Abstract
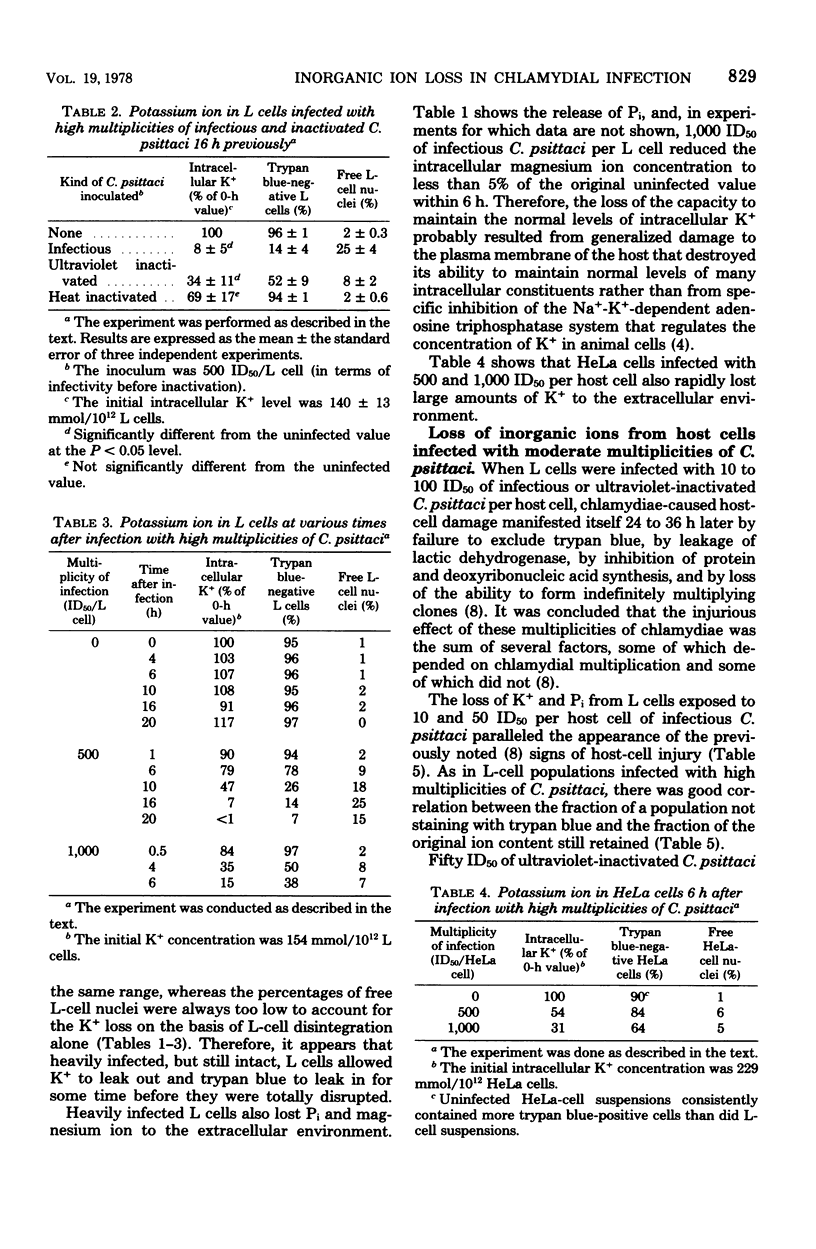
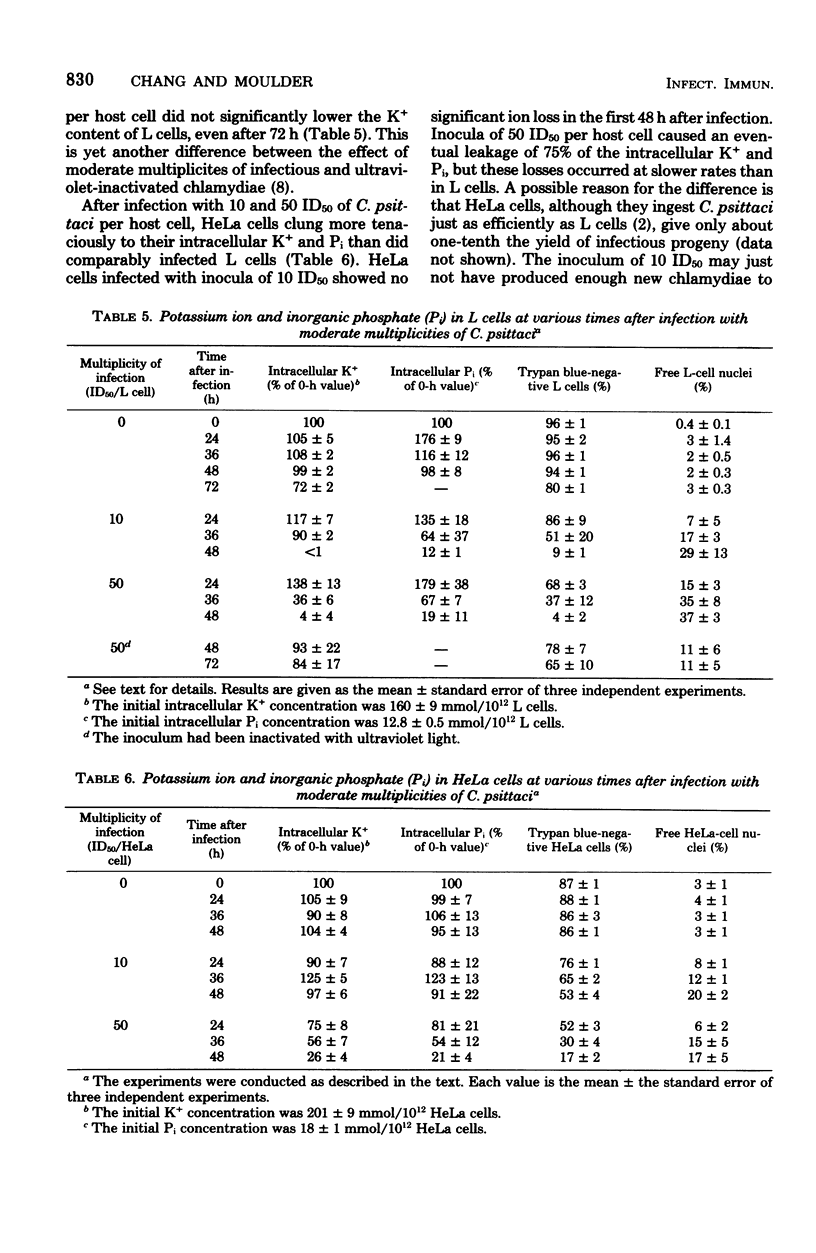
Mouse fibroblasts (L cells) infected with the 6BC strain of Chlamydia psittaci released potassium ion (K+) into the extracellular milieu in a way that depended on size of inoculum and time after infection. When the multiplicity of infection was 500 to 1,000 50% infectious units (ID50) per L cell, loss of intracellular K+ was first apparent 4 to 10 h after infection and was nearly complete at 6 to 20 h. Magnesium ion and inorganic phosphate (Pi) were also released. Similar multiplicities of ultraviolet-inactivated C. psittaci also caused release of K+. Leakage of inorganic ions probably resulted from immediate damage to the host-cell plasma membrane during ingestion of large numbers of chlamydiae. With multiplicities of 1 to 50 ID50 per L cell, ingestion of C. psittaci was not by itself enough to cause release of K+ and Pi from infected L cells. There was a delay of 36 to 72 h between infection and massive leakage of intracellular ions during which time the chlamydiae multiplied extensively. Fifty ID50 of ultraviolet-inactivated C. psittaci per L cell did not bring about significant leakage of K+, even after 72 h. The mechanism whereby these multiplicities of infection destroy the ability of host cells to retain intracellular molecules is not known. HeLa 229 cells also released K+ and Pi after infection, but these losses occurred more slowly than in comparably infected L cells, possibly because C. psittaci did not multiply as extensively in HeLa cells as it did in L cells. The significance of the inability of chlamydiae-infected cells to regulate the flow of molecules through their plasma membranes is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- HEMPLING H. G. Potassium transport in the Ehrlich mouse ascites tumor cell: evidence for autoinhibition by external potassium. J Cell Comp Physiol. 1962 Dec;60:181–198. doi: 10.1002/jcp.1030600302. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg K. R., Horoschak K. D., Moulder J. W. Toxicity of low and moderate multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1977 Nov;18(2):531–541. doi: 10.1128/iai.18.2.531-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Blyth W. A., Ballard R. C. Interactions of TRIC agents with macrophages: effects on lysosomal enzymes of the cell. J Hyg (Lond) 1974 Apr;72(2):297–309. doi: 10.1017/s0022172400023512. [DOI] [PMC free article] [PubMed] [Google Scholar]



