Abstract
Intracranial (i.c.) lymphocytic choriomeningitis virus (LCMV) infection of mice results in T cell-driven anorexia and weight loss, which is diminished in males compared to females. We investigated sex-specific effects on antigen-presenting cells (APCs) and T cells after i.c. LCMV infection. Numbers of LCMV-specific T cells, APC activation, and levels of inflammatory cytokines and chemokines in CSF were decreased in males compared to females. Orchidectomy enhanced these immune parameters in males, while dihydrotestosterone treatment of orchidectomized males decreased some of these parameters. These data suggest that qualitative and quantitative effects of androgens on APCs and T cells may contribute to the well-known, but poorly understood sex differences in immunity and autoimmunity.
Keywords: Viral infection, neuroimmunology, cytokines, T cells, MHC II, androgens
1. Introduction
Sex differences are apparent in susceptibility to both infection and autoimmune disease. For example, males are more susceptible to many bacterial, viral, fungal, and parasitic infections and have a higher risk for septic shock following multiple-injury trauma (Klein, 2000; Offner et al., 1999). In contrast, females are more susceptible to autoimmune diseases such as systemic lupus erythematosus (SLE), Graves’ disease, rheumatoid arthritis, and multiple sclerosis (MS), among others (Ansar Ahmed et al., 1986; Cutolo et al., 2004; Olsen and Kovacs, 1996; Whitacre, 2001). These sex differences are also observed in animal models of infection and autoimmune diseases (Ansar Ahmed et al., 1986; Harbuz et al., 1995; Klein, 2000; Roubinian et al., 1978; Voskuhl et al., 1996). Overall, this suggests that the activity of the immune system is more robust in females compared to males. Estrogens may contribute enhanced immune responses in females, as estrogens have been shown to enhance antibody-mediated responses to exogenous antigens (Brick et al., 1985), T cell cytotoxicity (Hu et al., 1988; Muller et al., 1995; Yang et al., 2006), cytokine and chemokine levels (Hu et al., 1988; Yang et al., 2006), and APC activation (Hu et al., 1988; Weinstein et al., 1984). However, estrogens have also been shown to be dampen the immune response in various in vitro and in vivo models, at both physiologic and supraphysiologic levels (Barreto et al., 2007; Bebo et al., 2001; Dimayuga et al., 2005; Ito et al., 2001). Thus, given the context, estrogens can have stimulatory or inhibitory effects on immune responses.
Conversely, substantial evidence in autoimmune models suggests that immune-suppressing effects of androgens underlie the observed sex differences in immunity. For example, removal of endogenous androgens from male mice via orchidectomy resulted in increased severity of experimental autoimmune encephalitis (EAE) (Bebo et al., 1998), arthritis (Harbuz et al., 1995), and autoimmune thyroiditis (Ansar Ahmed et al., 1986), while exogenous androgen administration was protective in EAE and other immune models (Ansar Ahmed et al., 1986; Fox, 1992; Harbuz et al., 1995; Palaszynski et al., 2004). Androgens have also been shown to decrease MHC II expression on APCs (Barreto et al., 2007; Matejuk et al., 2005), decrease T cell proliferation (Jacobson and Ansari, 2004; Matejuk et al., 2005), and alter the cytokine profile of activated T cells to decrease inflammation (Dalal et al., 1997; Liva and Voskuhl, 2001), possibly via the molecule PPARα (Dunn et al., 2007). Thus, in autoimmune models, it is clear that androgens dampen the immune response. However, in infectious models, relatively few studies have examined the effects of androgens on numbers of antigen-specific T cells and in vivo cytokine/chemokine production.
Sex differences have been reported in a well-studied mouse model of infection with lymphocytic choriomeningitis virus (LCMV) (Hildeman and Muller, 2000; Muller et al., 1995). Intracranial (i.c.) infection of wild-type mice with LCMV results in meningitis and death 6–8 days later. Disease is mediated by the immune response to the virus, as infection of irradiated or T cell-depleted mice leads to a persistent infection of multiple tissues without the development of lethal meningitis (Doherty and Zinkernagel, 1974; Hirsch et al., 1968; Rowe, 1956). Infection of β2-microglobulin-deficient mice (β2m−/− mice) results in persistent infection, meningitis, and the production of pro-inflammatory cytokines (Doherty et al., 1993; Fung-Leung et al., 1991; Hildeman et al., 1996; Lehmann-Grube et al., 1993). Meningitis in these mice occurs with a later onset and lower severity compared to wild-type mice (Doherty et al., 1993; Fung-Leung et al., 1991; Muller et al., 1995; Muller et al., 1992; Quinn et al., 1993). Interestingly, i.c. LCMV infection of β2m−/− mice also causes profound anorexia and weight loss (Hildeman and Muller, 2000; Kamperschroer and Quinn, 2002; Lehmann-Grube et al., 1993; Muller et al., 1995; Quinn et al., 1993), which are mediated by MHC class II-restricted, CD4+ T cells (Doherty et al., 1993; Lehmann-Grube et al., 1993; Muller et al., 1992; Quinn et al., 1993). Wild-type mice also undergo weight loss after infection but succumb to CD8+ T cell-mediated meningitis shortly thereafter (Hildeman and Muller, 2000), making it difficult to study of this particular aspect of disease. Anorexia and weight loss do not occur after intraperitoneal (i.p.) or intravenous (i.v.) infection with LCMV (Hildeman and Muller, 2000; Kamperschroer and Quinn, 2002), suggesting that interactions between the central nervous system (CNS) and the immune system are critical for disease.
Interestingly, male β2m−/− mice are less susceptible to LCMV-induced mortality and have decreased anorexia and weight loss compared to female β2m−/− mice (Hildeman and Muller, 2000; Muller et al., 1995). Susceptibility of male mice to disease can be increased either by orchidectomy and estrogen treatment (Muller et al., 1995) or prior vaccination against LCMV (Hildeman et al., 1997). Here, we took advantage of this model of LCMV infection in β2m−/− mice to study in more detail the specific aspects of the immune response (i.e., APC activation, antigen-specific T cell response) that are affected by sexual dimorphisms. Our results suggest that androgens dampen antigen-specific T cell expansion and, to a lesser extent, APC activation during viral infection. These results have implications for the often observed, but poorly understood sex differences in immune responses and susceptibility to autoimmunity.
2. Materials and Methods
2.1. Virus
The Armstrong-3 strain of LCMV was kindly provided by Dr. Rafi Ahmed (Emory University, Atlanta, GA). Virus was grown in BHK-21 cells (American Type Culture Collection, Manassas, VA), and viral titers of supernatants were determined by plaque assay on Vero cells (Ahmed et al., 1984). Virus was diluted in PBS prior to injection in mice.
2.2. Mice and virus infection
B6.129P2-B2mtm1Unc/J (β2m−/−) mice (Koller et al., 1990) were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were used within 8–16 weeks of age. Mice were housed and bred under specific pathogen-free conditions in the animal facility in the Cincinnati Children’s Hospital Research Foundation. Experimental procedures were reviewed and approved by the institutional animal care and use committee (IACUC) at the Cincinnati Children’s Hospital Research Foundation. For i.c. infection with LCMV, mice were anesthetized by i.p. injection of ketamine/xylazine (100 mg/ml ketamine + 20 mg/ml xylazine mixture in saline) and then injected i.c. with 1 × 103 pfu LCMV in 30 μl using a tuberculin syringe. Mock-infected mice received i.c. injections of 30 μl PBS.
2.3. Measurement of weight loss and food intake
β2m−/− mice were individually housed and allowed to acclimate for one week before infection. Mice were allowed ad libitum access to food and water, and mice and their food were weighed daily between 1200 and 1400h. 24-hour food intake was determined by weighing remaining food and subtracting that value from the previous day’s amount. Percent loss of body weight was calculated as [[(weight at day after infection) – (weight at day of LCMV infection)]/weight at day of LCMV infection] × 100.
2.4. Cell preparations
Brains from individual mice were harvested into balanced salt solution (BSS) and crushed through 100 μm strainers to generate single-cell suspensions. Cells were centrifuged for 5 minutes at 480 × g at 4°C. Cell pellets were resuspended in a mixture of 70% Percoll (GE Healthcare, Piscataway, NJ) in PBS and overlaid with 30% Percoll in PBS. After centrifugation at 480 × g at 4°C, cells were collected at the resulting interface and washed in BSS and S-MEM culture medium (Invitrogen, Carlsbad, CA) + 5% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) before use in experiments. Cervical lymph nodes from individual mice were harvested into BSS and crushed through 100 μm cell strainers to generate single-cell suspensions. Cells were centrifuged at 480 × g at 4°C and resuspended in S-MEM + 5% FBS before use in experiments.
2.5. Cell staining and flow cytometric analysis
Brain cells (3 × 105) from individual mice were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD11b, allophycocyanin (APC)-conjugated anti-CD11c, phycoerythrin – Cy7 (PE-Cy7)-conjugated anti-CD45, and phycoerythrin (PE)-conjugated anti-MHC class II antibodies (eBioscience, San Diego, CA or produced in house) in the presence of unlabeled blocking antibody (24.G2 supernatant produced in house) and fixed with 2% paraformaldehyde before analysis.
For analysis of LCMV-specific T cells, MHC class II tetrameric staining reagents were created as described (Crawford et al., 1998; Wojciechowski et al., 2006). The I-Ab gp61-80 tetramer detects T cells specific for LCMV glycoprotein amino acids 61-80 (Homann et al., 2001; Oxenius et al., 1995; Wojciechowski et al., 2006). 2 × 106 cervical lymph node cells or 6 × 105 brain mononuclear cells from each mouse were incubated with PE-conjugated I-Ab LCMV gp61-80 tetramer and stained with FITC-conjugated anti-CD44, PE-Cy7-conjugated anti-CD16/32, and APC-conjugated anti-CD4 antibodies (eBioscience or BD Biosciences, San Jose, CA).
For bromodeoxyuridine (BrdU) staining, mice were injected i.p. with 1 mg BrdU (Calbiochem, Gibbstown, NJ) dissolved in PBS on days 4, 5, 6, and 7 after infection. LN cells were stained for BrdU incorporation with a FITC anti-BrdU staining kit (BD Biosciences) according to manufacturer’s directions and were co-stained with I-Ab gp61-80 tetramer.
Data were acquired on a FACSCalibur (BD Biosciences) and analyzed using CellQuest Pro software (BD Biosciences). Live cells were determined by forward and side light scatter. For figures 2, 4, and 6, live cells were sent to CD4 vs. CD16/32 dot plots. CD4-positive, CD16/32-negative cells were sent to CD44 vs. I-Ab gp61-80 tetramer dot plots to determine the frequency of CD44-positive, tetramer-positive cells. For figure 2F, CD4-positive cells were sent to BrdU vs. I-Ab gp61-80 tetramer dot plots and tetramer-positive, BrdU-positive and-negative cells were sent to histograms for BrdU to determine the amount of BrdU incorporation. For figures 3, 5, and 7, live cells were sent to CD45 histograms to determine populations with bright (CD45high) and intermediate (CD45int) staining for CD45. CD45high and CD45int cells were sent to CD11c vs. CD11b dot plots as shown previously (Lin et al JV 2009). Populations from these plots were sent to MHC class II vs. CD11b dot plots to determine levels of MHC class II expression.
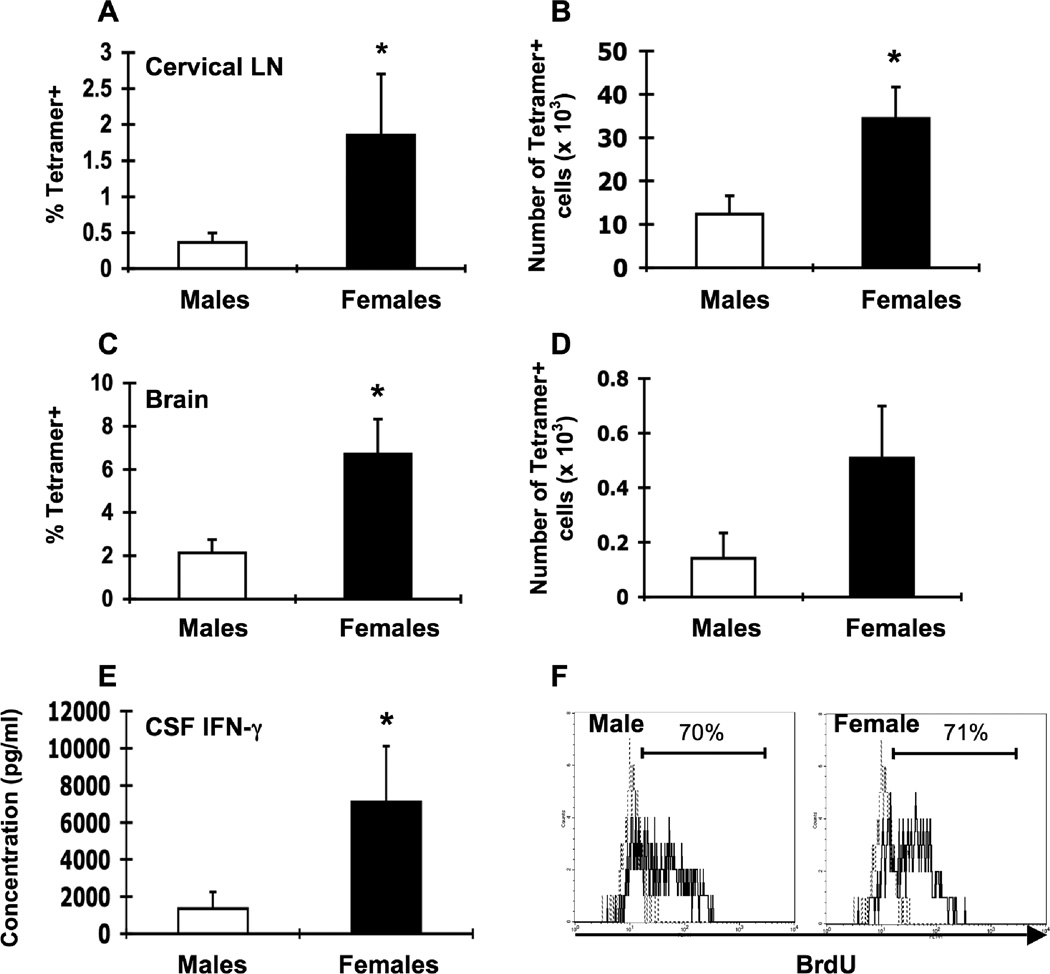
Figure 2.
Lower LCMV-specific T cell responses in male β2m−/− mice. Groups of male (white bars, n = 5–7) and female (black bars, n = 5–7) β2m−/− mice were infected i.c. with 1 × 103 pfu LCMV, sacrificed eight days later, and perfused with 10 ml PBS. Mononuclear cells from cervical LN and brains were isolated, stained with I-Ab gp61-80 tetramer, and analyzed by flow cytometry. Percentages (A, C) and total numbers (B, D) of tetramer-positive cells in the cervical LN (A, B) and brain (C, D) or infected males and infected females. (E) IFN-γ levels in the CSF. Data represent the mean ± SEM and are pooled from two independent experiments and are representative of at least three independent experiments with similar results. *p ≤ 0.05 (Mann-Whitney test, Minitab for Windows). (F) Groups of male and female β2m−/− mice were infected i.c. with 1 × 103 pfu LCMV. Mice received i.p. injections of 1 mg BrdU on days 4–7 after infection and proliferation of LCMV-specific T cells was assessed on day 8 by I-Ab gp61-80 tetramer staining and intracellular BrdU staining and flow cytometric analysis. Representative histograms show the frequency of CD4+ I-Ab gp61-80 gated cells that were BrdU+ in cervical LN from infected male (left) and female (right) mice (solid lines). Dotted lines represent BrdU staining in an infected mouse that did not receive BrdU injections.
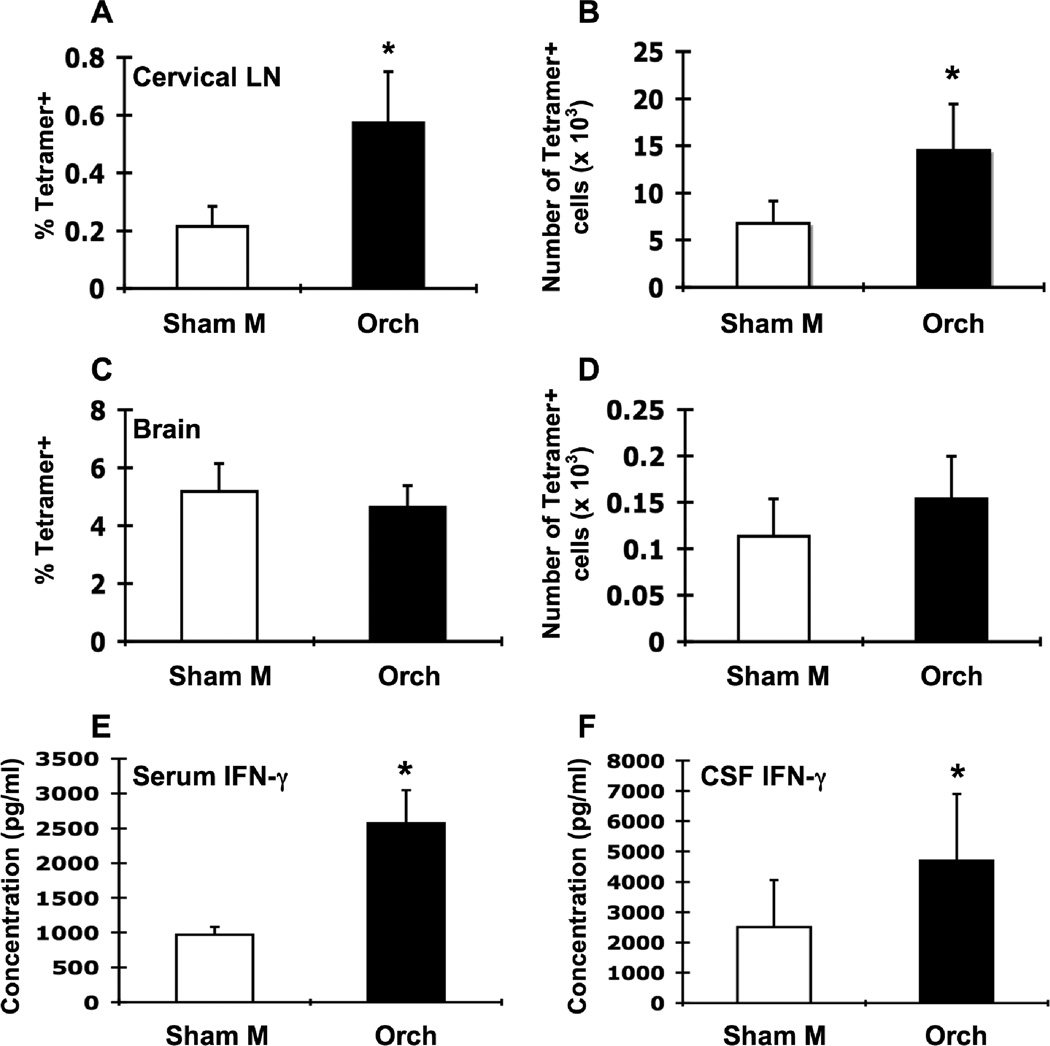
Figure 4.
Orchidectomy of male β2m−/− mice increases LCMV-specific T cell responses in the cervical LN. Groups of male β2m−/− mice were sham-operated (Sham M; white bars, n = 7) or orchidectomized (Orch; black bars, n = 7), infected i.c. with 1 × 103 pfu LCMV, sacrificed at day 8 of infection, and perfused with 10 ml PBS. Mononuclear cells from cervical LN and brains were isolated, stained with I-Ab gp61-80 tetramer, and analyzed by flow cytometry. Percentages (A, C) and total numbers (B, D) of tetramer-positive cells in the cervical LN (A, B) and brain (C, D) of infected sham-operated males and infected orchidectomized males. IFN-γ levels in the serum (E) and CSF (F). Data represent the mean ± SEM and are pooled from two independent experiments and are representative of at least three independent experiments with similar results. *p ≤ 0.05 (Mann-Whitney test, Minitab for Windows).
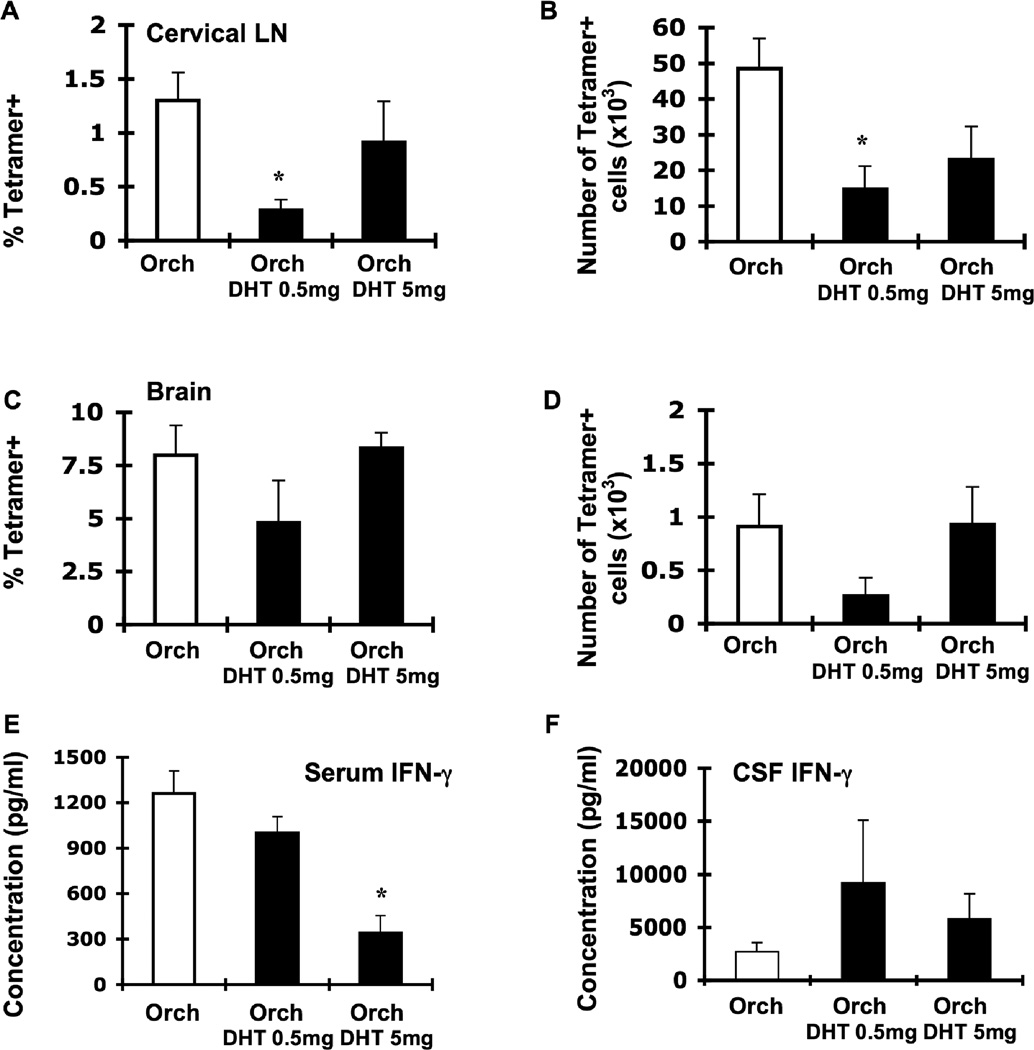
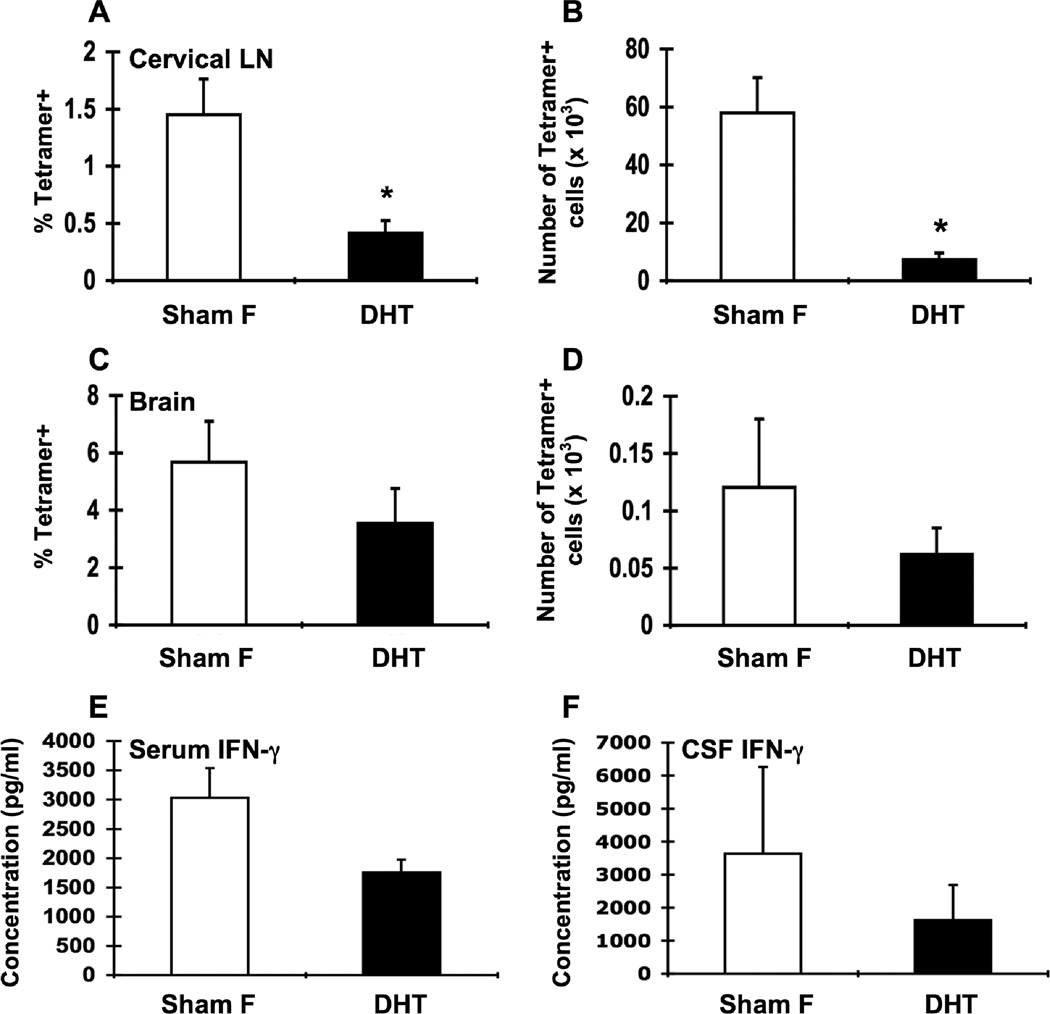
Figure 6.
Androgen replacement in orchidectomized male β2m−/− mice decreases LCMV-specific T cell responses in the cervical LN. Groups of male β2m−/− mice were orchidectomized and either sham-operated (Orch; white bars, n = 11 except figure E: n = 7) or implanted with DHT pellets (Orch DHT 0.5 mg and Orch DHT 5 mg; black bars, n = 5 for each dose except figure E: n = 6 for each dose). Animals were infected i.c. with 1 × 103 pfu LCMV, sacrificed at day 8 of infection, and perfused with 10 mL PBS. Mononuclear cells from cervical LN and brains were isolated, stained with I-Ab gp61-80 tetramer, and analyzed by flow cytometry. Percentages (A, C) and total numbers (B, D) of tetramer-positive cells in the cervical LN (A, B) and brain (C, D) of infected sham-operated orchidectomized males and infected DHT-treated orchidectomized males. IFN-γ levels in the serum (E) and CSF (F). Data represent the mean ± SEM and are pooled from two independent experiments. *p ≤ 0.02 (Mann-Whitney test, Minitab for Windows).
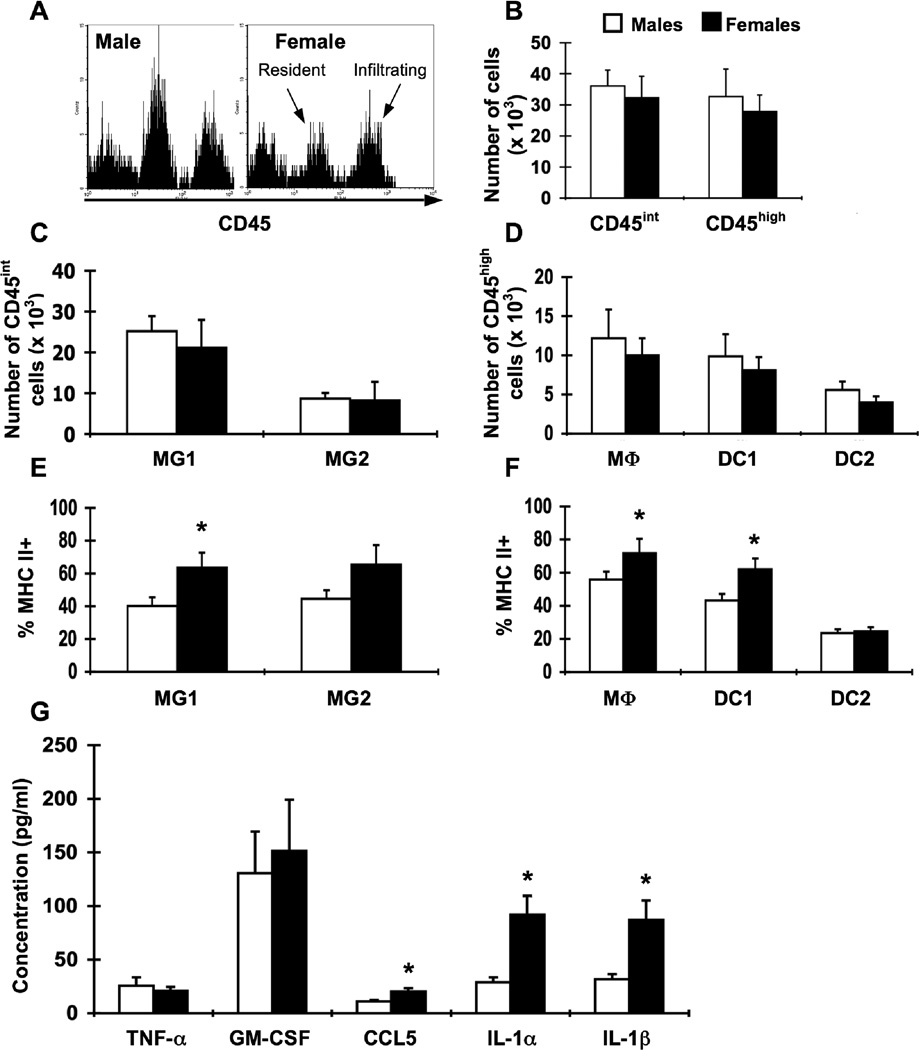
Figure 3.
Sex differences in APC activation in the brain at day 8 of i.c. LCMV infection. Groups of male (white bars, n = 5–7) and female (black bars, n = 5–7) β2m−/− mice were infected i.c. with 1 × 103 pfu LCMV, sacrificed eight days later, and perfused with 10 ml PBS. Mononuclear cells from brains were isolated, stained for APC markers, and analyzed by flow cytometry. (A) Representative histograms for CD45 expression on brain cells isolated from LCMV-infected male (left) and female (right) mice. Events in histograms were gated on live cells determined by forward and side light scatter. Peaks corresponding to resident CNS cells and infiltrating cells are indicated. (B) Numbers of CD45int and CD45high cells in the brain in infected male and female mice. (C) Numbers of CD45int cells in the brain among the identified APC populations (as defined in Table 1). (D) Numbers of CD45high cells in the brain among the identified APC populations (as defined in Table 1). (E) Percentages of MHC class IIhigh cells within resident APC populations in the brain. (F) Percentages of MHC class IIhigh cells within hematopoietic APC populations in the brain. (G) Levels of cytokines and chemokines in the CSF. CSF was harvested from individual perfused mice and analyzed by multiplex. Data represent the mean ± SEM and are representative of at least three independent experiments with similar results. *p ≤ 0.04 (Mann-Whitney test, Minitab for Windows).
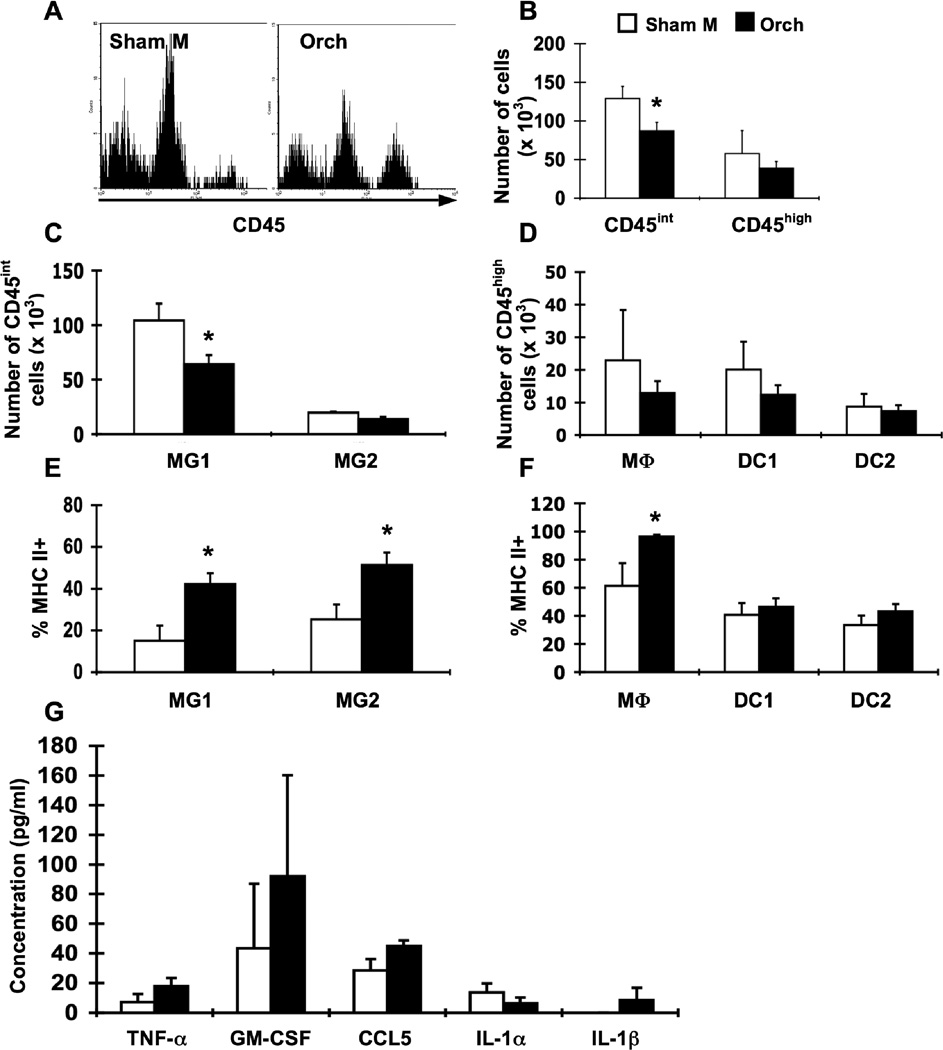
Figure 5.
Orchidectomy of male β2m−/− mice alters resident CNS cell populations and enhances APC activation. Groups of male β2m−/− mice were sham-operated (Sham M; white bars, n = 7) or orchidectomized (Orch; black bars, n = 7). Animals were infected i.c. with 1 × 103 pfu LCMV, sacrificed at day 8 of infection, and perfused with 10 ml PBS. Mononuclear cells from brains were isolated, stained for APC markers, and analyzed by flow cytometry. (A) Representative histograms for CD45 expression on brain cells isolated from LCMV-infected sham-operated males (left) and orchidectomized males (right). Events in histograms were gated on live cells as determined by forward and side light scatter. (B) Numbers of CD45int and CD45high cells in the brain in infected sham-operated males and orchidectomized males. Decreased numbers of CD45int cells are observed in orchidectomized males. (C) Numbers of CD45int cells in the brain among the identified APC populations (as defined in Table 1). Decreased numbers of MG1 microglia are observed in orchidectomized males. In the experiment shown, there is also a significant decrease in MG2 microglia, although this effect was minor and was not consistently observed. (D) Numbers of CD45high cells in the brain among the identified APC populations (as defined in Table 1). (E) Percentages of MHC class IIhigh cells within resident APC populations in the brain. (F) Percentages of MHC class IIhigh cells within hematopoietic APC populations in the brain. (G) Levels of cytokines and chemokines in the CSF. CSF was harvested from individual perfused mice and analyzed by multiplex. Data represent the mean ± SEM and are representative of at least two experiments with similar results. *p ≤0.04 (Mann-Whitney test, Minitab for Windows).
Figure 7.
DHT treatment of female β2m−/− mice decreases LCMV-specific T cell responses in the cervical LN. Groups of female β2m−/− mice were sham-operated (Sham F; white bars, n = 6) or implanted with DHT pellets (DHT; black bars, n = 6), infected i.c. with 1 × 103 pfu LCMV, sacrificed at day 8 of infection, and perfused with 10 ml PBS. Mononuclear cells from cervical LN and brains were isolated, stained with I-Ab gp61-80 tetramer, and analyzed by flow cytometry. Percentages (A, C) and total numbers (B, D) of tetramer-positive cells in the cervical LN (A, B) and brain (C, D) of infected sham-operated females and infected DHT-treated females. INF-γ levels in the serum (E) and CSF (F). Data represent the mean ± SEM and are representative of two independent experiments. *p ≤ 0.03 (Mann-Whitney test, Minitab for Windows).
2.6. CSF harvest
Mice were anesthetized by i.p. injection of ketamine/xylazine and perfused with 10 ml PBS. CSF was harvested from the cisterna magna as described previously (Fleming et al., 1983). Briefly, the skin, subcutaneous tissue, and muscle over the posterior neck were reflected to expose the arachnoid membrane covering the cisterna magna. A 27-guage needle attached to a capillary tube was used to puncture the membrane and slight suction applied to harvest CSF. Proper perfusion was confirmed by the absence of blood from the arteries surrounding the cisterna magna. CSF was harvested into 20 μl S-MEM culture medium.
2.7. Multiplex analysis of CSF
Detection of IL-1α, IL-1β, GM-CSF, TNF-α, CCL5, and IFN-γ in diluted CSF was performed using a Mouse Cytokine/Chemokine LINCOplex kit (Linco Research/Millipore, St. Charles, MO) according to manufacturer’s instructions. Samples from individual mice were analyzed in separate wells. Concentrations of analytes were calculated as (observed concentration) × [(amount CSF harvested)/(25 μl)].
2.8. Orchidectomy
Male β2m−/− mice underwent orchidectomy or sham surgery under isoflurane anesthesia. The fur over the scrotum was removed using an electric razor, the area was sterilized using betadine and isopropyl alcohol, and a central incision of the scrotum was made. Testicles were were retracted from the scrotum, sutured off with absorbable 4-O Vicryl suture (Ethicon/Johnson & Johnson, Cincinnati, OH), and removed, and the incision was closed with sutures. After surgery, mice were fed food containing doxycycline and given at least 10 days to recover before i.c. infection with LCMV. Sham-operated mice underwent the same procedure, except that the testicles were neither sutured off nor removed.
2.9. DHT pellet implantation
Orchidectomized male β2m−/− or intact female β2m−/− mice underwent implantation of a 21-day release 5α-dihydrotestosterone (DHT) pellet (Innovative Research of America, Sarasota, FL) or sham surgery under isoflurane anesthesia. The fur over the scapular area of the neck was removed using an electric razor and the area was sterilized using betadine and isopropyl alcohol. Pellets were implanted subcutaneously using forceps. After pellet implantation, the incision was closed with sutures. After surgery, mice were given at least 10 days to recover before i.c. infection with LCMV. Control orchidectomized male or intact female mice receiving sham surgery underwent the same procedure, except that no pellet was inserted.
2.10. Measurement of serum testosterone and estradiol levels
Blood was collected from mice via cardiac puncture into Microtainer serum separator tubes (Becton Dickinson, Franklin Lakes, NJ) just prior to perfusion of each mouse with PBS. Blood was allowed to clot at room temperature for at least 30 minutes. Tubes were spun at 16, 000 × g for 5 minutes and serum collected from the top of the tube. Serum was then used to determine estradiol and testosterone levels by Estradiol and Testosterone ELISA Kits (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s directions, except that an extra dilution of the testosterone standard was performed to give a standard curve starting at 250 pg/ml instead of 500 pg/ml. Serum was diluted 1:10—1:20 in assay diluent (provided in kits) before use. Inter- and intra-assay variation for both assays was < 20% and < 4%, respectively. The lower limit of detection for the testosterone assay was 10.24 pg/ml. There was 27% cross-reactivity with DHT in the testosterone ELISA. The lower limit of detection for the estradiol assay was 25.1 pg/ml.
2.11. IFN-γ ELISA on mouse serum
Serum was diluted 1:20—1:60 in S-MEM culture medium and incubated for 2 hours at room temperature in ELISA wells coated with 1 μg/ml AN18 antibody (produced in house). Recombinant murine IFN-γ (R&D Systems, Minneapolis, MN) was used as a standard, giving reliable sensitivity to 12.5 pg/ml. Secondary antibody (biotinylated R4.6A2; produced in house) was added at 500 ng/ml and incubated for 1 hour at room temperature. Strepdavidin HRP (eBioscience) was added at 1:1000 and incubated for 30 minutes at room temperature. TMB substrate (eBioscience) was added, and the plate read on a plate reader (Molecular Devices, Sunnayvale, CA) at 450 nm.
2.12. Statistical analyses
Statistical analyses were performed using a Mann-Whitney test. For weight loss studies, a repeated measures analysis of variance (ANOVA) was performed. For hormone studies, Student’s t-test was performed. Analyses were calculated using Minitab for Windows software, Release 14 (Minitab, Inc., State College, PA). All experiments were performed a minimum of two times. P-values less than or equal to 0.05 were considered significant.
3. Results
3.1. Males exhibit less severe immunopathologic, anorexic weight loss after i.c. LCMV infection
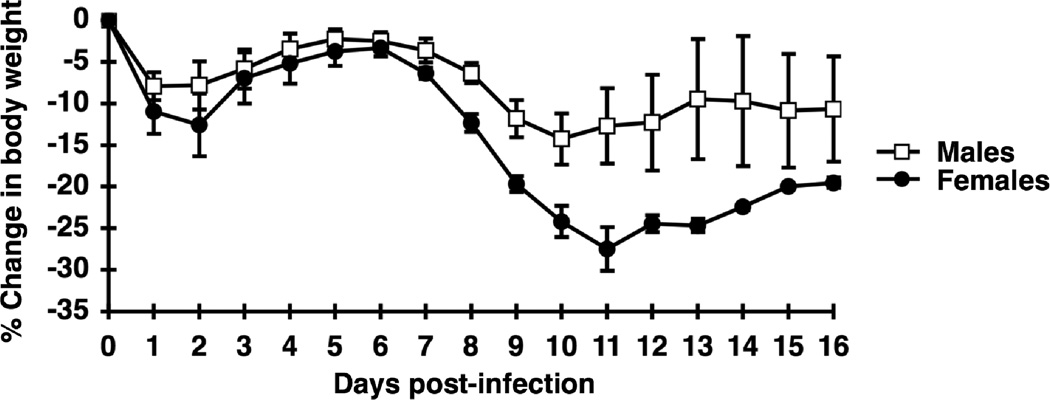
Previous studies have shown that female β2m−/− mice exhibit increased susceptibility to disease after i.c. LCMV infection, with a higher mortality rate and increased weight loss (Hildeman and Muller, 2000; Muller et al., 1995). These results were obtained with β2m−/− mice of mixed genetic background (129/Ola × C57BL/6). To confirm these results with mice backcrossed to C57BL/6 for 11 generations, we infected groups of backcrossed male and female β2m−/− mice i.c. with LCMV and monitored them daily for food intake and weight loss. Similar to previously published results, weight loss began at the same time in both male and female mice and progressed less in male mice, with male mice losing ~14% of their initial body weight compared to a ~27% loss in females (Fig. 1). Also consistent with previous results, weight loss was concurrent with a sharp decrease in food intake in both female and male mice, with a greater decrease in food intake in female mice compared to males (data not shown). Thus, our data confirm that male β2m−/− mice have less susceptibility to anorexic weight loss following i.c. LCMV infection and that the observed sex differences are not due to any effects of the 129/Ola background.
Figure 1.
Less severe immunopathologic weight loss in male β2m−/− mice. Male (open squares, n = 4) or female (closed circles, n =5) β2m−/− mice were infected i.c. with LCMV. Mice were allowed ad libitum access to food and monitored daily for food intake and weight changes. Percent loss of body weight was calculated as described in Materials and Methods. Data represent the mean ± SEM and are representative of three separate experiments with similar results. A significant difference was observed for the change in weight between male and female mice for days 7–16, p < 0.01 (repeated measures ANOVA, Minitab for Windows).
3.2. Males show decreased LCMV-specific T cell responses compared to females
As the T cell response is the cause of LCMV-induced disease (Doherty et al., 1993; Muller et al., 1992; Quinn et al., 1993), we examined immune responses in the CNS in LCMV-infected male and female β2m−/− mice at days 6, 8, and 12 after infection. At day 6 after infection, cytokines and infiltrating immune cells were barely detectable in the CNS (data not shown). Cytokines peaked on day 8 after infection and were dramatically decreased by day 12; immune cells were present at day 8 but failed to show sex differences beyond that time point (data not shown). To assess sex differences in the T cell response in the CNS after i.c. LCMV infection, we infected groups of male and female β2m−/− mice, sacrificed them eight days later, and stained cervical LN and mononuclear brain cells with tetramers that detect LCMV-specific T cells (I-Ab gp61-80 tetramers) (Homann et al., 2001; Oxenius et al., 1995; Wojciechowski et al., 2006). In the cervical LN and brain, the frequency and absolute number of LCMV-specific CD4+ T cells were 3–4 fold lower in male mice compared to females (Fig. 2A-D). To determine whether altered proliferation contributed to sex differences in T cell responses, we assessed T cell proliferation in vivo by infecting groups of male and female mice with LCMV and injecting them daily with BrdU. Incorporation of BrdU by LCMV-specific T cells was not different between males and females (Fig. 2F), suggesting that the observed sex differences in numbers of antigen-specific T cells was not due to differences in proliferation between days 4–7 after infection.
On day 8 after infection, males had lower levels of IFN-γ in the CSF compared to female mice (Fig. 2E). At this time point, IFN-γ production is T cell-dependent (Campbell et al., 1994; Frei et al., 1988; Kamperschroer and Quinn, 2002). Thus, at day 8 after infection, whether measured by MHC tetramer staining or by in vivo IFN-γ levels, these data suggest that male mice mount a less robust LCMV-specific T cell response.
3.3. Sex differences in APC activation and CSF cytokines after i.c. LCMV infection
The magnitude of the CD4+ T cell response is controlled by the quality of APCs and the cytokine milieu. To investigate sex differences in the APC and cytokine response to LCMV infection, we infected groups of male and female β2m−/− mice i.c. with LCMV, sacrificed them at 8 days after infection, and examined immune cell populations in the brain and cytokine levels in the CSF. We identified five populations of APCs, as defined in Table 1. Resident CNS APCs, such as microglia, display intermediate staining for the leukocyte common antigen CD45 (CD45int), while infiltrating cells, such as macrophages and dendritic cells (DCs), have high staining for CD45 (CD45high) (Ford et al., 1995; Sedgwick et al., 1991). Within the CD45int cells, we identified two subsets of microglia, one being CD11b+ CD11c- and the other CD11b+ CD11c+, as previously described by others (Ford et al., 1995; Reichmann et al., 2002; Remington et al., 2007; Sedgwick et al., 1991). Within the CD45high cells, we identified three separate populations of cells. Additional stains indicated that macrophages are the majority of the CD11b+ CD11c− population (Karman et al., 2004; Reichmann et al., 2002), while most of the CD11b+ CD11c+ cells represent myeloid dendritic cells (DC1) and most of the CD11b− CD11c+ cells are plasmacytoid dendritic cells (DC2) (Karman et al., 2004; Reichmann et al., 2002) (data not shown). We note that this analysis does not reflect the heterogeneity that exists within dendritic cell populations. Nonetheless, the two major dendritic cell subsets are represented in this analysis.
TABLE 1.
APC populations in brains of i.c. LCMV-infected β2m−/− mice.1
| Cell Type | CD11b | CD11c | CD45 |
|---|---|---|---|
| Microglia subset 1 (MG1) |
+ | − | int |
| Microglia subset 2 (MG2) |
+ | + | int |
| Macrophages (MΦ) |
+ | − | high |
| Dendritic cell subset 1 (DC1) |
+ | + | high |
| Dendritic cell subset 2 (DC2) |
− | + | high |
β2m−/− mice were infected i.c. with 1 × 103 pfu LCMV, sacrificed eight days later, and perfused with 10 mL PBS. Brains were removed and mononuclear cells isolated as described in Materials and Methods. Isolated cells (3 × 105 cells/well) were blocked with 24G2 supernatant and stained with fluorescently-tagged anti-CD11b, anti-CD11c, anti-CD45, and anti-MHC class II antibodies (eBioscience, San Diego, CA) and analyzed by flow cytometry. Five different populations of cells were identified. int = intermediate fluorescence intensity; high = high fluorescence intensity. References are within the text.
At day 8 after infection, no differences were observed in the number of CD45int or CD45high cells in the brain between male and female mice (Fig. 3A, B). There were also no differences in the distribution of cells within the CD45int or CD45high populations (Fig. 3C, D). However, APCs from brains of male mice were significantly less activated, as cell surface expression of MHC class II (Fig. 3E, F) and CD86 (data not shown) on MG1 microglia, macrophages, and DC1 were both significantly decreased compared to female mice. These data suggest that although male and female β2m−/− mice have similar numbers of APCs in the CNS after infection, the activation of APCs is significantly lower in male mice. The sex differences in APC activation are not likely due to differences in the ability of the APCs to respond to signals for upregulation of MHC class II, as IFN-γ-driven upregulation of MHC class II was similar in male and female APCs in vitro (data not shown).
APC activation and recruitment to sites of inflammation are affected by cytokines and chemokines. To assess sex differences in the CSF cytokine/chemokine milieu, CSF was harvested from infected male and female β2m−/− mice at day 8 after infection with LCMV and subjected to multiplex analysis. Males had two-fold lower levels of CCL5 (RANTES) and three-fold lower levels of IL-1α and IL-1β in the CSF at day 8 after infection compared to females. No differences were seen in levels of TNF-α and GM-CSF (Fig. 3G). Thus, in addition to decreased activation of APCs, male mice also had decreased levels of particular cytokines (IL-1) and chemokines (CCL5) compared to female mice.
3.4. Orchidectomy of male mice causes a more vigorous LCMV-specific T cell response
Given that androgens are able to influence APCs, T cells, and cytokines (Klein, 2000; Olsen and Kovacs, 1996), we examined the effect of androgen deficiency on these parameters of the immune response as well as anorexia and weight loss after i.c. LCMV infection. Male β2m−/− mice were orchidectomized or sham-operated and infected i.c. with LCMV. Orchidectomy was successful, as testosterone levels were reduced approximately three-fold in orchidectomized compared to sham-operated mice (111.4 ± 8.4 pg/ml and 389.5 ± 118.1 pg/ml, respectively; p < 0.03, Student’s t-test). Estrogen levels were unaffected by orchidectomy (data not shown). After LCMV infection, orchidectomized male mice had a significantly greater loss of body weight compared to sham-operated males (−9.9% ± 3.3% vs. −15.6% ± 2.2%; p < 0.001 for days 6–15, repeated measures ANOVA).
Orchidectomy increased the percentage and number of tetramer-positive cells in the cervical LN roughly 2-3-fold compared to sham-operated males on day 8 after infection (Fig. 4A, B), which were consistent findings over several experiments. IFN-γ levels were also significantly increased in serum of orchidectomized males compared to sham-operated males (Fig. 4E). No differences were observed in the percentage or number of tetramer-positive cells between orchidectomized and sham-operated males in the brain (Fig. 4C, D); however, IFN-γ levels in the CSF were significantly increased in orchidectomized males compared to shamoperated males. Together, these data suggest that testicular androgens are able to quantitatively and qualitatively suppress the T cell response to i.c. LCMV infection.
3.5. Orchidectomy of male mice alters resident CNS populations and causes enhanced activation of APCs
We next examined the effect of orchidectomy on APCs and cytokine production by assessing immune cell populations in the brain and CSF cytokines in orchidectomized and shamoperated males after LCMV infection. Interestingly, orchidectomized males exhibit decreased numbers of CD45int cells compared to sham-operated males, particularly within the MG1 microglial population (Fig. 5A, B, C); there was no difference in the number of CD45high cells nor the distribution of cells within CD45high populations (Fig. 5A, B, D). Orchidectomy substantially increased the activation status of APCs, as MHC class II levels were increased on microglial and macrophage populations (Fig. 5E, F). Thus, orchidectomy of male β2m−/− mice reduces resident APCs in the CNS and enhances APC activation in response to i.c. LCMV infection.
To determine whether orchidectomy affected in vivo cytokine production, CSF was harvested from infected orchidectomized and sham-operated males at day 8 of LCMV infection and examined by multiplex assay. Interestingly, orchidectomy did not significantly affect levels of cytokines and chemokines in the CSF (Fig. 5G); however, a trend towards increased levels of CCL5 was observed over multiple experiments, and when pooled from 2 experiments a significant difference in CCL5 levels was observed (p<0.04). Together, these data suggest that testicular androgens predominantly affect the overall numbers of viral-specific T cells and their production of IFN-γ.
3.6. DHT treatment of orchidectomized male mice results in decreased LCMV-specific T cell responses
To more directly test that androgens were responsible for controlling CD4+ T cell responses to LCMV, we determined whether replacement of androgens in orchidectomized male mice could reduce their immune responses following infection. To do this, we implanted pellets releasing 5α-dihydrotestosterone (DHT) into orchidectomized male β2m−/− mice. DHT pellets were chosen over testosterone pellets because DHT cannot be further metabolized to estrogen as can occur with testosterone (Palaszynski et al., 2004). Pellet implantation increased androgen levels compared to orchidectomized males (orchidectomized males: 98.3 ± 22.3 pg/ml; 0.5-mg DHT: 184.7 ± 35.6 pg/ml, p = 0.06 vs. orchidectomized males; 5-mg DHT: 1355.21 ± 164.0 pg/ml, p < 0.01). Androgen levels in orchidectomized mice receiving 0.5-mg DHT pellets were not significantly different than sham-orchidectomized or intact male mice (data not shown). Although no assay exists to directly measure DHT itself, the testosterone ELISA has a 27% cross-reactivity with DHT. Therefore, mice receiving 5mg DHT pellets likely had supraphysiologic levels of androgen, while mice receiving 0.5mg DHT pellets had nearly physiologic levels of androgen. In either case, levels of circulating androgens, as measured by serum testosterone, are effectively replenished following DHT pellet implantation.
Orchidectomized male mice receiving 0.5-mg DHT pellets had significantly lower percentages and numbers of tetramer-positive cells in the cervical LN compared to non-treated orchidectomized males (Fig. 6A, B). A similar trend in orchidectomized males receiving 5-mg DHT pellets was observed (Fig. 6A, B), although there was greater variability in the numbers and frequency of tetramer+ T cells in these mice. Serum IFN-γ levels were mildly lower in mice treated in 0.5-mg DHT pellets and were decreased approximately 4-fold in mice treated with 5-mg DHT pellets compared to non-treated orchidectomized males (Fig. 6E). No significant differences were seen between DHT-treated and non-treated orchidectomized males in the percentage or number of tetramer-positive cells in the brain or in CSF levels of IFN-γ (Fig. 6C, D, F). Thus, the enhanced T cell response to LCMV infection after orchidectomy was reversible by DHT treatment, suggesting that androgens regulate T cell responses to viral infection.
3.7. DHT treatment of orchidectomized males does not significantly affect numbers or activation of resident CNS APCs
We also examined the effect of androgen replacement on CNS APC populations and CSF cytokines after i.c. LCMV infection. DHT treatment of orchidectomized male mice resulted in no difference in the numbers or distribution of CD45int or CD45high cells in the brain compared to non-treated orchidectomized males (data not shown). Similarly, there were no significant differences seen in MHC class II expression on any of the CD45int or CD45high APCs, although there was a trend toward mildly decreased percentages of MHC class II-positive microglia in DHT-treated orchidectomized males, which repeated over several experiments (data not shown). There were also no significant effects of androgen replacement on levels of CSF cytokines and chemokines as measured by multiplex (data not shown).
3.8. DHT treatment of female mice results in decreased LCMV-specific T cell responses
As androgens contributed to regulation of T cell responses in male mice, we next determined if exogenous androgen could affect the immune response to LCMV in infected female mice. To do this, we implanted 5-mg DHT pellets into female β2m−/− mice. Pellet implantation caused an approximately 5-fold increase in serum testosterone levels compared to control females (613.8 ± 59.4 pg/ml in DHT-treated females vs. 125.9 ± 13.5 pg/ml in controls; p < 0.01, Student’s t-test); serum testosterone levels in DHT-treated females were not significantly different than intact males or sham-operated males. Estrogen levels were not affected by DHT treatment (data not shown). Thus, as assessed by serum testosterone, DHT treatment increased levels of circulating androgens in intact female mice, although the levels were likely supraphysiologic.
DHT treatment of female mice markedly decreased both the percentage and number of tetramer-positive cells in the cervical LN compared to control females (Fig. 7A, B). As expected, there was a strong trend toward decreased serum levels of IFN-γ in DHT-treated females compared to control females (Fig. 7E); this trend was consistently observed in multiple experiments. No significant differences were seen between DHT-treated and control females in the percentage or number of tetramer-positive cells in the brain or in CSF levels of IFN-γ (Fig. 7C, D, F). However, we consistently observed mild decreases in these parameters in DHT-treated females across multiple experiments. Consistent with our experiments in male mice, these data suggest that androgens are able to quantitatively suppress the T cell response to i.c. LCMV infection in female mice.
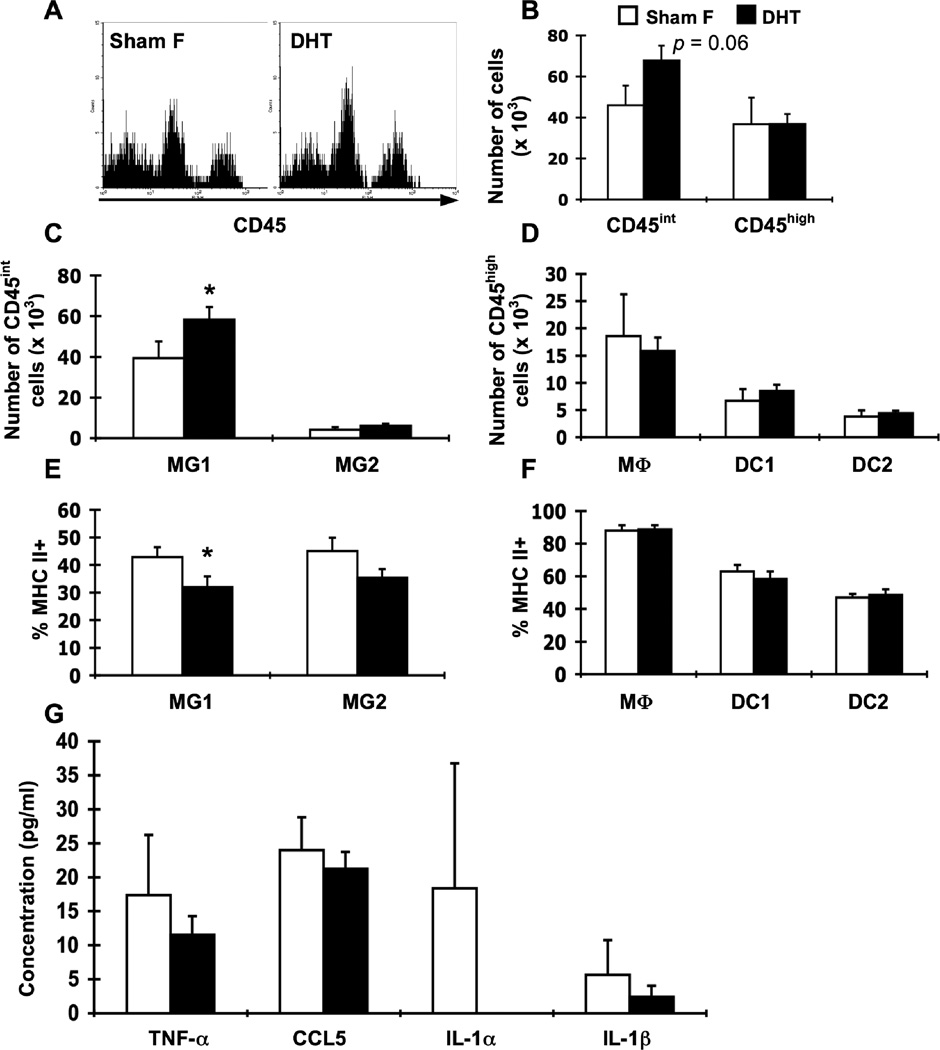
3.9. DHT treatment of females increases numbers and decreases activation of resident CNS APCs
We next examined the effect of exogenous androgens on CNS APC populations and CSF cytokines after i.c. LCMV infection. DHT treatment resulted in a trend toward increased numbers of CD45int cells in the brain compared to control females (Fig. 8A, B), which was observed in multiple experiments. The increase was within the MG1 microglial cells and not the MG2 population (Fig. 8C). No differences were seen between DHT-treated females and control females in the numbers of CD45high cells nor in the distribution of CD45high cells among different populations (Fig. 8A, B, D). Similarly, DHT-treated females had decreased percentages of MHC class II-positive microglia, but there were no differences seen in MHC class II expression on any of the CD45high APCs (Fig 8E, F). Together, these data suggest that exogenous androgen administration to female β2m−/− mice can alter the quantity and quality of resident CNS APCs after i.c. LCMV infection.
Figure 8.
DHT treatment of female β2m−/− mice alters resident CNS cell populations and decreases APC activation. Groups of female β2m−/− mice were sham-operated (Sham F; white bars, n = 8) or implanted with DHT pellets (DHT; black bars, n = 8). Animals were infected i.c. with 1 × 103 pfu LCMV, sacrificed at day 8 of infection, and perfused with 10 ml PBS. Mononuclear cells from brains were isolated, stained for APC markers, and analyzed as by flow cytometry. (A) Representative histograms for CD45 expression on brain cells isolated from LCMV-infected sham-operated females (left) and DHT-treated females (right). Events in histograms were gated on live cells as determined by forward and side light scatter. (B) Numbers of CD45int and CD45high cells in the brain in infected DHT-treated females and sham-operated females. (C) Numbers of CD45int cells in the brain among the identified APC populations (as defined in Table 1). (D) Numbers of CD45high cells in the brain among the identified APC populations (as defined in Table 1). (E) Percentages of MHC class IIhigh cells within resident APC populations in the brain. (F) Percentages of MHC class IIhigh cells within hematopoietic APC populations in the brain. (G) Levels of cytokines and chemokines in the CSF. CSF was harvested from perfused mice and analyzed by multiplex. Data represent the mean ± SEM and are representative of two independent experiments. *p ≤ 0.05 (Mann-Whitney test, Minitab for Windows).
To determine whether androgen treatment affects in vivo production of cytokines, CSF was harvested from infected DHT-treated females and control females 8 days post-LCMV infection and assayed for cytokines and chemokines by multiplex assay. Levels of CSF cytokines and chemokines were not significantly affected by exogenous androgen administration (Fig. 8G). Together, these data again suggest specific effects of androgens on the quantity and quality of the T cell response. Interestingly, these effects appear to modulate APC activation as assessed by MHC class II upregulation, but not cytokine/chemokine levels in the CSF.
4. Discussion
Male mice are clearly less susceptible to LCMV-induced anorexia and weight loss compared to female mice. Here, we further characterized specific aspects of the immune response between male and female mice and the role played by androgens on these parameters. We previously showed that estrogen could directly enhance the cytotoxic activity of male T cells in short-term CTL assays (Muller et al., 1995), suggesting that T cells are the primary targets of sex hormones in vitro. However, androgen and estrogen receptors are expressed by a wide variety of immune cells (Dimayuga et al., 2005; Liva and Voskuhl, 2001; Paharkova-Vatchkova et al., 2004; Suenaga et al., 1998), and it is possible that sex hormones have direct effects on both T cells and APCs that contribute to disease susceptibility.
In this report, we show that androgens have suppressive effects on the immune response to LCMV, particularly with regards to antigen-specific T cells, which is consistent with our previous studies showing that vaccination enhances the susceptibility of male mice to LCMV-induced disease (Hildeman et al., 1997). Here, we found that male mice have lower LCMV-specific T cell responses and less severe LCMV-mediated disease compared to female mice, and that orchidectomy of male mice was sufficient to increase the percentage and number of LCMV-specific T cells in the cervical lymph node. A caveat to the DHT replacement experiments is the difficulty in determining whether they can achieve physiologic levels of “testosterone”. Indeed, our experiments with 5mg pellets were likely supraphysiologic. Nonetheless, we showed that (i) orchidectomy of male mice could increase viral specific T cell responses; (ii) restoration of near physiologic levels of “testosterone” (0.5mg DHT pellets) could decrease viral specific T cell responses demonstrates that androgens contribute to control of T cell responses to viral infection. Although we did not find significant decreases in viral-specific T cell responses in orchidectomized mice treated with supraphysiologic doses of “testosterone”, the overall trend was there despite substantial variability in that experiment. On the other hand, female mice have less overall variability to viral infection (Hildeman et al., 1997) and supraphysiologic doses of DHT in female mice were able to significantly decrease their viral-specific T cell responses. Thus, we have shown that androgens can contribute to regulation of T cell responses in male mice and can be manipulated to control T cell responses in female mice.
The cervical lymph nodes are the draining lymph nodes for the CNS, and thus, the site at which CNS immune responses are initiated (Widner et al., 1988), one possibility is that T cell priming is less efficient in male than female mice. In this scenario, less antigen-specific naïve T cells are productively activated in male mice. A second possibility is that androgens inhibit T cell proliferation (Jacobson and Ansari, 2004; Matejuk et al., 2005; McMurray et al., 2001), although ours and other studies (Bebo et al., 1998; Chien et al., 2006) suggest that androgens do not affect T cell proliferation. However, we recognize the insensitivity of the BrdU assay and cannot rule out the possibility that proliferation was slightly less robust in male mice. Alternatively, androgens may affect T cell survival; testosterone has been shown to increase apoptosis in T cell lines (McMurray et al., 2001) and double positive thymocytes (Guevara Patino et al., 2000), perhaps by decreasing expression of the anti-apoptotic molecule Bcl-2 (Huber et al., 1999). Finally, it is possible that androgens could subtly affect all of these parameters, and the summation of these effects contributes to the observed decreases in LCMV-specific T cell responses.
While alterations in androgen levels were sufficient to affect generation of T cell responses in the cervical lymph node, neither orchidectomy nor exogenous DHT administration significantly altered percentages or numbers of LCMV-specific T cells in the brain, regardless of dose, suggesting that trafficking of cells to the CNS was unaffected by androgen levels. Levels of CCL5, a major chemokine important for T cell entry into the CNS (Glabinski et al., 1998; Lane et al., 2000), were increased in female β2m−/− mice compared to males, but no differences were found in CCL5 levels between groups in which only androgen levels differed. Estrogens, rather than androgens, may affect the ability of T cells to traffic to the CNS after LCMV infection. Indeed, in vivo studies have demonstrated estrogens’ abilities to upregulate CCL5 and other chemokines, as well as their receptors (Ma et al., 2007; Mo et al., 2005).
Orchidectomy alone was also sufficient to increase the infection-induced activation of CNS resident (i.e., microglia) and infiltrating (e.g., macrophages) APCs with regards to surface expression of MHC class II. In addition, DHT treatment of female mice was sufficient to decrease infection-induced expression of MHC class II on microglia, but not infiltrating APCs. Thus, androgens may act on immune cells in at least three ways: i) Androgens decrease T cell IFN-γ production which, in turn, decreases APC activation; ii) Androgens affect APC activation, which then affects T cell production of IFN-γ; or iii) Androgens affect both APCs and T cells, which can then also affect each other. Current work in our laboratory suggests that IFN-γ from T cells acts on APCs to drive upregulation of MHC II but does not affect numbers of LCMV-specific T cells (Lin et al J. Virol 2009), supportive of hypothesis #1 above. However, both androgens and estrogens may still be acting directly on APCs to alter expression of MHC class II, as observed in other studies (Barreto et al., 2007; Matejuk et al., 2005; Paharkova-Vatchkova et al., 2004; Yang et al., 2006). Future studies using mice in which androgen receptors can be deleted specifically in T cells will be required to delineate between these possibilities.
Our study also suggests that androgens can positively impact proliferation and/or survival of populations of resident CNS microglia. Although there was no difference in the number of CD45int cells in the brain after i.c. LCMV infection between males and females, removal of endogenous androgens in males via orchidectomy decreased the number of CD45int cells compared to sham-operated males, and addition of exogenous androgens to female mice via DHT pellet implantation increased the number of CD45int cells compared to control females. Microglia are able to undergo proliferation and apoptosis in response to various stimuli (Jones et al., 1997; Remington et al., 2007; Wirenfeldt et al., 2007), but little is known about the effects of sex hormones on these processes in microglia. Possible roles of physiologic levels of estrogens on microglial survival/proliferation and the balance of estrogens’ effects with the effects of androgens must also be considered.
The CD45int population that was mostly affected was the CD11b+ CD11c- MG1 microglia, which are thought to be CNS-derived microglia as opposed to the bone marrow-derived CD11b+ CD11c+ MG2 microglia (Remington et al., 2007; Simard and Rivest, 2004). We observed that when numbers of MG1 microglia were decreased, there was also an increase in CSF IFN-γ (orchidectomized males); conversely, an increase in numbers of MG1 microglia correlated with trends toward decreased levels of CSF IFN-γ (DHT females). Studies have shown immunosuppressive roles for microglia: after viral infection, CD11b+ CD45int CXCR3+ microglia secrete IL-10 and TGF-β (Li et al., 2006), and primary microglial cultures express indoleamine 2,3-dioxygenase (IDO) after IFN-γ stimulation (Yadav et al., 2007). Thus, future experiments will examine the potential role of MG1 microglia and whether or not they play an immunosuppressive role in this model.
While the majority of the cytokines examined in this study were unaffected by alterations in androgen levels, we observed specific effects of androgens on production of IFN-γ. Interestingly, orchidectomy had a more significant effect in males than DHT treatment did in intact females; orchidectomized males exhibit IFN-γ levels in the serum and CSF that were significantly different from sham-operated males and similar to those seen in females, while IFN-γ levels in DHT treated females were not significantly different from control females and did not reach the IFN-γ levels observed in male mice. In contrast, DHT treatment of females did confer a male phenotype with regards to percentages and numbers of LCMV-specific T cells in the cervical LN, while orchidectomy was unable to increase percentages or numbers of T cells in male mice to the levels seen in females. Thus, it seems that orchidectomy of male mice affects the quality of the T cell response more than the quantity, while DHT treatment of intact females affects the quantity more than the quality. We note that while orchidectomy can effectively decrease serum androgen levels in a male mouse and while DHT treatment can effectively increase serum androgen levels in a female mouse, other, non-androgenic factors that influence the development of the T cell response still differ between the sexes and may play subtle roles. For example, estrogens may have stronger effects on IFN-γ production than androgens due to estrogen-responsive elements in the IFN-γ promoter (Ciccarone et al., 1990; Fox et al., 1991). ADORA THE PREVIOUS SENTENCE DOESN’T REALLY JIVE WITH THE STATEMENT IN THE YELLOW HIGHLIGHTED SENTENCE ABOVE. I THINK WE COULD JUST REMOVE THE YELLOW SENTENCE WHAT DO YOU THINK ? Alternatively, leptin, which circulates at higher concentrations in females (Saad et al., 1997) and can promote and sustain inflammatory T cell responses (Lord et al., 1998; Matarese et al., 2001), may also contribute to sex differences in immune responses. However, we and others have previously observed that leptin levels are decreased after LCMV infection (HILDEMAN ET AL VIRAL IMMUNOL KAMPERSHROER ET AL JI), suggesting that leptin does not contribute to sex specific differences in T cell responses. Despite the complex interactions of systems affecting the immune response, we have shown that androgens are able to have a substantial effect on the immune response to viral infection.
In conclusion, our results demonstrate that compared to female β2m−/− mice, males have significantly decreased CD4+ T cell responses after i.c. LCMV infection in vivo, leading to decreased immunopathology and disease. This aspect of LCMV infection is intriguing considering the decreased prevalence and severity of several autoimmune diseases in men, which may be at least partly explained by diminished CD4+ T cell activity (Olsen and Kovacs, 1996; Whitacre, 2001). Sex differences in the immune response to LCMV are also seen at the level of APC activation, which may have feedback effects on the T cell response. The observed sex differences are likely mediated by suppressive effects of androgens, the modulation of which are sufficient to affect the accumulation of antigen-specific T cells and their interaction with APCs through levels of IFN-γ and MHC class II expression. The results of this work may lend insight during the interpretation of results from clinical trials that present conflicting conclusions with regards to the efficacy (Petri et al., 2004; Sicotte et al., 2007) or non-efficacy (Gordon et al., 2008; van Vollenhoven et al., 1999) of androgens in the treatment of autoimmune diseases. This study also provides a basis for further investigation of sex differences in immune responses, immunopathology, and neuroimmunology, which may have significant implications for development of therapies for immune-mediated disease, as well as furthering our understanding of sex differences in susceptibility to autoimmune diseases.
Acknowledgements
This work was supported by generous start-up funds from the Division of Immunobiology, a Trustee Grant from Cincinnati Children’s Hospital Research Foundation, and Public Health Service Grant NS051798 (D.A.H.).
We thank Drs. Michael Jordan, Marsha Wills-Karp, and Bob Colbert for critical review of the manuscript. We also thank Dr. Pulak Tripathi for help in generation of the I-Ab gp61-80 tetramer, and Joe Drennan and Rachael Logsdon for assistance with experiments.
References
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar Ahmed S, Young PR, Penhale WJ. Beneficial effect of testosterone in the treatment of chronic autoimmune thyroiditis in rats. J Immunol. 1986;136:143–147. [PubMed] [Google Scholar]
- Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25:3039–3046. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- Brick JE, Wilson DA, Walker SE. Hormonal modulation of responses to thymus-independent and thymus-dependent antigens in autoimmune NZB/W mice. J Immunol. 1985;134:3693–3698. [PubMed] [Google Scholar]
- Campbell IL, Hobbs MV, Kemper P, Oldstone MB. Cerebral expression of multiple cytokine genes in mice with lymphocytic choriomeningitis. J Immunol. 1994;152:716–723. [PubMed] [Google Scholar]
- Chien EJ, Chang CP, Lee WF, Su TH, Wu CH. Non-genomic immunosuppressive actions of progesterone inhibits PHA-induced alkalinization and activation in T cells. J Cell Biochem. 2006;99:292–304. doi: 10.1002/jcb.20858. [DOI] [PubMed] [Google Scholar]
- Ciccarone VC, Chrivia J, Hardy KJ, Young HA. Identification of enhancer-like elements in human IFN-gamma genomic DNA. J Immunol. 1990;144:725–730. [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, Straub RH. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–638. doi: 10.1191/0961203304lu1094oa. [DOI] [PubMed] [Google Scholar]
- Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, Bruce-Keller AJ. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161:123–136. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Doherty PC, Hou S, Southern PJ. Lymphocytic choriomeningitis virus induces a chronic wasting disease in mice lacking class I major histocompatibility complex glycoproteins. J Neuroimmunol. 1993;46:11–17. doi: 10.1016/0165-5728(93)90228-q. [DOI] [PubMed] [Google Scholar]
- Doherty PC, Zinkernagel RM. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19:89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, Steinman L. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JO, Ting JY, Stohlman SA, Weiner LP. Improvements in obtaining and characterizing mouse cerebrospinal fluid. Application to mouse hepatitis virus-induced encephalomyelitis. J Neuroimmunol. 1983;4:129–140. doi: 10.1016/0165-5728(83)90017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Fox HS. Androgen treatment prevents diabetes in nonobese diabetic mice. J Exp Med. 1992;175:1409–1412. doi: 10.1084/jem.175.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- Frei K, Leist TP, Meager A, Gallo P, Leppert D, Zinkernagel RM, Fontana A. Production of B cell stimulatory factor-2 and interferon gamma in the central nervous system during viral meningitis and encephalitis. Evaluation in a murine model infection and in patients. J Exp Med. 1988;168:449–453. doi: 10.1084/jem.168.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung WP, Kundig TM, Zinkernagel RM, Mak TW. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabinski AR, Tuohy VK, Ransohoff RM. Expression of chemokines RANTES, MIP-1alpha and GRO-alpha correlates with inflammation in acute experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 1998;5:166–171. doi: 10.1159/000026333. [DOI] [PubMed] [Google Scholar]
- Gordon C, Wallace DJ, Shinada S, Kalunian KC, Forbess L, Braunstein GD, Weisman MH. Testosterone patches in the management of patients with mild/moderate systemic lupus erythematosus. Rheumatology (Oxford) 2008;47:334–338. doi: 10.1093/rheumatology/kem342. [DOI] [PubMed] [Google Scholar]
- Guevara Patino JA, Marino MW, Ivanov VN, Nikolich-Zugich J. Sex steroids induce apoptosis of CD8+CD4+ double-positive thymocytes via TNF-alpha. Eur J Immunol. 2000;30:2586–2592. doi: 10.1002/1521-4141(200009)30:9<2586::AID-IMMU2586>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Perveen-Gill Z, Lightman SL, Jessop DS. A protective role for testosterone in adjuvant-induced arthritis. Br J Rheumatol. 1995;34:1117–1122. doi: 10.1093/rheumatology/34.12.1117. [DOI] [PubMed] [Google Scholar]
- Hildeman D, Muller D. Immunopathologic weight loss in intracranial LCMV infection initiated by the anorexigenic effects of IL-1beta. Viral Immunol. 2000;13:273–285. doi: 10.1089/08828240050144617. [DOI] [PubMed] [Google Scholar]
- Hildeman D, Salvato M, Whitton JL, Muller D. Vaccination protects beta 2 microglobulin deficient mice from immune mediated mortality but not from persisting viral infection. Vaccine. 1996;14:1223–1229. doi: 10.1016/s0264-410x(96)00028-x. [DOI] [PubMed] [Google Scholar]
- Hildeman D, Yanez D, Pederson K, Havighurst T, Muller D. Vaccination against persistent viral infection exacerbates CD4+ T-cell-mediated immunopathological disease. J Virol. 1997;71:9672–9678. doi: 10.1128/jvi.71.12.9672-9678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch MS, Murphy FA, Hicklin MD. Immunopathology of lymphocytic choriomeningitis viurs infection of newborn mice. Antithymocyte serum effects on glomerulonephritis and wasting disease. J Exp Med. 1968;127:757–766. doi: 10.1084/jem.127.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Hu SK, Mitcho YL, Rath NC. Effect of estradiol on interleukin 1 synthesis by macrophages. Int J Immunopharmacol. 1988;10:247–252. doi: 10.1016/0192-0561(88)90055-0. [DOI] [PubMed] [Google Scholar]
- Huber SA, Kupperman J, Newell MK. Estradiol prevents and testosterone promotes Fas-dependent apoptosis in CD4+ Th2 cells by altering Bcl 2 expression. Lupus. 1999;8:384–387. doi: 10.1177/096120339900800511. [DOI] [PubMed] [Google Scholar]
- Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167:542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- Jacobson JD, Ansari MA. Immunomodulatory actions of gonadal steroids may be mediated by gonadotropin-releasing hormone. Endocrinology. 2004;145:330–336. doi: 10.1210/en.2003-0510. [DOI] [PubMed] [Google Scholar]
- Jones LL, Banati RB, Graeber MB, Bonfanti L, Raivich G, Kreutzberg GW. Population control of microglia: does apoptosis play a role? J Neurocytol. 1997;26:755–770. doi: 10.1023/a:1018514415073. [DOI] [PubMed] [Google Scholar]
- Kamperschroer C, Quinn DG. The role of proinflammatory cytokines in wasting disease during lymphocytic choriomeningitis virus infection. J Immunol. 2002;169:340–349. doi: 10.4049/jimmunol.169.1.340. [DOI] [PubMed] [Google Scholar]
- Karman J, Ling C, Sandor M, Fabry Z. Dendritic cells in the initiation of immune responses against central nervous system-derived antigens. Immunol Lett. 2004;92:107–115. doi: 10.1016/j.imlet.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Grube F, Lohler J, Utermohlen O, Gegin C. Antiviral immune responses of lymphocytic choriomeningitis virus-infected mice lacking CD8+ T lymphocytes because of disruption of the beta 2-microglobulin gene. J Virol. 1993;67:332–339. doi: 10.1128/jvi.67.1.332-339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Gang Z, Yuling H, Luokun X, Jie X, Hao L, Li W, Chunsong H, Junyan L, Mingshen J, Youxin J, Feili G, Boquan J, Jinquan T. Different neurotropic pathogens elicit neurotoxic CCR9- or neurosupportive CXCR3-expressing microglia. J Immunol. 2006;177:3644–3656. doi: 10.4049/jimmunol.177.6.3644. [DOI] [PubMed] [Google Scholar]
- Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Ma LJ, Guzman EA, DeGuzman A, Muller HK, Walker AM, Owen LB. Local cytokine levels associated with delayed-type hypersensitivity responses: modulation by gender, ovariectomy, and estrogen replacement. J Endocrinol. 2007;193:291–297. doi: 10.1677/JOE-06-0024. [DOI] [PubMed] [Google Scholar]
- Matarese G, Sanna V, Di Giacomo A, Lord GM, Howard JK, Bloom SR, Lechler RI, Fontana S, Zappacosta S. Leptin potentiates experimental autoimmune 31 encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol. 2001;31:1324–1332. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol. 2005;174:2387–2395. doi: 10.4049/jimmunol.174.4.2387. [DOI] [PubMed] [Google Scholar]
- McMurray RW, Suwannaroj S, Ndebele K, Jenkins JK. Differential effects of sex steroids on T and B cells: modulation of cell cycle phase distribution, apoptosis and bcl-2 protein levels. Pathobiology. 2001;69:44–58. doi: 10.1159/000048757. [DOI] [PubMed] [Google Scholar]
- Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol. 2005;174:6023–6029. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- Muller D, Chen M, Vikingsson A, Hildeman D, Pederson K. Oestrogen influences CD4+ T-lymphocyte activity in vivo and in vitro in beta 2-microglobulin-deficient mice. Immunology. 1995;86:162–167. [PMC free article] [PubMed] [Google Scholar]
- Muller D, Koller BH, Whitton JL, LaPan KE, Brigman KK, Frelinger JA. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science. 1992;255:1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–938. doi: 10.1001/archsurg.134.9.935. discussion 938-940. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Oxenius A, Bachmann MF, Ashton-Rickardt PG, Tonegawa S, Zinkernagel RM, Hengartner H. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur J Immunol. 1995;25:3402–3411. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Petri MA, Mease PJ, Merrill JT, Lahita RG, Iannini MJ, Yocum DE, Ginzler EM, Katz RS, Gluck OS, Genovese MC, Van Vollenhoven R, Kalunian KC, Manzi S, Greenwald MW, Buyon JP, Olsen NJ, Schiff MH, Kavanaugh AF, Caldwell JR, Ramsey-Goldman R, St Clair EW, Goldman AL, Egan RM, Polisson RP, Moder KG, Rothfield NF, Spencer RT, Hobbs K, Fessler BJ, Calabrese LH, Moreland LW, Cohen SB, Quarles BJ, Strand V, Gurwith M, Schwartz KE. Effects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. 2004;50:2858–2868. doi: 10.1002/art.20427. [DOI] [PubMed] [Google Scholar]
- Quinn DG, Zajac AJ, Frelinger JA, Muller D. Transfer of lymphocytic choriomeningitis disease in beta 2-microglobulin-deficient mice by CD4+ T cells. Int Immunol. 1993;5:1193–1198. doi: 10.1093/intimm/5.10.1193. [DOI] [PubMed] [Google Scholar]
- Reichmann G, Schroeter M, Jander S, Fischer HG. Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. J Neuroimmunol. 2002;129:125–132. doi: 10.1016/s0165-5728(02)00184-4. [DOI] [PubMed] [Google Scholar]
- Remington LT, Babcock AA, Zehntner SP, Owens T. Microglial recruitment, activation, and proliferation in response to primary demyelination. Am J Pathol. 2007;170:1713–1724. doi: 10.2353/ajpath.2007.060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe WP. Protective effect of pre-irradiation on lymphocytic choriomeningitis infection in mice. Proc Soc Exp Biol Med. 1956;92:194–198. doi: 10.3181/00379727-92-22425. [DOI] [PubMed] [Google Scholar]
- Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82:579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicotte NL, Giesser BS, Tandon V, Klutch R, Steiner B, Drain AE, Shattuck DW, Hull L, Wang HJ, Elashoff RM, Swerdloff RS, Voskuhl RR. Testosterone treatment in multiple sclerosis: a pilot study. Arch Neurol. 2007;64:683–688. doi: 10.1001/archneur.64.5.683. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. Faseb J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- Suenaga R, Evans MJ, Mitamura K, Rider V, Abdou NI. Peripheral blood T cells and monocytes and B cell lines derived from patients with lupus express estrogen receptor transcripts similar to those of normal cells. J Rheumatol. 1998;25:1305–1312. [PubMed] [Google Scholar]
- van Vollenhoven RF, Park JL, Genovese MC, West JP, McGuire JL. A double-blind, placebo-controlled, clinical trial of dehydroepiandrosterone in severe systemic lupus erythematosus. Lupus. 1999;8:181–187. doi: 10.1191/096120399678847588. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Pitchekian-Halabi H, MacKenzie-Graham A, McFarland HF, Raine CS. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann Neurol. 1996;39:724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- Weinstein Y, Ran S, Segal S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol. 1984;132:656–661. [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Widner H, Moller G, Johansson BB. Immune response in deep cervical lymph nodes and spleen in the mouse after antigen deposition in different intracerebral sites. Scand J Immunol. 1988;28:563–571. doi: 10.1111/j.1365-3083.1988.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Wirenfeldt M, Dissing-Olesen L, Anne Babcock A, Nielsen M, Meldgaard M, Zimmer J, Azcoitia I, Leslie RG, Dagnaes-Hansen F, Finsen B. Population control of resident and immigrant microglia by mitosis and apoptosis. Am J Pathol. 2007;171:617–631. doi: 10.2353/ajpath.2007.061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav MC, Burudi EM, Alirezaei M, Flynn CC, Watry DD, Lanigan CM, Fox HS. IFN-gamma-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia. 2007;55:1385–1396. doi: 10.1002/glia.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Hu Y, Hou Y. Effects of 17beta-estradiol on the maturation, nuclear factor kappa B p65 and functions of murine spleen CD11c-positive dendritic cells. Mol Immunol. 2006;43:357–366. doi: 10.1016/j.molimm.2005.02.012. [DOI] [PubMed] [Google Scholar]