Abstract
Background
Recent epidemiological studies suggest that periodontitis is a major risk factor for renal failure and cerebral infarction. The aim of this study was to evaluate the association among periodontitis, renal failure, and cerebral infarction, focusing on microbiological and immunological features.
Methods
Twenty-one patients treated with hemodialysis (HD) were enrolled in this study. They were 8 with diabetic nephropathy and 13 with non-diabetic nephropathy. Blood examination, periodontal examination, brain magnetic resonance image (MRI), and dental radiography were performed on all patients. Subgingival plaque, saliva, and blood samples were analyzed for the periodontal pathogens, Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Porphyromonas gingivalis (P. gingivalis), and Prevotella intermedia (P. intermedia) using quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA).
Results
We found that the patients with diabetic nephropathy had more A. actinomycetemcomitans compared with non-diabetic nephropathy (P = 0.038) in dental plaque. Furthermore, the patients with diabetic nephropathy showed a significantly higher incidence of cerebral infarction compared with those with non-diabetic nephropathy (P = 0.029). Clinical oral and radiographic scores tended to be higher among patients in the diabetic nephropathy group than in the non-diabetic nephropathy group.
Conclusions
Periodontal pathogens, particularly A. actinomycetemcomitans, may play a role, at least a part, in the development of cerebral infarction in Japanese HD patients with diabetic nephropathy.
Keywords: Periodontitis, Aggregatibacter actinomycetemcomitans, Diabetic nephropathy, Cerebral infarction
Background
Chronic kidney disease (CKD) is a growing public health problem that is associated with an increased risk of cardiovascular disease and mortality [1]. Reduced kidney function is associated with cardiovascular events, even when dysfunction is mild. The vascular changes in CKD patients consist not only in atherosclerosis but also in arteriosclerosis associated with both medial and intimal vascular calcification [2]. CKD is caused by a progressive and irreversible decline in the number of functioning nephrons. The patients develop end-stage renal disease (ESRD) once the damage passes the point of compensation. Therefore, hemodialysis (HD) treatment and kidney transplantation are life-saving medical procedures in these patients.
Recently, some studies demonstrated a high prevalence of periodontitis in individuals with all stages of CKD [3,4]. Periodontitis, one of the most common infections in humans, is caused by subgingival infection with predominantly gram-negative anaerobic bacteria in disease susceptible individuals. Because, this disease contributes to systemic inflammation [5], the periodontal treatment markedly reduces systemic inflammation.
Recent evidence shows that chronic inflammation may cause protein-energy malnutrition and progressive atherosclerosis in HD patients [6]. Chronic periodontal inflammation may also contribute to the chronic systemic inflammatory burden associated with CKD [7]. Thus, periodontopathic bacteria may play a key role in the progression of CKD.
Periodontal disease is known as an independent risk factor for cerebral ischemia [8]. CKD is associated with a high prevalence of stroke [9], and the presence of silent cerebral infarction increased markedly as estimated glomerular filtration rate decreased [10]. However, there was no study to clarify the pathophysiological relationship between chronic periodontitis and cerebral infarction in patients with renal failure.
Therefore, the purpose of the present study was to investigate the association among chronic periodontitis, cerebral infarction and cause of ESRD within Japanese HD patients, focusing on the microbiological and immunological features of this disease.
Methods
Study population
We conducted the present study of all HD patients who admitted to the hemodialysis unit at Saku Central Hospital, Nagano, Japan. Exclusion criteria included (i) known systemic diseases, (ii) history and/or presence of other infections, (iii) systemic antibiotic, immunosuppressive or periodontal treatment in the preceding 6 months prior to the sample collection, and (iv) disagreement with the present study. Informed consent was obtained from each subject after providing them with verbal and written explanations of the nature of the study. The study was approved by the Ethics Committees of Saku Central Hospital, University of Tokyo (ID 2947) and Tokyo Medical and Dental University (ID 546). Data of age, gender, height, dry weight, smoking status, cause of ESRD, dialysis prescription, and the use of drugs, including statins, antihypertensives, anticoagulants, and antiplatelets were collected from the medical records.
Periodontal examination
This cross-sectional study was conducted between November and December 2011. Periodontal examination was performed by one experienced dentist who was masked to the clinical systemic findings of these patients. Full-mouth clinical measurements including probing pocket depth (PPD), clinical attachment level (CAL), and bleeding on probing (BOP) were recorded at 6 sites on each tooth using a manual probe (PCP-UNC 15, Hu-Friedy Manufacturing Co., Chicago, IL, USA). Oral specimens (subgingival plaque and saliva) were taken at the same time. A full-mouth set of 10 periapical radiograms was also obtained from each patient using the isometric method. Alveolar bone loss was measured using the 10 dental X-ray films. Patients underwent a standard phase of nonsurgical periodontal treatment.
Sample collection and preparation
Laboratory data were taken from all subjects within a few days of the clinical examination during stable outpatient HD sessions. Blood samples were drawn from the arterial end of the vascular access immediately before initiation of HD, then stored at -70°C until assay. All subjects underwent a complete blood count, blood chemistry analysis, and several measures of lipid metabolism including total cholesterol (TC), triglycerides (TG), and high- and low-density lipoprotein (HDL, LDL). The serum levels of high-sensitivity C-reactive protein (hs-CRP) were also measured. Whole blood was subjected to microbiological analysis. Blood samples were also subjected to determine the specific serum IgG antibody responses to the periodontal pathogens tested. Subgingival plaque and saliva samples were collected during a periodontal examination. Subgingival plaque samples were collected from the deepest pockets in each quadrant and pooled for microbiological analysis. After supragingival debridement, subgingival plaque was collected by inserting a sterile paper point (No. 30) into the pocket until resistance was felt and was kept in place for 30 seconds. Paper points with plaque samples were transferred to a sterile vial and unstimulated saliva (500 μL) was also collected from each patient in a sterile tube. All samples were kept in a freezer at -80°C until used for the extraction of bacterial DNA. Dialysis clearance of urea was expressed as Kt/Vurea, according to Daugirdas [11] in HD. The characteristics of the subjects, including age, sex, smoking status, and biological, hematologic, and dialysis-related data, are listed in Table 1.
Table 1.
Baseline characteristics of the study populations
| DM (n=8) | Non-DM (n=13) | P value* | |
|---|---|---|---|
| Characteristics |
|
|
|
| Age (y) |
63.0 (57.2, 68.5) |
64.0 (45.5, 74.5) |
0.913 |
| Height (cm) |
166.8 (160.0, 171.3) |
167.0 (156.6, 169.4) |
0.772 |
| Dry weight (kg) |
68.4 (58.4, 81.1) |
57.0 (53.8, 65.0) |
0.051 |
| Female sex |
2(25.0) |
3 (23.1) |
0.920 |
| Smoking |
1(12.5) |
2 (15.4) |
0.854 |
| Drugs |
|
|
|
| Antihypertensives |
7(87.5) |
13 (100) |
0.192 |
| Antiplatelets |
5(62.5) |
5 (38.5) |
0.284 |
| Anticoagulants |
1(12.5) |
3 (23.1) |
0.549 |
| Statins |
3(37.5) |
3 (23.1) |
0.477 |
| Biochemical data |
|
|
|
| TP (g/dL) |
6.9 (6.7, 7.3) |
6.5 (6.1, 6.9) |
0.064 |
| Alb (g/dL) |
4.2 (3.8, 4.3) |
4.0 (3.7,4.4) |
0.689 |
| AST (IU/L) |
9.5 (9.0, 20.0) |
11.0 (6.5, 14.0) |
0.445 |
| ALT (IU/L) |
12.0 (9.3, 17.5) |
9.0 (8.0, 12.5) |
0.094 |
| LDH (IU/L) |
193.5 (182.8, 213.5) |
171.0 (148.5, 194.5) |
0.076 |
| ALP (IU/L) |
318.0 (193.8, 348.8) |
168.0 (157.5, 241.5) |
0.039 |
| BUN (mg/dL) |
61.0 (56.3, 72.3) |
60.0 (50.5, 68.5) |
0.405 |
| Cr (mg/dL) |
11.8 (9.7, 13.4) |
12.2 (10.2, 13.8) |
0.799 |
| UA (mg/dL) |
7.6 (5.4, 8.9) |
7.6 (6.5, 8.0) |
0.744 |
| β2-MG (mg/L) |
21.7 (18.2, 22.9) |
21.0 (20.0, 24.5) |
0.799 |
| NT-proBNP (pg/mL) |
3323 (1473, 6494) |
2657 (826, 12645) |
0.638 |
| Ca (mg/dL) |
8.8 (8.5, 9.6) |
9.1 (8.9, 9.5) |
0.611 |
| P (mg/dL) |
5.9 (5.0, 6.9) |
5.4 (4.9, 6.2) |
0.537 |
| iPTH (pg/mL) |
152.5 (70.8, 259.8) |
174.0 (82.0, 223.0) |
0.914 |
| Fe (μg/dL) |
64.5 (55.5, 82.8) |
73.0 (50.0, 94.0) |
0.612 |
| Ferritin (μg/L) |
74.5 (30.3, 241.8) |
245.0 (131.0, 393.5) |
0.120 |
| hs-CRP (mg/dL) |
0.10 (0.06, 0.31) |
0.06 (0.03, 0.13) |
0.403 |
| TG (mg/dL) |
129.0 (91.3, 190.0) |
100.0 (77.0, 141.0) |
0.277 |
| TC (mg/dL) |
152.0 (123.3, 184.0) |
163.0 (145.5, 201.0) |
0.246 |
| LDL-C (mg/dL) |
79.5 (54.5, 94.0) |
93.0 (72.5, 123.5) |
0.246 |
| HDL-C (mg/dL) |
39.0 (27.5, 54.5) |
42.0 (39.0, 51.5) |
0.663 |
| BS (mg/dL) |
148.0 (98.3, 195.5) |
107.0 (96.5, 116.5) |
0.180 |
| HbA1c (%) |
7.0 (6.6, 7.9) |
5.2 (4.8, 5.3) |
0.0002 |
| Hematologic data |
|
|
|
| WBC (103/μL) |
5.55 (4.90, 6.25) |
5.10 (4.15, 5.85) |
0.310 |
| Hb (g/dL) |
11.8 (11.0, 12.6) |
11.0 (10.5, 12.1) |
0.232 |
| Ht (%) |
37.0 (34.0, 38.9) |
34.1 (33.2, 36.2) |
0.168 |
| PLt (104/μL) |
19.1 (17.4, 23.6) |
15.0 (10.6, 17.9) |
0.020 |
| Dialysis parameters |
|
|
|
| HD duaration (y) |
5.2 (2.4, 6.3) |
4.1 (1.1, 13.7) |
0.971 |
| Kt/Vurea | 1.32 (1.25, 1.42) | 1.31 (1.21, 1.45) | 0.968 |
Data are presented in number (%) or median (interquartile range).
*p-values were calculated by Mann-Whitney test for continuous variables or Fishers exact test for categorical variables.
Abbreviations: DM diabetes mellitus, TP total protein, Alb albumin, AST aspartate aminotransferase, LDH lactase dehydrogenase, ALP alkaline phosphatase, BUN blood urea nitrogen, Cr reatinine, UA urinary acid, β2-MG beta2-microglobulin, NT-proBNP N-terminal pro-brain natriuretic peptide, Ca calcium, P phosphorus, iPTH intact parathyroid hormone, Fe ferrum, hsCRP high-sensitivity C-reactive protein, TG triglyceride, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, BS blood sugar, HbA1c hemoglogin A1c, WBC white blodd cell, Hb hemoglobin, Ht hematocrit, PLt platelet, HD hemodialysis, Kt/Vurea Kt/V for urea.
Quantitative real-time polymerase chain reaction (qRT-PCR) assay
Periodontopathogens were identified using a quantitative real-time polymerase chain reaction (qRT-PCR) [12] based on 16S rRNA genes. Bacteria-specific primer pairs based on the species-specific region on the 16S rRNA genes are shown in Table 2[13,14]. Each 50 μL PCR reaction mixture contained 5 μL of the sample, 5 μL of 10 x PCR buffer (TaKaRa, Shiga, Japan), 1.25 units of TaqDNApolymerase (TaKaRa), 0.2 mM of each deoxyribonucleotide (TaKaRa), 1.0 mM of each primer, and 1.0 mM MgCl2 for Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) or 1.5 mM MgCl2 for Porphyromonas gingivalis (P. gingivalis). PCR amplification was performed in a DNA thermal cycler (PTC-200, MJ Research, Boston, MA). The temperature profile for A. actinomycetemcomitans and Prevotella intermedia (P. intermedia) included an initial step at 95°C for 2 minutes followed by 36 cycles of 94°C for 30 seconds, 55°C for 1 minute, 72°C for 2 minutes, and a final step at 72°C for 10 minutes. The PCR temperature profile for P. gingivalis included an initial step at 95°C for 2 minutes followed by 36 cycles of 95°C for 30 seconds, 60°C for 1 minute, 72°C for 1 minute, and a final step at 72°C for 2 minutes.
Table 2.
Species-specific and ubiquitous primers for PCR
| Species | Primers pairs (5’ to 3’) | Amplicon size in bp |
|---|---|---|
|
Aggregatibacter antinomycetemcomitans |
AAACCCATCTCTGAGTTCTTCTTC |
557 |
| ATGCCAACTTGACGTTAAT |
|
|
|
Porphyromonas gingivalis |
ACTGTTAGCAACTACCGATGT |
404 |
| AGGCAGCTTGCCATACTGCG |
|
|
|
Prevotella intermedia |
TCAACATCTCTGTATCCTGCGT |
575 |
| TTTGTTGGGGAGTAAAGCGGG |
Abbreviations: PCR polymerase chain reaction.
Serum IgG antibody titer measurement
Specific serum IgG titers were measured by ELISA using sonicated whole cell extracts of each periodontopathogen. Briefly, the microtiter plates were coated with sonicated whole cell extracts of P. gingivalis ATCC 33277, A. actinomycetemcomitans ATCC 33384 and P. intermedia ATCC 25611. The 96-well microtiterplates (EIA plate, Costar, Cambridge, MA) were coated with sonicated extracts (10 μg/mL) in a carbonate buffer, and incubated for 2 hours at 37°C. After blocking with 2% BSA in carbonate buffer, the plates were washed 3 times with PBS-T (1 × PBS, 0.05% Tween 20, pH 7.2). Serially diluted reference positive control serum (25 to 214, 100 μL per well) and single diluted (210 for P. gingivalis and A. actinomycetemcomitans, and 28 for P. intermedia) patient serum were added into each well in duplicate and the plates were incubated for 2 hours at 37°C. Following incubation, the plates were washed again 3 times. Subsequently, 100 μL per well of alkaline phosphatase-conjugated goat anti-human IgG (Sigma Chemical Co., USA) was added. Following incubation, the plates were washed 3 times and developed with phosphate substrate (Sigma104). The optical density was read using a Microplate Reader (SOFT MaxTM) at 405 nm with a 650 nm reference wavelength. Antibody titer was calculated according the method of Wang et al. [15].
Magnetic resonance imaging (MRI) evaluation of the brain
All subjects received an MRI of the brain. The slice thickness was 5 mm with an interslice gap of 1 mm. Criteria of cerebral infarction was defined as a low-intensity area on the T1-weighted image and as a high-intensity area on the T2-weighted image [16]. Cerebral infarction included both symptomatic and silent cerebral infarction. We defined silent cerebral infarction as a focal area ≧3 mm and <20 mm in diameter in both T1- and T2- weighted scans, while dilated Virchow-Robin spaces were excluded. Cerebral infarction history was determined by checking the medical records of all subjects, independent of their MRI outcome. We defined silent cerebral infarction as evidence on MRI of one or more infarctions, without a history of a stroke.
Statistical analysis
Baseline characteristics of the study populations were presented in number (%) for categorical variables or median (interquartile range) for continuous variables. Differences in continuous and categorical variables were examined with Mann–Whitney test and Fishers exact test for two group comparisons, respectively. We evaluated statistical correlations between cause of ESRD (the diabetic and non-diabetic groups) and periodontal parameters and serum IgG titers specific to them using Mann–Whitney test. We used Fishers exact test to evaluate statistical correlations between cause of ESRD and cerebral infarction. We analyzed statistical correlations between cause of ESRD and 3 periodontopathogens in saliva and subgingival plaque using both Mann–Whitney and Fishers exact test. The alpha level was set at 0.05. All statistical analyses were performed with the aid of statistical software (SPSS Statistics®, Version 20, IBM).
Results
Characteristics of the study population
Seventeen patients of 149 HD patients in Saku Central Hospital were excluded from the study because they had at least one of the following exclusion criteria; (i) known systemic diseases, (ii) history and/or presence of other infections, or (iii) systemic antibiotic, immunosuppressive or periodontal treatment in 6 months prior to the sample collection. Only 21 of 132 HD patients consented to this study. These patients (16 males, 5 females) with a median duration of 4.7 years of HD therapy (from 0.3 to 27.6 years) were enrolled for analysis. The demographic characteristics of the study population are presented in Table 1. Patient age ranged from 40 to 86 years (median 64.0 years). Primary renal diseases of the study population were as follows: diabetic nephropathy (38%), chronic glomerulonephritis (14%), and hypertensive glomerulosclerosis (10%), and unknown (38%). There were 8 HD patients with diabetic nephropathy (2 females and 6 males, median age 63.0 years) and 13 with non-diabetic nephropathy (3 females and 10 males, median age 64.0 years). The 21 HD patients included 8 with diabetic nephropathy and 13 with non-diabetic nephropathy. The majority of HD patients were undergoing 4 hours of HD 3 times/week. HD was prescribed in these patients with single-use hollow-fiber dialyzers equipped with polysulfone or polymethylmethacrylate membranse. The dialysate used was a standard ionic composition and bicarbonate-based buffer.
Periodontal evaluation
Subjects who had at least 1 site with a tooth pocket depth of ≥ 4 mm and/or showed bone loss on the radiograms were considered to have periodontitis. In the present study, most subjects had periodontitis. Representative periapical radiogram findings of HD patients are presented in Figure 1. We evaluated the statistical correlations between cause of ESRD and clinical oral and radiographic parameters. The number of missing teeth, % of sites with PPD ≥ 4 mm, % of sites exhibiting BOP, and % of sites with bone loss ≥ 25% on radiograms also tended to be higher among patients in the diabetic nephropathy group than in the non-diabetic nephropathy group. However, the differences were not statistically significant (Table 3).
Figure 1.
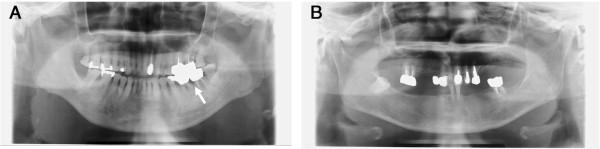
Periapical radiogram. Representative periapical radiogram findings of non-diabetic (A) and diabetic (B) nephropathy patients are shown. Mild horizontal and vertical alveolar bone resorption was found in a non-diabetic (A) nephropathy patient. Moderate horizontal alveolar bone resorption and a lot of missing teeth were found in a diabetic (B) nephropathy patient. An arrow indicates vertical alveolar bone resorption.
Table 3.
Clinical oral and radiographic data of the study populations
| DM (n=8) | Non-DM (n=13) | P value* | |
|---|---|---|---|
| Number of missing teeth |
3.0 (1.0, 12.3) |
2.0 (0, 4.0) |
0.340 |
| % of sites with PPD ≥ 4 mm |
3.3 (0.6, 6.0) |
3.1 (1.2, 5.1) |
0.942 |
| % of sites with CAL ≥ 4 mm |
15.2 (12.9, 22.4) |
9.1 (4.2, 33.5) |
0.158 |
| % of sites exhibiting BOP |
30.1 (1.7, 47.1) |
14.0 (7.8, 33.0 ) |
0.717 |
| % of sites with bone loss ≥ 25 |
18.7 (5.8, 45.0) |
4.2 (3.7, 16.0) |
0.137 |
| % on radiograms |
Data are presented in median (interquartile range).
*p-values were calculated by Mann-Whitney test.
Abbreviations: DM diabetes mellitus, PPD probing pocket depth, CAL clinical attachment level, BOP bleeding on probing.
Microbiological evaluation
The results of PCR analysis on subgingival plaque and saliva samples for all subjects are shown in Figure 2 and Tables 4 and 5. Although each pathogen did not demonstrate the statistical differences between the two groups, A. actinomycetemcomitans in the diabetic nephropathy group tended to have a higher prevalence rate compared to the non-diabetic nephropathy group in both saliva and plaque. Moreover, the patients with diabetic nephropathy had significantly more A. actinomycetemcomitans quantitatively compared to the patients with non-diabetic nephropathy (P = 0.038) in dental plaque. P. gingivalis and P. intermedia in both dental plaque and saliva did not differ quantitatively between the diabetic and non-diabetic groups. There were 5 (56%) and 3 (33%) patients who were positive for A. actinomycetemcomitans salivary and subgingivally of the 9 patients with cerebral infarction, respectively.
Figure 2.
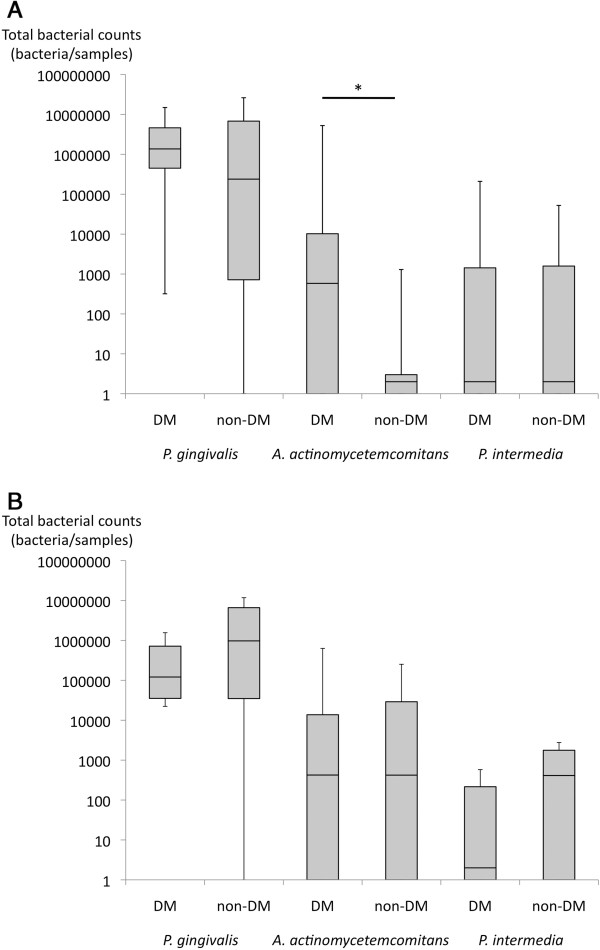
PCR analysis. The results of PCR analysis of subgingival plaque (A) and saliva (B) are demonstrated. An asterisk (*) indicates a significant difference. A boxplot shows median, and interquatile range between 25th and 75th percent. Data are presented in number (%) or median (interquartile range). P-values were calculated by Mann–Whitney test.
Table 4.
Number (%) of patients who were positive for each pathogen in saliva
| A. actinomycetemcomitans | P. intermedia | P. gingivalis | |
|---|---|---|---|
| DM (n=8) |
4 (50) |
2 (25) |
7 (88) |
| Non-DM (n=13) | 5 (38) | 6 (46) | 9 (69) |
Abbreviations: DM diabetes mellitus.
Table 5.
Number (%) of patients who were positive for each pathogen in subgingival plaque
| A. actinomycetemcomitans | P. intermedia | P. gingivalis | |
|---|---|---|---|
| DM (n=8) |
5 (63) |
3 (38) |
8 (100) |
| Non-DM (n=13) | 3 (23) | 5 (38) | 10 (77) |
Abbreviations: DM daibetes mellitus.
Serum IgG titers to periodontopathogens
Serum IgG titers specific to the 3 periodontopathogens are shown in Figure 3. No statistical difference was found in the IgG titers between the diabetic and non-diabetic groups.
Figure 3.
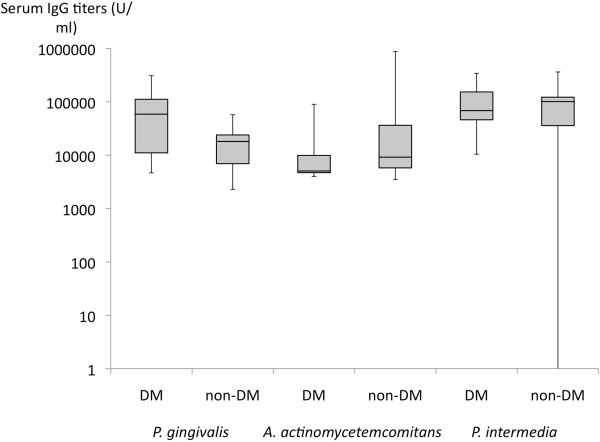
ELISA analysis. The results of serum IgG antibody titers specific to the 3 periodontopathogens measured by ELISA analysis are demonstrated.
The median serum anti-A. actinomycetemcomitans antibody levels were 90026 in the 5 patients who were salivary positive for it, while the levels were 4891 in the 3 patients who were subgingivally positive for it.
Brain MRI evaluation
Representative brain MRI findings (T2-WI) of HD patients are presented in Figure 4. It was found that 75% of the patients with diabetic nephropathy (6/8) had cerebral infarction, whereas 23% of those with non-diabetic nephropathy (3/13) had cerebral infarction (P = 0.029). Among patients with cerebral infarction, all showed lacunar infarction. Eight patients had silent cerebral infarction and only one patient with diabetic nephropathy had symptomatic cerebral infarction.
Figure 4.
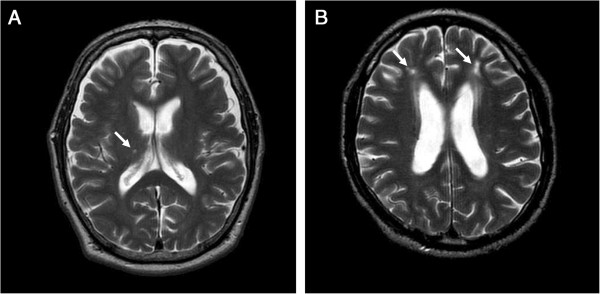
Brain MRI. Representative brain MRI findings (T2-WI) of non-diabetic (A) and diabetic (B) nephropathy patients are shown. Arrows indicate cerebral infarctions.
Discussion
The present study demonstrated the periodontal and brain status of 21 HD patients. Our results showed that patients with diabetic nephropathy had significantly more A. actinomycetemcomitans compared to patients with non-diabetic nephropathy in dental plaque. We also found that the patients with diabetic nephropathy showed a significantly higher incidence of cerebral infarction compared to those with non-diabetic nephropathy.
Kshirsagar et al. reported a retrospective HD cohort in which moderate to severe periodontal disease was associated with a 5-fold increase in cardiovascular mortality after 18 months of follow-up [17]. Furthermore, a large-scale systemic review that included 8 case-controlled and 18 cross-sectional reports suggested that periodontal disease may be associated modestly with atherosclerosis, myocardial infarction, and cerebrovascular disease [18]. The cardiovascular risk seemed to be highest among those who showed both evidence of some chronic, low-grade infection, and elevated CRP levels [19].
Takeuchi et al. evaluated the composition of subgingival microbiota in 81 patients with CKD with that in 62 healthy individuals by performing PCR with gingival crevicular fluid [20]. They found that Tannerella forsythia (T. forsythia), Treponema denticola (T. denticola), Prevotella nigrescens, and Candida albicans were more frequent in patients with CKD than in controls. Bastos et al. showed that red bacterial complex (P. gingivalis, T forsythia, and T. denticola) were more frequent in patients with chronic periodontitis and CKD than in healthy individuals [21]. Wara-aswapati et al. also suggested that red complex bacteria were predominant periodontal pathogens of the moderate to severe form of chronic periodontitis in a Thai population [22]. The presence of red bacterial complex was associated with the severity of disease. Therefore, these pathogens should be considered as targets for the prevention and treatment of periodontal disease.
It is commonly known that individuals with diabetes mellitus (DM) are at risk of periodontitis [23]. The high prevalence of periodontitis among diabetic patients is mainly due to their high susceptibility to infection. The implications for oral health and the provision of dental care for people with DM are significant, because numerous studies have demonstrated an association between DM and periodontitis [24]. Furthermore, DM patients with periodontal disease have an increased risk of severe systemic disease compared to those without periodontal disease [25]. A recent meta-analysis of the efficacy of periodontal treatment on glycemic control in diabetic patients suggested that such treatment could lead to significant reductions of glycated hemoglobin [26].
Ghizoni et al. reported that stroke patients had deeper pockets, more severe attachment loss, increased BOP, increased plaque index, and in their pockets harbored increased levels of P. gingivalis[27]. These findings suggest that periodontal disease is a risk factor for the development of cerebral hemorrhage or infarction.
A. actinomycetemcomitans is a gram-negative, facultatively anaerobic coccobaccillus and is considered to be the major etiologic agent of localized aggressive periodontitis [28]. It also contributes to chronic periodontitis. Studies in Chinese, Korean, and southern Thai populations have shown high detection frequencies of A. actinomycetemcomitans ranging approximately from 40% to 75% of the sampling sites [29-32]. In contrast, a study in a Japanese population reported that less than 10% of the chronic periodontitis diseased site was positive for A. actinomycetemcomitans[33]. Furthermore, this microorganism has been isolated from several other non-oral infections, including endocarditis [34] and pericarditis [35]. Recently, these species were identified in atheromatous plaques of cardiovascular disease patients [36], suggesting a possible role for P. gingivalis and A. actinomycetemcomitans in the development of this lesion.
These results are supported by recent studies showing that elevated serum anti-A. actinomycetemcomitans antibody levels predicts stroke [37] and coronary heart disease [38]. A. actinomycetemcomitans possesses a number of putative virulence factors, including a leukotoxin that targets and destroys specific host immune cells. Leukotoxin is also involved in the adhesion of A. actinomycetemcomitans[39]. The organism takes advantage of its high adhesiveness and is capable of rapid invasion and spread through eukaryotic cells [40]. Ten et al. reported that periodontitis patients infected with A. actinomycetemcomitans harbored A. actinomycetemcomitans-specific T-cells in peripheral blood, and T-cells expressed RANK ligand (RANKL) in response to A. actinomycetemcomitans[41]. RANKL from T-cells stimulates vascular smooth muscle cells to produce matrixmetalloproteinase-9. These cells destabilize atherosclerotic plaque and were reported to be elevated in coronary artery plaque in patients with acute myocardial infarction [42].
In the present study, we selected A. actinomycetemcomitans, P. intermedia, and P. gingivalis for analysis after the previous study, which reported an association between serum anti-periodontal pathogen antibody and ischemic stroke. Hosomi et al. reported that anti-P. intermedia antibody was associated with carotid artery atherosclerosis, and that anti-P. intermedia antibody may be associated with atherothrombotic stroke through its association with carotid artery atherosclerosis [43]. It was found that patients with diabetic nephropathy had significantly more A. actinomycetemcomitans and cerebral infarction compared to patients with non-diabetic nephropathy in dental plaque. The results of degree of periodontitis also tended to be higher among patients in the diabetic nephropathy group, although the difference was not significant. Therefore, we thought that A. actinomycetemcomitans may play a role, at least a part, in the development of cerebral infarction in Japanese HD patients with diabetic nephrology. However, some of the results were not consistent with our hypothesis. Increased systemic inflammation as a pathogenic link was not supported by serum hs-CRP levels and the IgG titers against A. actinomycetemcomitans. Because periodontal A. actinomycetemcomitans infection is generally a local burden, it may not influence systemic inflammatory reaction until periodontitis becomes more advanced. At this point, we cannot clarify the discrepancy between A. actinomycetemcomitans infection and hs-CRP levels. Further investigation is required.
The virulence of A. actinomycetemcomitans is still not well understood, but it is able to produce a heat-labile leukotoxin, which belongs to the repeat-in-toxin (RTX) family. The gene ltxA encodes a structural leukotoxin and genes ltxB and ltxD encode proteins required for its secretion. Gene ltxC encodes an acyltransferase that is responsible for the modification of proto-toxin to the active toxin [44]. Moreover, the leukotoxin production has been associated with evasion against the defense cells of the periodontal tissues [45,46]. In this study, we cannot reveal the virulence mechanisms among the dialysis, DM, and cerebral infarction. Thus, further investigation is needed to clarify the pathological mechanisms.
We have some limitations in this study. Firstly, it is difficult to reveal a causal relationship among periodontal A. actinomycetemcomitans infection, diabetic nephropathy, and cerebral infarction, because this is a cross-sectional observation study. Secondly, some statistical analyses were incomplete because the sample size was too small to calculate. Thus, further investigation is required.
Conclusions
Periodontal pathogens, particularly A. actinomycetemcomitans, may play a role, at least a part, in the development of cerebral infarction in Japanese HD patients with diabetic nephropathy.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MM performed the statistical analysis and drafted the manuscript. JS conceived of the study, and participated in its design and coordination and helped to draft the manuscript. SY participated in its design and coordination. RM performed periodontal examination. NA and NA carried out the microbiological evaluation and immunoassays. MI, NK, HA, IK, YI, and MI contributed to the discussion and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Minoru Murakami, Email: murakami11108510@yahoo.co.jp.
Jun-ichi Suzuki, Email: junichisuzuki-circ@umin.ac.jp.
Satoshi Yamazaki, Email: zm99st@bma.biglobe.ne.jp.
Masaya Ikezoe, Email: ike-chan@vega.ocn.ne.jp.
Rintaro Matsushima, Email: rainbow@mvc.biglobe.ne.jp.
Norihiko Ashigaki, Email: ashigaki.peri@tmd.ac.jp.
Norio Aoyama, Email: aoyaperi@tmd.ac.jp.
Naho Kobayashi, Email: kobnperi@tmd.ac.jp.
Kouji Wakayama, Email: kj-wakayama@s9.dion.ne.jp.
Hiroshi Akazawa, Email: akazawah-tky@umin.ac.jp.
Issei Komuro, Email: komuro_tky2000@yahoo.co.jp.
Yuichi Izumi, Email: y-izumi.peri@tmd.ac.jp.
Mitsuaki Isobe, Email: isobemi.cvm@tmd.ac.jp.
Acknowledgments
We would like to thank Dr. Tomoya Hanatani, Dr. Asuka Sekinishi, Ms. Noriko Tamura and Ms. Yasuko Matsuda for their excellent assistance.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (No. 25870198), Ministry of Education, Culture, Sports, Science and Technology of Japan, Mitsui Life Social Welfare Foundation, Daiwa Security Health Foundation, Mitsui Sumitomo Marine Welfare Foundation, Institute of Geriatric Dentistry Foundation, Institute of St. Luka Life Science Foundation, Health Welfare Foundation, Taiyo Life Welfare Foundation, and the 8020 Foundation.
References
- Hostetter TH. Chronic kidney disease predicts cardiovascular disease. N Engl J Med. 2004;351:1344–1346. doi: 10.1056/NEJMe048211. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Biondi ML, Galassi A, Cusi D, Brancaccio D, Gallieni M. Vascular calcification and cardiovascular outcome in dialysis patients: the role of gene polymorphisms. Blood Purif. 2010;29:347–351. doi: 10.1159/000302722. [DOI] [PubMed] [Google Scholar]
- Kshirsagar AV, Moss KL, Elter JR, Beck JD, Offenbacher S, Falk RJ. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study. Am J Kidney Dis. 2005;45:650–657. doi: 10.1053/j.ajkd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Fisher MA, Taylor GW, Shelton BJ, Jamerson KA, Rahman M, Ojo AO, Sehgal AR. Periodontal disease and other nontraditional risk factors for CKD. Am J Kidney Dis. 2008;51:45–52. doi: 10.1053/j.ajkd.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251–264. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- Stenvinkel P. Inflammation in end-stage renal disease: the hidden enemy. Nephrology. 2006;11:36–41. doi: 10.1111/j.1440-1797.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, Lutz R, Bultmann S, Preusch M, Dorfer CE. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D. [DOI] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hirawa N, Yatsu K, Kobayashi Y, Yamamoto Y, Saka S, Andoh D, Toya Y, Yasuda G, Umemura S. Relationship between silent brain infarction and chronic kidney disease. Nephrol Dial Transplant. 2009;24:201–207. doi: 10.1093/ndt/gfn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT. The post: pre-dialysis plasma urea nitrogen ratio to estimate K.t/V and NPCR: mathematical modeling. Int J Artif Organs. 1989;12:411–419. [PubMed] [Google Scholar]
- Jervoe-Storm PM, AlAhdab H, Koltzscher M, Fimmers R, Jepsen S. Quantification of periodontal pathogens by paper point sampling from the coronal and apical aspect of periodontal lesions by real-time PCR. Clinical oral investigations. 2010;14:533–541. doi: 10.1007/s00784-009-0333-x. [DOI] [PubMed] [Google Scholar]
- Maeda H, Fujimoto C, Haruki Y, Maeda T, Kokeguchi S, Petelin M, Arai H, Tanimoto I, Nishimura F, Takashiba S. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS immunol Med Microbiol. 2003;39:81–86. doi: 10.1016/S0928-8244(03)00224-4. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Takeuchi Y, Umeda M, Ishikawa I, Benno Y. Rapid detection and quantification of five periodontopathic bacteria by real-time PCR. Microbiol Immunol. 2001;45:39–44. doi: 10.1111/j.1348-0421.2001.tb01272.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Kawashima Y, Nagasawa T, Takeuchi Y, Kojima T, Umeda M, Oda S, Ishikawa I. Elevated serum IgG titer and avidity to Actinobacillus actinomycetemcomitans serotype c in Japanese periodontitis patients. Oral Microbiol Immunol. 2005;20:172–179. doi: 10.1111/j.1399-302X.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- Zhu YC, Dufouil C, Tzourio C, Chabriat H. Silent brain infarcts: a review of MRI diagnostic criteria. Stroke. 2011;42:1140–1145. doi: 10.1161/STROKEAHA.110.600114. [DOI] [PubMed] [Google Scholar]
- Kshirsagar AV, Craig RG, Moss KL, Beck JD, Offenbacher S, Kotanko P, Klemmer PJ, Yoshino M, Levin NW, Yip JK, Almas K, Lupovici EM, Usvyat LA, Falk RJ. Periodontal disease adversely affects the survival of patients with end-stage renal disease. Kidney Int. 2009;75:746–751. doi: 10.1038/ki.2008.660. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8:38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- Roivainen M, Viik-Kajander M, Palosuo T, Toivanen P, Leinonen M, Saikku P, Tenkanen L, Manninen V, Hovi T, Manttari M. Infections, inflammation, and the risk of coronary heart disease. Circulation. 2000;101:252–257. doi: 10.1161/01.CIR.101.3.252. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Ishikawa H, Inada M, Shinozuka O, Umeda M, Yamazaki T, Yoshikawa M, Sasaki S. Study of the oral microbial flora in patients with renal disease. Nephrology. 2007;12:182–190. doi: 10.1111/j.1440-1797.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- Bastos JA, Diniz CG, Bastos MG, Vilela EM, Silva VL, Chaoubah A, Souza-Costa DC, Andrade LC. Identification of periodontal pathogens and severity of periodontitis in patients with and without chronic kidney disease. Arch Oral Biol. 2011;56:804–811. doi: 10.1016/j.archoralbio.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Wara-aswapati N, Pitiphat W, Chanchaimongkon L, Taweechaisupapong S, Boch JA, Ishikawa I. Red bacterial complex is associated with the severity of chronic periodontitis in a Thai population. Oral Dis. 2009;15:354–359. doi: 10.1111/j.1601-0825.2009.01562.x. [DOI] [PubMed] [Google Scholar]
- Ohlrich EJ, Cullinan MP, Leichter JW. Diabetes, periodontitis, and the subgingival microbiota. J Oral Microbiol. 2010;2: . doi: 10.3402/jom.v2i0.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson H, Lindholm E, Lindh C, Groop L, Bratthall G. Type 2 diabetes and risk for periodontal disease: a role for dental health awareness. J Clin Periodontol. 2006;33:408–414. doi: 10.1111/j.1600-051X.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- Shultis WA, Weil EJ, Looker HC, Curtis JM, Shlossman M, Genco RJ, Knowler WC, Nelson RG. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306–311. doi: 10.2337/dc06-1184. [DOI] [PubMed] [Google Scholar]
- Darre L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabetes Metab. 2008;34:497–506. doi: 10.1016/j.diabet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Ghizoni JS, Taveira LA, Garlet GP, Ghizoni MF, Pereira JR, Dionisio TJ, Brozoski DT, Santos CF, Sant'Ana AC. Increased levels of Porphyromonas gingivalis are associated with ischemic and hemorrhagic cerebrovascular disease in humans: an in vivo study. J Appl Oral Sci. 2012;20:104–112. doi: 10.1590/S1678-77572012000100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051X.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- He J, Huang W, Pan Z, Cui H, Qi G, Zhou X, Chen H. Quantitative analysis of microbiota in saliva, supragingival, and subgingival plaque of Chinese adults with chronic periodontitis. Clin oral investig. 2012;16:1579–1588. doi: 10.1007/s00784-011-0654-4. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Baelum V, Luan WM, Madianos PN, Chen X, Fejerskov O, Dahlen G. Subgingival microbiota in adult Chinese: prevalence and relation to periodontal disease progression. J Periodontol. 1997;68:651–666. doi: 10.1902/jop.1997.68.7.651. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Teanpaisan R, Obiechina NS, Pithpornchaiyakul W, Pongpaisal S, Pisuithanakan S, Baelum V, Fejerskov O, Dahlen G. Periodontal microbiota and clinical periodontal status in a rural sample in southern Thailand. Eur J Oral Sci. 2002;110:345–352. doi: 10.1034/j.1600-0722.2002.21361.x. [DOI] [PubMed] [Google Scholar]
- Choi BK, Park SH, Yoo YJ, Choi SH, Chai JK, Cho KS, Kim CK. Detection of major putative periodontopathogens in Korean advanced adult periodontitis patients using a nucleic acid-based approach. J Periodontol. 2000;71:1387–1394. doi: 10.1902/jop.2000.71.9.1387. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Umeda M, Ishizuka M, Huang Y, Ishikawa I. Prevalence of periodontopathic bacteria in aggressive periodontitis patients in a Japanese population. J Periodontol. 2003;74:1460–1469. doi: 10.1902/jop.2003.74.10.1460. [DOI] [PubMed] [Google Scholar]
- Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14:177–207. doi: 10.1128/CMR.14.1.177-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz EA, Pugsley MP, Turbes PG, Clark RB. Pericarditis caused by Actinobacillus actinomycetemcomitans. J Infect Dis. 1987;155:152–153. doi: 10.1093/infdis/155.1.152. [DOI] [PubMed] [Google Scholar]
- Kozarov E, Sweier D, Shelburne C, Progulske-Fox A, Lopatin D. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect. 2006;8:687–693. doi: 10.1016/j.micinf.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Alfthan G, Rissanen H, Reunanen A, Asikainen S, Knekt P. Antibodies to periodontal pathogens and stroke risk. Stroke. 2004;35:2020–2023. doi: 10.1161/01.STR.0000136148.29490.fe. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Nyyssonen K, Alfthan G, Salonen R, Laukkanen JA, Salonen JT. Serum antibody levels to Actinobacillus actinomycetemcomitans predict the risk for coronary heart disease. Arterioscler Thromb Vasc Biol. 2005;25:833–838. doi: 10.1161/01.ATV.0000157982.69663.59. [DOI] [PubMed] [Google Scholar]
- Henderson B, Nair SP, Ward JM, Wilson M. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol. 2003;57:29–55. doi: 10.1146/annurev.micro.57.030502.090908. [DOI] [PubMed] [Google Scholar]
- Meyer DH, Lippmann JE, Fives-Taylor PM. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg WJ, Yndestad A, Oie E, Smith C, Ueland T, Ovchinnikova O, Robertson AK, Muller F, Semb AG, Scholz H, Andreassen AK, Gullestad L, Damås JK, Frøland SS, Hansson GK, Halvorsen B, Aukrust P. Enhanced T-cell expression of RANK ligand in acute coronary syndrome: possible role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2006;26:857–863. doi: 10.1161/01.ATV.0000204334.48195.6a. [DOI] [PubMed] [Google Scholar]
- Higo S, Uematsu M, Yamagishi M, Ishibashi-Ueda H, Awata M, Morozumi T, Ohara T, Nanto S, Nagata S. Elevation of plasma matrix metalloproteinase-9 in the culprit coronary artery in patients with acute myocardial infarction: clinical evidence from distal protection. Circ J. 2005;69:1180–1185. doi: 10.1253/circj.69.1180. [DOI] [PubMed] [Google Scholar]
- Hosomi N, Aoki S, Matsuo K, Deguchi K, Masugata H, Murao K, Ichihara N, Ohyama H, Dobashi H, Nezu T, Ohtsuki T, Yasuda O, Soejima H, Ogawa H, Izumi Y, Kohno M, Tanaka J, Matsumoto M. Association of serum anti-periodontal pathogen antibody with ischemic stroke. Cerebrovasc Dis. 2012;34:385–392. doi: 10.1159/000343659. [DOI] [PubMed] [Google Scholar]
- Lally ET, Hill RB, Kieba IR, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–361. doi: 10.1016/S0966-842X(99)01530-9. [DOI] [PubMed] [Google Scholar]
- Fine DH, Kaplan JB, Kachlany SC, Schreiner HC. How we got attached to Actinobacillus actinomycetemcomitans: a model for infectious diseases. Periodontol 2000. 2006;42:114–157. doi: 10.1111/j.1600-0757.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Schreiner HC, Furgang D, Fine DH. Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients. J Clin Microbiol. 2002;40:1181–1187. doi: 10.1128/JCM.40.4.1181-1187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


