Abstract
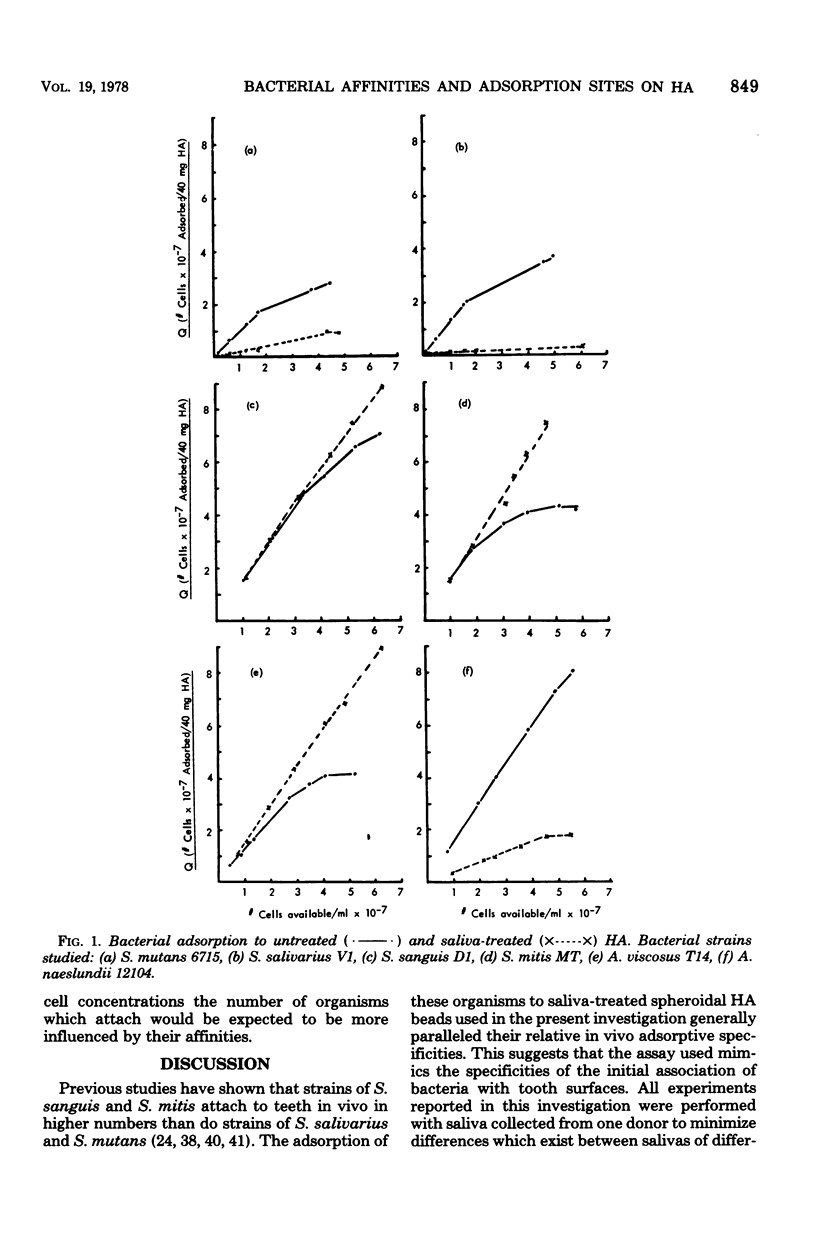
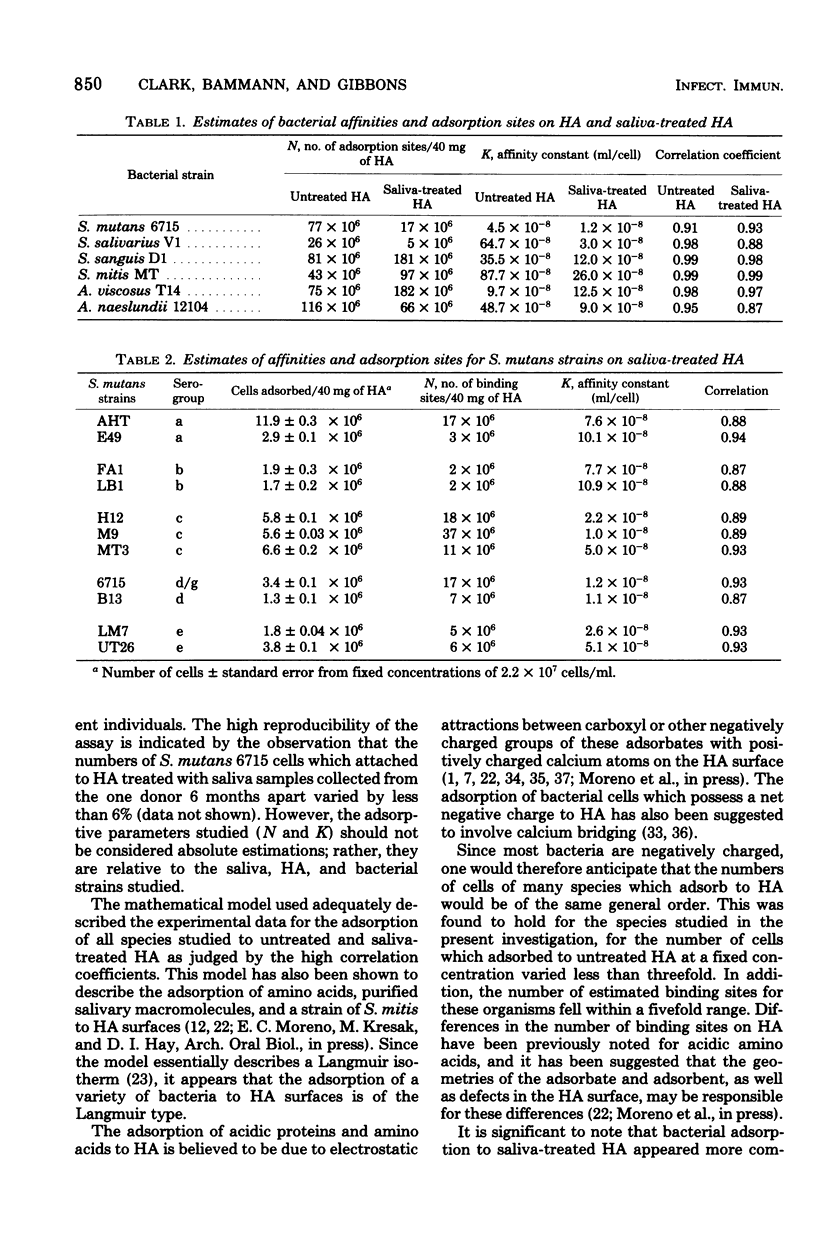
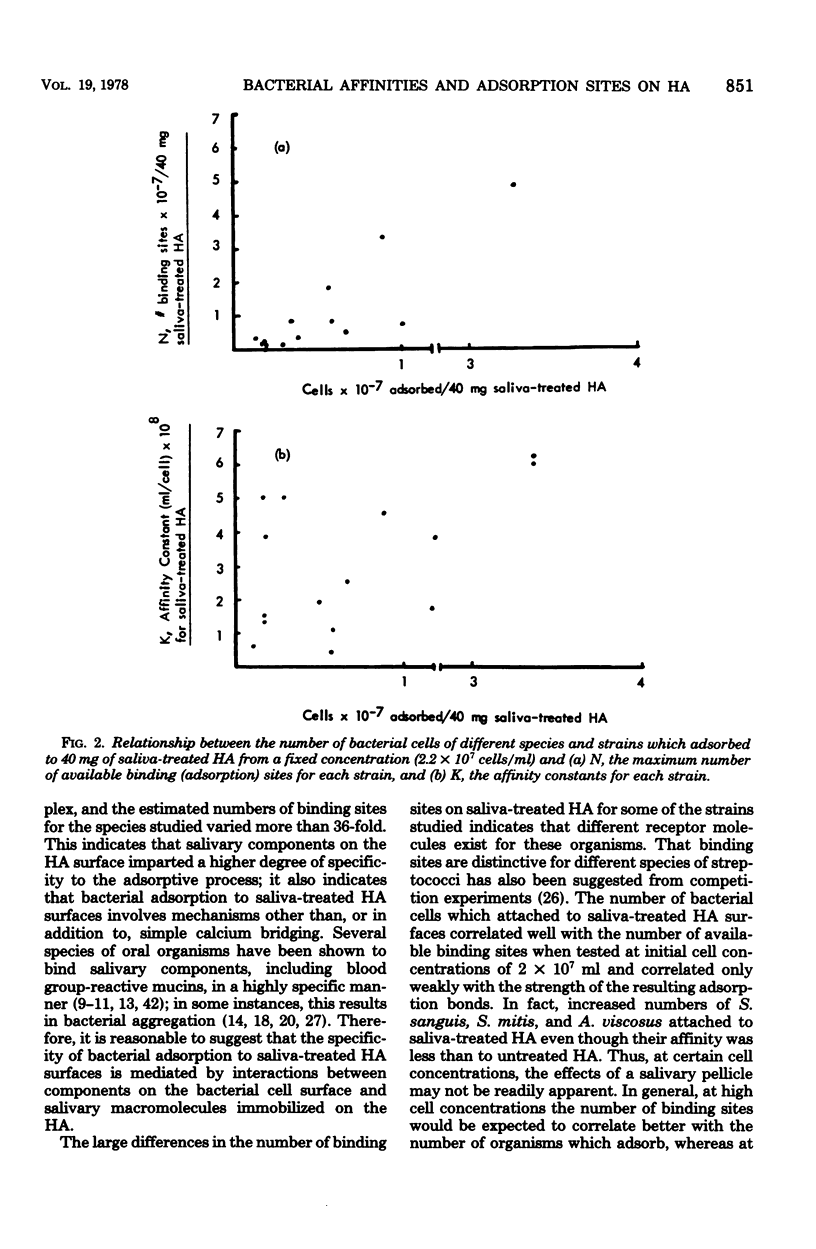
The adsorption of strains of prominent oral bacteria to hydroxyapatite (HA) surfaces was studied by use of an adsorption model based on the Langmuir adsorption isotherm; this permitted comparative estimates of the number of adsorption sites and the strength of the adsorption bonds on untreated and salivatreated HA surfaces for strain of Streptococcus mutans, S. salivarius, S. sanguis, S. mitis, Actinomyces viscosus, and A. naeslundii. The experimental data closely followed the adsorption model as judged by the high correlation coefficients obtained for all strains studied. Adsorption to untreated HA was similar for strains of the six species studied, suggesting that a common adsorption mechanism, possibly Ca2+ bridging, may exist for attachment to HA. More complex interactions appeared to be involved in bacterial adsorption to saliva-treated HA since adsorption of the strains tested at unsaturating cell concentrations varied more than 30-fold. This indicates that adsorbed salivary components on HA surfaces impart a higher order of specificity for subsequent bacterial adsorption. Fewer cells of strains of S. mutans, S. salivarius, and A. naeslundii adsorbed to saliva-treated HA than to untreated HA because adsorbed salivary components presented fewer adsorption sites. Substantially higher numbers of cells of strains of S. sanguis, S. mitis, and A. viscosus adsorbed to saliva-treated HA because the film of adsorbed salivary components increased the number of adsorption sites for these strains. The affinity constants for all but one strain studied were lower on saliva-treated HA than on untreated HA. The number of bacterial cells which adsorbed to saliva-treated HA more closely related to the number of available binding sites than to the strength of their adsorption bonds when tested at an initial concentration of 2 × 107 organisms/ml. Although some differences were observed in the adsorption of strains of S. mutans representative of five serological groups, the numbers which attached to saliva-treated HA did not vary widely; this suggests that factors other than their ability to attach to a pellicle-covered HA surface may be responsible for their varying geographic distribution in human populations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G., Giro M. G., Gaillard C. Chromatography of polypeptides and proteins on hydroxyapatite columns: some new developments. Biochim Biophys Acta. 1972 Oct 31;278(3):409–420. doi: 10.1016/0005-2795(72)90001-3. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Camacho Martínez F., Armijo Moreno M., Naranjo Sintes R., Serrano Ortega S., de Dulanto F. Hipodermitis nodular subaguda migratriz. Actas Dermosifiliogr. 1977 May-Jun;68(5-6):283–292. [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embery G., Rolla G. Chemistry of the sulfate groups in a sulfated glycoprotein from rabbit submandibular gland. Scand J Dent Res. 1977 May;85(4):241–246. doi: 10.1111/j.1600-0722.1977.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Ericson T., Magnusson I. Affinity for hydroxyapatite of salivary substances inducing aggregation of oral streptococci. Caries Res. 1976;10(1):8–18. doi: 10.1159/000260185. [DOI] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Ericson T. Salivary glycoproteins. Composition and adsorption to hydroxylapatite in relation to the formation of dental pellicles and calculus. Acta Odontol Scand. 1968 May;26(1):3–21. doi: 10.3109/00016356809004577. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hay D. I. The adsorption of salivary proteins by hydroxyapatite and enamel. Arch Oral Biol. 1967 Aug;12(8):937–946. doi: 10.1016/0003-9969(67)90088-x. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Kashket S., Guilmette K. M. Aggregation of oral streptococci in the presence of concanavalin A. Arch Oral Biol. 1975 May-Jun;20(5-6):375–379. doi: 10.1016/0003-9969(75)90030-8. [DOI] [PubMed] [Google Scholar]
- Keene H. J., Shklair I. L., Mickel G. J., Wirthlin M. R. Distribution of Streptococcus mutans biotypes in five human populations. J Dent Res. 1977 Jan;56(1):5–10. doi: 10.1177/00220345770560011101. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Competitive binding among oral strptococci to hydroxyapatite. J Dent Res. 1977 Feb;56(2):157–165. doi: 10.1177/00220345770560021001. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Ericson T., Pruitt K. Effect of slivary agglutinins on bacterial colonization of tooth surfaces. Caries Res. 1976;10(2):113–122. doi: 10.1159/000260195. [DOI] [PubMed] [Google Scholar]
- Mayhall C. W. Amino acid composition of experimental salivary pellicles. J Periodontol. 1977 Feb;48(2):78–91. doi: 10.1902/jop.1977.48.2.78. [DOI] [PubMed] [Google Scholar]
- Mayhall C. W., Butler W. T. The carbohydrate composition of experimental salivary pellicles. J Oral Pathol. 1976 Nov;5(6):358–370. doi: 10.1111/j.1600-0714.1976.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Mayhall C. W. Concerning the composition and source of the acquired enamel pellicle of human teeth. Arch Oral Biol. 1970 Dec;15(12):1327–1341. doi: 10.1016/0003-9969(70)90021-x. [DOI] [PubMed] [Google Scholar]
- Olsson J., Krasse B. A method for studying adherence of oral streptococci to solid surfaces. Scand J Dent Res. 1976 Jan;84(1):20–28. doi: 10.1111/j.1600-0722.1976.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolla G., Embery G. Sulfated glycoproteins in the acquired pellicle and in plaque from Macaca fascicularis demonstrated with labeled sulfate. Scand J Dent Res. 1977 May;85(4):237–240. doi: 10.1111/j.1600-0722.1977.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Rölla G., Melsen B. Desorption of protein and bacteria from hydroxyapatite by fluoride and monofluorophosphate. Caries Res. 1975;9(1):66–73. doi: 10.1159/000260144. [DOI] [PubMed] [Google Scholar]
- Rölla G., Melsen B., Sönju T. Sulphated macromolecules in dental plaque in the monkey Macaca irus. Arch Oral Biol. 1975 May-Jun;20(5-6):341–343. doi: 10.1016/0003-9969(75)90024-2. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Sundström B., Zelander T., Lobo R. Some observations on the morphology of in vitro decalcified incipient enamel caries. Odontol Revy. 1972;23(1):1–7. [PubMed] [Google Scholar]
- Sönju T., Rölla G. Chemical analysis of the acquired pellicle formed in two hours on cleaned human teeth in vivo. Rate of formation and amino acid analysis. Caries Res. 1973;7(1):30–38. doi: 10.1159/000259822. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



