associated with severe visual loss. The incidence of endophthalmitis varies with the surgical procedure performed. The most reported categories of endophthalmitis are following cataract surgery/intraocular lens implantation and following intravitreal injections. A review of the literature indicates the incidence in these scenarios to vary from 0.07% to 0.4% for cataract surgery and from 0.038% to 0.065% for intravitreal injections.1–5 Endophthalmitis following pars plana vitrectomy (PPV) is an uncommon cause of endophthalmitis (Figure 1). The reported rates of post-PPV endophthalmitis have been decreasing over the last decade. In this review, the incidence of post-PPV endophthalmitis, risk factors for occurrence, microbiological profile, and treatment outcomes in the sutureless PPV era are reported.
Figure 1.
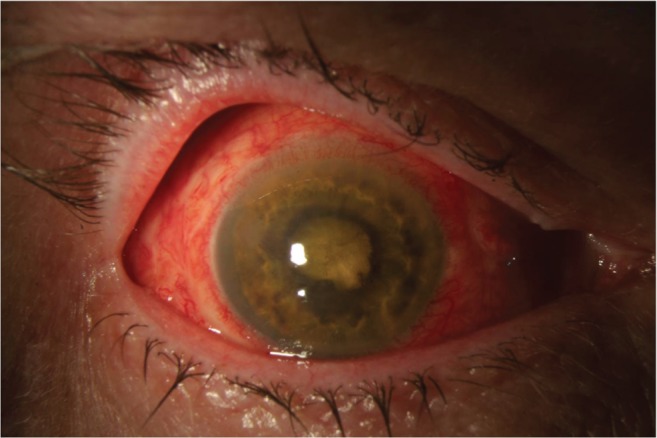
Anterior segment photograph of a patient with endophthalmitis following pars plana vitrectomy operated for rhegmatogenous retinal detachment. Cultures were positive for Bacillus cereus. Visual acuity was light perception after treatment.
Incidence
Endophthalmitis following PPV is relatively uncommon. Over the years, there have been various studies which reported incidences of endophthalmitis after PPV (Table 1).6–13 The incidence ranges between 0.03% and 0.14% for 20 G PPV. None of the cases had additional concurrent surgery.
Table 1.
Incidence of endophthalmitis following pars plana vitrectomy in 20 G surgery
| Reference | Year | Case occurrence | Incidence |
|---|---|---|---|
| Ho and Tolentino6 | 1984 | 4/2,817 | 0.14% |
| Cohen et al8 | 1995 | 18/12,216 | 0.15% |
| Aaberg et al7 | 1998 | 3/6,557 | 0.04% |
| Zhang et al9 | 2003 | 3/7,000 | 0.04% |
| Eifrig et al10 | 2004 | 6/15,326 | 0.03% |
| Sakamoto et al45 | 2004 | 1/1,886 | 0.05% |
| Joondeph et al11 | 2005 | 5/10,397 | 0.04% |
| Mollan et al12 | 2009 | 2/5,278 | 0.03% |
| Chen et al17 | 2009 | 1/3,046 | 0.03% |
| Scott et al21 | 2011 | 1/4,403 | 0.02% |
Incidence in transconjunctival sutureless vitrectomy
The first reported case of endophthalmitis in transconjunctival sutureless vitrectomy (TSV) surgery was in 2005 following a 25 G PPV (Table 2).14 The vitreous sample was negative on Gram stain and culture. After intravitreal and oral antibiotics, vision increased from hand motion close to face to 6/6 at the 3-week follow-up visit.
Table 2.
Incidence of endophthalmitis in transconjunctival sutureless vitrectomy
| Reference | Year | Case occurrence | Incidence |
|---|---|---|---|
| Shaikh et al16 | 2007 | 2/129 | 1.55% |
| Kunimoto and Kaiser15 | 2007 | 1/443 | 0.22% |
| Scott et al18 | 2008 | 1/119 | 0.84% |
| Shimada et al19 | 2008 | 1/3,343 | 0.03% |
| Chen et al17 | 2009 | 1/431 | 0.23% |
| Hu et al20 | 2009 | 1/1,424 | 0.07% |
| Scott et al21 | 2011 | 2/4,151 | 0.04% |
| Mutoh et al46 | 2012 | 4/502 | 0.79% |
Older studies comparing 25 G with 20 G PPV generally reported higher rates in the 25 G group. Kunimoto and Kaiser compared endophthalmitis rates after 20 G and 25 G PPV in 2007 and reported 0.23% in 25 G cases as compared with 0.018% in 20 G cases, which was statistically significant.15 Both the groups had final median visual acuity of counting fingers. Shaikh et al reviewed 129 consecutive cases of 20 G and 129 consecutive cases of 25G PPV in 2007 and reported two cases (1.55%) of endophthalmitis in the 25 G group and none in the 20 G group.16 Chen et al in 2009 reported a nonsignificantly higher rate of endophthalmitis in 25 G (0.23%) compared with 20 G (0.03%) PPV.17 Scott et al in a multicenter study 2008 reported a significantly higher rate of endophthalmitis in 25G TSV (0.84%) versus 20 G PPV (0.03%).18 Of these, most cases (9/11) in the TSV group that developed endophthalmitis had straight incisions which may have been a possible risk factor.
However, other studies, and especially newer studies, have reported no significant difference in endophthalmitis rates between the two groups, perhaps reflecting advances in wound construction and improvements in surgical technique.19–21 There are also reports which show very rare occurrence of endophthalmitis post TSV.22,23
In a 2-year, prospective, nationwide surveillance study from the UK, the rate of endophthalmitis after PPV was 28 per 48,433 (0.058%). There were no statistically significant differences in the rates between small gauge TSV and 20 G PPV.24 Culture positivity was reported in 60.7% of cases. Outcomes were generally poor, with 29.6% of eyes having final visual outcomes of light perception, no light perception, or evisceration.25
Predisposing factors
There are certain factors that may increase the incidence of post-PPV endophthalmitis (Table 3). These include inadequate wound closure, postoperative hypotony, vitreous incarceration at a sclerotomy site, aqueous intraocular tamponade, additional concomitant intraocular procedures, and surgeon learning curve.
Table 3.
Risk factors associated with endophthalmitis after pars plana vitrectomy
| Surgery-related risk factors | Patient-related risk factors |
|---|---|
| Inadequate wound closure | Diabetes mellitus or other causes of immune compromise |
| Hypotony | Trauma or excessive eye rubbing |
| Vitreous incarceration | Noncompliance with treatment |
| Endotamponade agent (air, gas, or silicone oil) | |
| Subconjunctival and intravitreal injections | |
| Surgeon learning curve (non-beveled sclerotomies) |
Inadequate wound closure and hypotony
In the first reported case of endophthalmitis following TSV, the authors speculated lack of adequate wound closure and wound leak to be possible contributory factors.14 The hypotony from a sclerotomy leak may allow ingress of microorganisms into the vitreous cavity from the ocular surface.26–28 An optical coherence tomography study reported the benefits of a sutured sclerotomy over an unsutured one with respect to wound closure.29 Other studies have reported ingress of India ink across unsutured sclerotomies while no ink was shown to enter the globe in cases of sutured sclerotomies.30–34
Sclerotomy site vitreous incarceration (vitreous wick)
Another potential risk factor for endophthalmitis may be vitreous incarceration at a sclerotomy site. Endoscopic evaluation of autopsied vitrectomized eyes have reported vitreous incarceration at the port sites.34,35 In an unsutured wound, this can allow communication of ocular surface bacteria to the inside of the globe by a “vitreous wick” phenomenon wherein microorganisms can migrate from the exterior into the vitreous cavity along a strand of vitreous prolapsing out of the unsutured sclerotomy wound.36
Type of intraocular tamponade
In earlier reports,15,18 no intraocular gas or oil tamponade following PPV was used. It was hypothesized that balanced salt solution is likely to provide poorer wound integrity at the sclerotomy site compared with tamponade agents due to different surface tension properties. Higher surface tension properties of air, gas, and silicone oil may prevent fluid from egressing across the sclerotomy.37 It is also suspected that air, gas, and silicone oil do not support bacterial growth as well as balanced salt solution.
Contamination of the vitreous cavity
Vitreous contamination rates by microorganisms are reported to be significantly higher during TSV than during 20 G surgery (22% versus 2.4%).38,39 Although the penetrating instruments in their study were not cultured, the most common organism isolated in their study was Propionibacterium acnes which is consistent with residual ocular surface flora. The lower incidences of bacterial contamination during 20 G surgery may be associated with less risk of surgical instruments directly contacting the conjunctiva.
Associated pharmacotherapy
Subconjunctival antibiotics are sometimes used at the conclusion of intraocular procedures. With emergence of TSV, many centers have done away with this practice. In addition, some surgeons use intravitreal triamcinolone, dexamethasone, or bevacizumab injections at the end of surgery. These additional injections, along with the practice of eliminating subconjunctival antibiotics, could potentially increase the risk of endophthalmitis.18
Surgeon learning curve
As in any other ophthalmic surgery, vitrectomy also has a learning curve. In one study, surgeons beginning to transition from 20 G to TSV surgeries were reported to face higher rates of hypotony and wound leakage, which are potential risk factors for endophthalmitis.27 A common challenge during the learning curve is a poorly constructed incision, which may lead to postoperative wound leakage.
Diabetes mellitus
There is a theoretical association between diabetes mellitus and an increased risk of endophthalmitis following PPV. As diabetics are relatively immunologically compromised, there is a potential for an increased rate of procedure-related infections. Diabetics are relatively more likely to have longer surgeries for proliferative diabetic retinopathy and associated complications such as traction retinal detachment. These surgeries may have multiple insertions and removal of instruments and may be combined with cataract surgery, increasing the risk of endophthalmitis. In most studies describing endophthalmitis, a substantial proportion of the patients were diabetic.6,8,10–12,15,17–19,40
Microbiological spectrum
Multiple studies have implicated a variety of microorganisms. The reported bacteria include coagulase-negative staphylococci, Pseudomonas species, Propionibacterium, enterococci, and Bacillus species. Most studies report that coagulase-negative staphylococci is the most common organism.40–44
Clinical features
Clinical features of endophthalmitis following PPV are similar to those of endophthalmitis following cataract surgery. Typically patients present acutely with a hypopyon and dense vitritis. Some cases, however, may demonstrate a delayed presentation, perhaps due to the lack of vitreous and consequent lack of severe posterior segment inflammation until later in the course of the disease. Possibly because of coexisting advanced vitreoretinal diseases, the visual outcomes after treatment of endophthalmitis following PPV are often poor.
Prophylaxis
As in any case of endophthalmitis, early detection and prompt treatment are important elements in having a successful clinical outcome. The following preoperative, intraoperative, and postoperative measures can be taken to theoretically reduce the incidence of post-PPV endophthalmitis.34
Preoperative preparation
Preoperative preparation of lids and lashes with povidone-iodine 10% solution is highly recommended as it has been shown to reduce ocular flora in these areas. In patients with reported iodine allergy, chlorhexidine gluconate can be considered for the eye lids.25 However, povidone-iodine 5% should be used directly on the ocular surface.
Usage of lid speculum and adhesive surgical drape should ensure that no eye lashes are exposed in the surgical field. If a few lashes stay exposed after draping, consider redraping or removing the exposed lashes. In patients with active ipsilateral eye lid infection (stye or severe blepharitis), elective vitreous surgery can be postponed until the inflammation is treated.
Intraoperative care
Some centers use antibiotics in the infusion fluid, but this is a controversial practice. At this time, there have been no reported studies comparing the incidence of post-PPV endophthalmitis in eyes with or without antibiotics in the infusion fluid.
During small-gauge TSV, it is recommended to displace the conjunctiva before making a trocar entry. After entry, the conjunctiva can be released to its original position. This ensures that the conjunctival entry and the scleral entry wound are not in the same line, which may reduce risks of bacterial entry into the vitreous cavity.
During small-gauge TSV, scleral incisions should be beveled and not perpendicular to the scleral surface. A beveled incision offers better wound closure, reducing risks of sclerotomy leak.
After small-gauge TSV cannula removal, it is important to check for wound leak or vitreous wicking. If the eye goes soft or there is egress of air bubbles, the sclerotomy should be sutured. One may consider suturing the conjunctiva and the sclerotomy separately in such cases.
Postoperative care
Complaints of pain and blurred vision should alert the surgeon to look for subtle signs of endophthalmitis. The Endophthalmitis Vitrectomy Study did not enroll patients with post-PPV endophthalmitis, so its results do not directly apply to these cases. Nevertheless, certain principles apply. Vitreous tap and inject is quick, inexpensive, and does not require access to an operating room or specialized vitrectomy equipment. Prior to antibiotic injection, a vitreous cavity sample should be obtained for culture and sensitivity. If the vitreous cavity is filled with gas or silicone oil, a sample from the anterior chamber can be taken. A negative microbiology report does not rule out endophthalmitis. In case of doubt, one can repeat the sampling and treat with a broad-spectrum intravitreal antibiotic combination.
Culture positivity
Various studies have demonstrated culture positivity rates in cases of post-PPV endophthalmitis (Table 4). Culture positivity is very varied across studies. On average, about 70% of all samples across studies have shown a positive microbiology culture report. In a large multicenter study, Cohen et al8 had 16 culture-positive cases out of 18 cases (89%) of endophthalmitis post vitrectomy. Abi-Ayad et al have shown the lowest incidence of 29% (4/14 cases).
Table 4.
Culture positivity rates in endophthalmitis after pars plana vitrectomy
| Reference | Year | Culture positivity rate | Culture-positive cases (n) | Predominant organism |
|---|---|---|---|---|
| Cohen et al8 | 1995 | 89% | 16/18 | CNS |
| Aaberg et al7 | 1998 | 100% | 3/3 | CNS |
| Eifrig et al10 | 2004 | 83% | 5/6 | Staphylococcus aureus |
| Joondeph et al11 | 2005 | 100% | 5/5 | CNS |
| Abi-Ayad et al40 | 2007 | 29% | 4/14 | CNS |
| Scott et al18 | 2008 | 100% | 1/1 | CNS |
| Shimada et al19 | 2008 | 100% | 2/2 | MRSA, Enterococcus faecalis |
| Chen et al17 | 2009 | 50% | 1/2 | Staphylococcus aureus |
| Mollan et al12 | 2009 | 0% | – | None |
| Scott et al21 | 2011 | 50% | 2/3 | CNS |
| Mutoh et al46 | 2012 | 0% | – | None |
Abbreviations: CNS, coagulase-negative staphylococci; MRSA, methicillin-resistant Staphylococcus aureus.
Visual outcomes
Visual outcomes after treatment of post-PPV endophthalmitis are varied in the literature. In general, the outcomes are worse than that after cataract surgery, perhaps due to the underlying retinal pathology and associated poor visual potential. Although good visual outcomes have been reported in a few patients, most large studies show poor post-treatment visual gain (Table 5).
Table 5.
Visual acuity outcomes in reported series of post-pars plana vitrectomy endophthalmitis after treatment
| Reference | Diagnosis at time of PPV | Year | Cases (n) | Visual outcome at last visit |
|---|---|---|---|---|
| Cohen et al8 | ERM, MH, PDR | 1995 | 18/12,216 | 3 EV, 6 NLP, 1 HM, 1 LP, 2 20/400, 1 20/50, 1 20/30, 1 20/25 and 2 20/20 |
| Aaberg et al7 | – | 1998 | 3/6,557 | All eyes NLP |
| Eifrig et al10 | PDR, macular pucker, recurrent RD | 2004 | 6/15,326 | 3 NLP, 1 LP, 1 2/200 and 1 20/200 |
| Joondeph et al11 | VH, MH, ERM, RD | 2005 | 5/10,397 | 2 NLP, 1 HM, 1 20/200 and 1 20/50 |
| Shaikh et al16 | ERM | 2007 | 2/129 | One 20/400, one 20/40 |
| Scott et al18 | ERM, PDR, CRVO, disc pit | 2008 | 13/7,682 | 1 LP, 1 HM, 1 20/400, 1 5/200, 2 20/150, 1 20/100, 2 20/20, 2 20/40, 2 20/30 |
| Shimada et al19 | ERM | 2008 | 2/6,935 | Both cases NLP |
| Chen et al17 | VH, TRD with VH | 2009 | 2/3,477 | One 20/200, one 20/125 |
| Mollan et al12 | MH, PDR | 2009 | 2/5,278 | One 1/60, one 6/12 |
| Scott et al21 | RD, MH, ERM | 2011 | 3/8,554 | One HM, one 20/100, one 20/40 |
| Mutoh et al46 | CME, ERM | 2012 | 4/502 | One 20/30, one 20/100, one 20/20, one 20/25 |
Abbreviations: PPV, pars plana vitrectomy; NLP, no light perception; LP, light perception; HM, hand motion vision; ERM, epiretinal membrane; RD, retinal detachment; VH, vitreous hemorrhage; CRVO, central retinal vein occlusion; PDR, proliferative diabetic retinopathy; CME, cystoid macular edema; MH, macular hole; TRD, traction retinal detachment; EV, eviscerated.
Conclusion
Endophthalmitis after PPV is a rare but potentially very serious event. The outcomes are often poor despite prompt and appropriate treatment. The risk was potentially higher in the initial years of TSV surgeries, which could be hypothesized to be due to leaking sclerotomies. However, most recent studies have reported very low rates.
Footnotes
Disclosure
SGS has been a consultant for Bausch & Lomb and Santen, and has received lecture fees from ThromboGenics. The other authors have no conflicts of interest to report in this work.
References
- 1.Ferro JF, de Pablos M, Logrono MJ, et al. Postoperative contamination after using vancomycin and gentamicin during phacoemulsification. Arch Ophthalmol. 1997;115:165–170. doi: 10.1001/archopht.1997.01100150167003. [DOI] [PubMed] [Google Scholar]
- 2.Aaberg TM, Jr, Flynn HW, Jr, Murray TG. Intraocular ceftazidime as an alternative to the aminoglycosides in the treatment of endophthalmitis. Arch Ophthalmol. 1994;12:18–19. doi: 10.1001/archopht.1994.01090130028003. [DOI] [PubMed] [Google Scholar]
- 3.Kattan HM, Flynn HW, Jr, Pflugfelder SC, et al. Nosocomial endophthalmitis survey: current incidence of infection after intraocular surgery. Ophthalmology. 1991;98:227–238. [PubMed] [Google Scholar]
- 4.Hughes DS, Hill RJ. Infectious endophthalmitis after cataract surgery. Br J Ophthalmol. 1994;78:227–232. doi: 10.1136/bjo.78.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Causative organisms and possible treatment strategies. Retina. 2011;31:654–661. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- 6.Ho PC, Tolentino FI. Bacterial endophthalmitis after closed vitrectomy. Arch Ophthalmol. 1984;102:207–210. doi: 10.1001/archopht.1984.01040030157016. [DOI] [PubMed] [Google Scholar]
- 7.Aaberg TM, Flynn HW, Jr, Schiffman J, Newton J. Nosocomial acute-onset postoperative endophthalmitis survey. A 10-yr review of incidences and outcomes. Ophthalmology. 1998;105:1004–1010. doi: 10.1016/S0161-6420(98)96000-6. [DOI] [PubMed] [Google Scholar]
- 8.Cohen SM, Flynn HW, Jr, Murray TG, Smiddy WE. Endophthalmitis after pars plana vitrectomy. The post vitrectomy endophthalmitis study group. Ophthalmology. 1995;102:702–712. doi: 10.1016/s0161-6420(95)30965-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Ding X, Hu J, Gao R. Clinical features of endophthalmitis after vitreoretinal surgery. Yan Ke Xue Bao. 2003;19:39–43. Chinese. [PubMed] [Google Scholar]
- 10.Eifrig CW, Scott IU, Flynn HW, Jr, Smiddy WE, Newton J. Endophthalmitis after pars plana vitrectomy: incidence, causative organisms, and visual acuity outcomes. Am J Ophthalmol. 2004;138:799–802. doi: 10.1016/j.ajo.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Joondeph BC, Blanc JP, Polkinghorne PJ. Endophthalmitis after pars plana vitrectomy: a New Zealand experience. Retina. 2005;25:587–589. doi: 10.1097/00006982-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Mollan SP, Mollan AJ, Konstantinos C, Durrani OM, Butler L. Incidence of endophthalmitis following vitreoretinal surgery. Int Ophthalmol. 2009;29:203–205. doi: 10.1007/s10792-008-9202-x. [DOI] [PubMed] [Google Scholar]
- 13.Wykoff CC, Parrott MB, Flynn HW, Jr, et al. Nosocomial acute-onset postoperative endophthalmitis at a university teaching hospital. 2010;150:392–398. doi: 10.1016/j.ajo.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Taylor SR, Aylward GW. Endophthalmitis following 25-gauge vitrectomy. Eye. 2005;19:1228–1229. doi: 10.1038/sj.eye.6701737. [DOI] [PubMed] [Google Scholar]
- 15.Kunimoto DY, Kaiser RS, Wills Retina Service Incidence of endophthalmitis after 20 and 25 gauge vitrectomy. Ophthalmology. 2007;114:2133–2137. doi: 10.1016/j.ophtha.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Shaikh S, Ho S, Richmond PP, Olson JC, Barnes CD. Untoward outcomes in 25-gauge versus 20-gauge vitreoretinal surgery. Retina. 2007;27:1048–1053. doi: 10.1097/IAE.0b013e3180592bb7. [DOI] [PubMed] [Google Scholar]
- 17.Chen JK, Khurana RN, Nguyen QD, Do DV. The incidence of endophthalmitis following transconjunctival sutureless 25 vs 20 gauge vitrectomy. Eye. 2009;23:780–784. doi: 10.1038/eye.2008.160. [DOI] [PubMed] [Google Scholar]
- 18.Scott IU, Flynn HW, Jr, Dev S, et al. Endophthalmitis after 25 gauge and 20 gauge pars plana vitrectomy: incidence and outcomes. Retina. 2008;28:138–142. doi: 10.1097/IAE.0b013e31815e9313. [DOI] [PubMed] [Google Scholar]
- 19.Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Incidence of endophthalmitis after 20 and 25 gauge vitrectomy: causes and prevention. Ophthalmology. 2008;115:2215–2220. doi: 10.1016/j.ophtha.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Hu AYH, Bourges JL, Shah SP, et al. Endophthalmitis after pars plana vitrectomy. Ophthalmology. 2009;116:1360–1365. doi: 10.1016/j.ophtha.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 21.Scott IU, Flynn HW, Jr, Acar N, et al. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2011;249:377–380. doi: 10.1007/s00417-010-1505-8. [DOI] [PubMed] [Google Scholar]
- 22.Parolini B, Romanelli F, Prigione G, Pertile G. Incidence of endophthalmitis in a large series of 23-gauge and 20-gauge transconjunctival pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009;247:895–898. doi: 10.1007/s00417-009-1063-0. [DOI] [PubMed] [Google Scholar]
- 23.Haas A, Seidel G, Steinbrugger I, et al. Twenty-three-gauge and 20-gauge vitrectomy in epiretinal membrane surgery. Retina. 2010;30:112–116. doi: 10.1097/IAE.0b013e3181b32ebf. [DOI] [PubMed] [Google Scholar]
- 24.Park JC, Ramasamy B, Shaw S, Prasad S, Ling RH. A prospective and nationwide study investigating endophthalmitis following pars plana vitrectomy: incidence and risk factors. Br J Ophthalmol. 2014;94:529–533. doi: 10.1136/bjophthalmol-2013-304485. [DOI] [PubMed] [Google Scholar]
- 25.Wykoff CC, Flynn HW, Jr, Han DP. Allergy to povidone-iodine and cephalosporins: the clinical dilemma in ophthalmic usage. Am J Ophthalmol. 2011;151:4–6. doi: 10.1016/j.ajo.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Aylward GW. Sutureless vitrectomy. Ophthalmologica. 2011;225:67–75. doi: 10.1159/000317910. [DOI] [PubMed] [Google Scholar]
- 27.Fujii GY, De Juan E, Humayun MS, et al. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology. 2002;109:1814–1820. doi: 10.1016/s0161-6420(02)01119-3. [DOI] [PubMed] [Google Scholar]
- 28.Acar N, Kapran Z, Unver Y, Altan T, Ozdogan S. Early postoperative hypotony after 25-gauge sutureless vitrectomy with straight incisions. Retina. 2008;28:545–552. doi: 10.1097/IAE.0b013e318162b008. [DOI] [PubMed] [Google Scholar]
- 29.Chen D, Lian Y, Cui L, Lu F, Ke Z, Song Z. Sutureless vitrectomy incision architecture in the immediate post-operative period evaluated in vivo using optical coherence tomography. Ophthalmology. 2010;117:2003–2009. doi: 10.1016/j.ophtha.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 30.Gupta OP, Maguire JI, Eagle RC, Jr, et al. The competency of pars plana vitrectomy incisions: a comparative histologic and spectrophotometric analysis. Am J Ophthalmol. 2009;147:243–250. doi: 10.1016/j.ajo.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Singh RP, Bando H, Brasil OF, et al. Evaluation of wound closure using different incision techniques with 23-gauge and 25-gauge microincision vitrectomy systems. Retina. 2008;28:242–248. doi: 10.1097/IAE.0b013e318156dea3. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JT. Advantages and limitations of small gauge vitrectomy. Surv Ophthalmol. 2011;56:162–172. doi: 10.1016/j.survophthal.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Meredith TA. Antimicrobial pharmacokinetics in endophthalmitis treatment: studies of ceftazidime. Trans Am Ophthalmol. 1993;91:653–699. [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser RS, Prenner J, Scott IU, et al. The Microsurgical Safety Task Force: evolving guidelines for minimizing the risk of endophthalmitis associated with microincisional vitrectomy surgery. Retina. 2010;30:692–699. doi: 10.1097/IAE.0b013e3181db8bf7. [DOI] [PubMed] [Google Scholar]
- 35.Nagpal M, Wartikar S, Nagpal K. Comparisons of clinical outcomes and wound dynamics of sclerotomy ports of 20, 23, and 25 gauge vitrectomy. Retina. 2009;29:225–231. doi: 10.1097/IAE.0b013e3181934908. [DOI] [PubMed] [Google Scholar]
- 36.Chen SD, Mohammed Q, Bowling B, Patel CK. Vitreous wick syndrome – a potential cause of endophthalmitis after intravitreal injection of triamcinolone through the pars plana. Am J Ophthalmol. 2004;137:1159–1160. doi: 10.1016/j.ajo.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Chiang A, Kaiser R, Avery R, et al. Endophthalmitis in microincision vitrectomy. Outcomes of gas-filled eyes. Retina. 2011;31:1513–1517. doi: 10.1097/IAE.0b013e3182209290. [DOI] [PubMed] [Google Scholar]
- 38.Tominaga A, Oshima Y, Wakabayashi T, Sakaguchi H, Hori Y, Maeda N. Bacterial contamination of the vitreous cavity associated with transconjunctival 25-gauge microincision vitrectomy surgery. Ophthalmology. 2010;117:811–817. doi: 10.1016/j.ophtha.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Fujii GY, Juan E, Jr, Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109:1807–1812. doi: 10.1016/s0161-6420(02)01179-x. [DOI] [PubMed] [Google Scholar]
- 40.Abi-Ayad N, Gambrelle J, Duquesne N, Fleury J, Grange JD, Kodjikian L. Endophthalmitis after plana vitrectomy: incidence, microbiology and visual outcomes. J Fr Ophthalmol. 2007;30:397–402. doi: 10.1016/s0181-5512(07)89610-0. French. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Berrocal MH, Arévalo JF, Carpentier C, et al. Endophthalmitis after pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina. 2011;31:673–678. doi: 10.1097/IAE.0b013e318203c183. [DOI] [PubMed] [Google Scholar]
- 42.Oshima Y, Kadonosono K, Yamaji H, et al. Multicenter survey with a systematic overview of acute-onset endophthalmitis after transconjunctival microincision vitrectomy surgery. Japan Microincision Vitrectomy Surgery Study Group. Am J Ophthalmol. 2010;150:716–725.e1. doi: 10.1016/j.ajo.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Wani VB, Al Sabti K, Kumar N, et al. Endophthalmitis after vitrectomy and vitrectomy combined with phacoemulsification: incidence and visual outcomes. Eur J Ophthalmol. 2009;19:1044–1049. doi: 10.1177/112067210901900623. [DOI] [PubMed] [Google Scholar]
- 44.Taban M, Ufret-Vincenty RL, Sears JE. Endophthalmitis after 25-gauge transconjunctival sutureless vitrectomy. Retina. 2006;26:830–831. doi: 10.1097/01.iae.0000244272.13890.cc. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto T, Enaida H, Kubota T, et al. Incidence of acute endophthalmitis after triamcinolone-assisted pars plana vitrectomy. Am J Ophthalmol. 2004;138:137–138. doi: 10.1016/j.ajo.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 46.Mutoh T, Kadoya K, Chikuda M. Four cases of endophthalmitis after 25-gauge pars plana vitrectomy. Clin Ophthalmol. 2012;6:1393–1397. doi: 10.2147/OPTH.S35123. [DOI] [PMC free article] [PubMed] [Google Scholar]


