ABSTRACT
Objectives:
Levels of stool fatty acid soaps and beneficial bacteria differ between formula-fed and breast-fed infants; addition of specific formula ingredients may reduce these differences. This study evaluated the effects of a term infant formula containing high sn-2 palmitate term infant formula (sn-2) or an identical formula supplemented with oligofructose (OF) at 2 concentrations (sn-2+3 g/L OF, sn-2+5 g/L OF) on stool composition, stool characteristics, and fecal bifidobacteria.
Methods:
Healthy, term formula-fed infants 7 to 14 days old (n = 300) were randomized in a double-blind manner to receive standard formula (control), sn-2, sn-2+3 g/L OF, or sn-2+5 g/L OF for 8 weeks. Human milk (HM)–fed infants (n = 75) were studied in parallel. Stool samples were collected from all subjects at week 8 for fatty acid soaps and mineral content, and from a subset at baseline and week 8 for bifidobacteria. Stool characteristics were assessed via 3-day diary.
Results:
The sn-2 group had 46% less stool soap palmitate (P < 0.001) and softer stools than control (20% more mushy soft stools, P = 0.026; 50% fewer formed stools, P = 0.003). Addition of OF resulted in even fewer formed stools versus control (65% fewer for sn-2+3 g/L OF, 79% fewer for sn-2+5 g/L OF), with 5 g/L OF more closely resembling that of HM-fed infants. Both sn-2 (P < 0.05) and sn-2 with OF groups (P < 0.01) had significantly higher fecal bifidobacteria concentrations than control at week 8, not differing from HM-fed infants.
Conclusions:
High sn-2-palmitate formulas led to reduced stool soaps, softer stools, and increased bifidobacteria, whereas addition of OF further improved stool consistency. Those modifications brought outcomes in formula-fed infants closer to that in HM-fed infants.
Keywords: bifidobacteria, fatty acid soaps, infant formula, oligofructose, sn-2-palmitate, stool consistency
Differences in bowel habits and gastrointestinal (GI) tolerance between human milk (HM)–fed and formula-fed infants are well recognized. HM-fed infants typically have frequent, soft stools, whereas formula-fed infants have less frequent, firm or formed stools (1,2) that may be difficult to pass. Although improvements have been made in the composition and performance of infant formulas, constipation and relatively hard stools remain common in formula-fed infants (3). Fat blends used in infant formula may contribute to these problems, and use of fat blends more closely resembling HM fat can improve infants’ stooling patterns and consistency (4–7). The addition of prebiotics to infant formulas has also led to softer stools and may provide additional health benefits such as support of a more favorable gut microbiota (8–11).
Triglycerides, the major source of energy in both HM and infant formula, comprised 3 fatty acids esterified to a 3-carbon glycerol backbone. The stereospecific position of fatty acids on the glycerol, which is identified as sn-1 (outer carbon), sn-2 (center carbon), or sn-3 (outer carbon), differs in HM fat compared with vegetable oils used in infant formula (12). Palmitic acid, an abundant saturated fatty acid in both HM and infant formula (approximately 20% of total fatty acids), is predominantly esterified (approximately 70%) at the sn-2 position in HM, but primarily esterified at the sn-1 and sn-3 positions in fat blends commonly used in infant formulas (13–15). Pancreatic lipase preferentially hydrolyzes fatty acids in the sn-1 and sn-3 positions, yielding 2 free fatty acids and the corresponding sn-2 monoglyceride, which is well absorbed regardless of the fatty acid attached. Thus, palmitic acid in HM is well absorbed given its predominant esterification in the sn-2 position. In contrast, intestinal digestion of infant formula yields relatively high levels of free palmitic acid that can bind calcium in the intestine, forming fatty acid soaps that are excreted in the feces (16) and are associated with hard, infrequent stools, and unnecessary loss of both fatty acids and calcium (3,4,17). Use of a vegetable oil blend rich in sn-2 palmitate (sn-2) in infant formula yields a positional distribution of palmitic acid that more closely resembles that of HM. Infants who consumed a formula containing high sn-2 palmitate had reduced fecal excretion of palmitic acid soaps and softer stools (4–7).
Oligofructose (OF) is a nondigestible, inulin-derived carbohydrate consisting of short chains of fructose polymers (18). OF decreases GI transit time and increases fecal weight and water-holding capacity (19). OF can beneficially affect the host by selectively stimulating the growth and/or activity of 1 or a limited number of bacteria (eg, bifidobacteria) in the colon (20,21). Studies in term and preterm infants indicate that infant formulas with OF are well tolerated, promote a bifidobacteria-dominant fecal flora, and may increase stool softness (8–10,22).
We hypothesized that formula with high sn-2 palmitate in combination with OF would promote improved GI function. This study evaluated stool composition, stool characteristics, fecal bifidobacteria, and GI tolerance in infants receiving a control formula versus infants receiving formulas containing a fat blend with elevated levels of sn-2 palmitate, either alone or in combination with 2 different concentrations of OF.
METHODS
This was a randomized controlled double-blind study of healthy, term formula-fed infants receiving 1 of 4 study formulas for 8 weeks. A nonrandomized HM-fed group was included as a reference. Clinic visits were scheduled at baseline, week 4, and week 8, with telephone follow-ups midway between clinic visits.
Demographic and household characteristics were collected at the baseline visit. Anthropometrics (weight, length, and head circumference) were obtained and a parent questionnaire, the Infant Gastrointestinal Symptom Questionnaire (IGSQ), was administered at the baseline, week 4, and week 8 visits. Stool characteristics (consistency and frequency) were assessed via a stool diary completed by parents/guardians on 3 consecutive days immediately before the week 4 and week 8 visits. Infant stool samples were collected by parents/caregivers at home during a 5-day period before the week 8 visit and analyzed for stool soap fatty acids and mineral content. Additional stool samples were collected from a subset (n = 170) during the baseline and week 8 visits and analyzed for bifidobacteria.
The study was conducted between April 2009 and September 2009 at Muntinlupa Health Center, Muntinlupa City, the Philippines, in accordance with Good Clinical Practice, International Conference on Harmonization guidelines, and the provisions of the Declaration of Helsinki and its amendments. The protocol and informed consent form were approved by the National Ethics Committee and the Bureau of Food and Drug in the Philippines. Written, informed consent was obtained from the parent or legal guardian of each infant before enrollment.
Participants
Infants were healthy, term (gestational age ≥37 weeks) singleton, ages 7 to 14 days, weight-for-age ≥5th percentile according to Filipino reference standards, and exclusively consuming and tolerating a cow's-milk–based infant formula (for formula-fed groups) or exclusively consuming HM (for the HM-fed group). Breast-feeding was encouraged, and only when the mother had clearly decided not to breast-feed was she approached for inclusion of her infant in the formula-fed groups. Main exclusion criteria included the use of medication(s) known or suspected to affect fat digestion, absorption, and/or metabolism; supplements containing calcium; suppositories; bismuth-containing medications; herbal supplements; or medications that may neutralize or suppress gastric acid secretion.
Study Feedings
Infants enrolled in the HM reference group continued to receive HM ad libitum. Formula-fed infants were assigned to receive ad libitum 1 of the following using validated randomization software: control—a bovine milk-based, whey-predominant, α-lactalbumin-enriched term formula with a 100% vegetable fat blend (S-26 GOLD; Wyeth Nutrition, Askeaton, Ireland); sn-2—a high sn-2 palmitate formula (control formula modified to contain 60% vegetable fat blend and 40% high sn-2 palmitate fat blend [Betapol; Loders Croklaan, Wormerveer, the Netherlands]); sn-2+3 g/L OF—a high sn-2 palmitate formula supplemented with 3.0 g/L OF (Orafti P95; BENEO-ORAFTI, Tienen, Belgium); sn-2+5 g/L OF—a high sn-2 palmitate formula supplemented with 5.0 g/L OF (Orafti P95). Formulas were produced in powder form. To ensure double blinding, all formulas were packaged in an identical manner except for package number. Formula labels provided preparation and storage instructions in both English and Filipino. All formulas met Codex Alimentarius standards for infant formula (23). Table 1 lists fatty acid profiles, percentage of sn-2 palmitate, and selected nutrient levels in the formulas.
TABLE 1.
Composition of study formulas
| Control | sn-2 | sn-2+3g/L OF | sn-2+5g/L OF | |
| Energy, kcal/L | 654 | 642 | 660 | 671 |
| Protein, g/L | 14.1 | 13.8 | 14.3 | 13.9 |
| Total carbohydrate, g/L | 70.2 | 66.7 | 72.5 | 74.8 |
| Fat, g/L | 35.2 | 35.5 | 34.7 | 35.1 |
| Calcium, mg/L | 473.7 | 457.5 | 439.4 | 414.1 |
| Phosphorous, mg/L | 275.0 | 267.5 | 255.0 | 256.7 |
| Magnesium, mg/L | 53.8 | 53.0 | 51.9 | 53.1 |
| Fatty acids, wt% of total | ||||
| C12:0 (lauric) | 8.2 | 5.7 | 5.9 | 5.4 |
| C14:0 (myristic) | 4.2 | 3.0 | 3.1 | 3.1 |
| C16:0 (palmitic) | 21.3 | 22.8 | 23.0 | 23.2 |
| C18:0 (stearic) | 4.2 | 4.0 | 4.0 | 4.1 |
| C18:1 (oleic) | 38.9 | 42.3 | 42.2 | 42.6 |
| C18:2 (linoleic) | 19.0 | 18.4 | 18.0 | 18.2 |
| C18:3 (linolenic) | 1.3 | 1.5 | 1.5 | 1.5 |
| C20:4 (arachidonic) | 0.4 | 0.4 | 0.4 | 0.4 |
| C22:6 (docosahexaenoic) | 0.2 | 0.2 | 0.2 | 0.2 |
| %C16:0 in sn-2 position | 11.7 | 35.9 | 36.6 | 36.9 |
OF = oligofructose; sn-2 = high sn-2 palmitate infant formula.
Stool Characteristics
In a 3-day diary, parents/caregivers recorded the number of bowel movements and stool consistency using standardized pictures of stools corresponding to a validated 5-point scale (1 = watery, 2 = runny, 3 = mushy soft, 4 = formed, or 5 = hard) (2,3).
Stool Biochemical Composition
Standardized procedures were provided to parents/caregivers to collect infant stool samples at home during a 5-day period before the week 8 visit. Infants were fitted with diapers containing a strip of Tegaderm tape (3M, St Paul, MN) to facilitate retention of stool in the diaper. Freshly passed stool was placed into an amber plastic bag, weighed on a portable scale, and stored in a home freezer until ≥30 g had been collected. Frozen samples were brought to the clinic visit in a collection-kit cooler bag, then shipped on dry ice to Covance Laboratories (Madison, WI) for analysis. Samples were analyzed for soap fatty acids using a modification (ie, acetic acid replaced hydrochloric acid to convert soaps to free fatty acids) of an earlier method (3). The limit of fatty acid quantitation was defined as the concentration corresponding to the lowest C16:0 calibration standard (0.5 mg/g). The acceptance criteria with respect to recovery and coefficients of variability for fatty acids were ±30% for the performance of replicate analyses related to accuracy as compared with previously established values of targets in quality control samples. Repeatability relative standard deviations (SDs) for a dry quality control level of C16:0 ranged from 2.9% to 12.6%. Total soap fatty acids were calculated as the sum of individual soap fatty acids. Results were expressed as milligrams per gram of dry stool weight. Standard analytical procedures were used to determine stool moisture and solids. Calcium, phosphorous, and magnesium concentrations were determined by inductively coupled plasma emission spectrometry following AOAC International Official Methods of Analysis (24,25).
Fecal Bifidobacteria
An additional 3 g of fresh stool was collected from a subset of infants during baseline and week 8 visits. Samples were collected with a scoop attached to the inner aspect of the lid of a sterile polypropylene vial and immediately stored in a −20°C freezer. Frozen samples were shipped on dry ice to NIZO Food Research (Ede, the Netherlands) where they were fixed for fluorescent in situ hybridization analysis. Samples were then shipped on dry ice to University Medical Center Groningen, Groningen, the Netherlands, where fluorescent in situ hybridization analyses were conducted. Total number of bifidobacteria was analyzed with probe BIF164 as described previously (26). Quantitative determination was conducted by automated process; a cumulative coefficient of variation was used to assess quality of automated counting. If cumulative coefficient of variation was >15%, the sample was recounted. Data were reported as bacteria counts per gram of wet stool weight. Parents or legal guardians gave consent for this component of the study.
GI Tolerance
The IGSQ is a 21-item standardized, validated, interviewer-assisted questionnaire that allows parents to describe the frequency and intensity of their infant's GI signs and symptoms of distress during the previous 7 days. The questionnaire includes 5 domains: stooling, vomiting, crying, fussiness, and flatulence. An individual score for each question and an overall index score were calculated. The IGSQ index score is a measure of total GI symptom burden; it contains a subset of 13 questions from the IGSQ that are summed to produce a single score. Potential values for the IGSQ index score range from 13 to 65, wherein 13 indicates low and 65 indicates high GI burden (27).
GI study events were recorded during clinic visits and telephone calls throughout the study. A study event was defined as any untoward, undesired, or unplanned event in the form of signs, symptoms, disease, or laboratory or physiological observations occurring in an infant. The severity of study events and whether they were related to study feedings were determined by the investigators. A subset of symptoms related to the digestive system and GI tolerance were identified of particular interest a priori: hard stool, constipation, difficulty having a bowel movement, acute diarrhea, chronic diarrhea, spitting up, regurgitation, vomiting, gastroesophageal reflux disease, colic, and crying/neonatal abnormal crying. To ensure consistency in diagnosis, investigators were provided with standard definitions for these symptoms.
Anthropometry
Infants were weighed without a diaper on an electronic infant scale (Seca 374, Hamburg, Germany) and measurements were recorded to nearest gram. Recumbent length was measured on a pediatric length board (Ellard Instrumentation, Washington, DC) to the nearest 0.1 cm. Head circumference was measured using a pediatric tape measure (Seca 212) to the nearest 0.1 cm. Measures were taken twice at each visit and the mean was calculated.
Statistical Analyses
Data analysis was conducted as outlined in an a priori statistical analysis plan using SAS software version 8.2 or later (SAS Institute, Cary, NC). At least 66 infants per group was required to achieve 90% power (α = 0.05) to detect a mean difference of 6.0 mg of stool soap fatty acids per 100 mg of dry stool weight (SD 9) between the control and sn-2 groups, and to detect a mean difference of 0.4 in stool consistency scores (SD 0.7) between the sn-2 group and either of the sn-2+OF groups. Assuming a 15% dropout/nonevaluable rate, approximately 300 formula-fed infants (75/group) were required to achieve 264 evaluable formula-fed infants (66/group).
Endpoints for stool composition and characteristics and IGSQ scores were evaluated in the efficacy evaluable population, defined as all randomly assigned infants who took ≥1 feeding of study formula and had a measurable postbaseline value for the endpoint being analyzed. Fecal bifidobacteria data were analyzed in a per-protocol population. Initially, analyses were conducted including all 4 randomized formula groups and excluding the nonrandomized HM reference group. The prespecified comparison of primary interest was between sn-2 and control. Subsequently, each formula group was compared individually with the HM group. The primary efficacy endpoint was stool composition; palmitic acid and total soap fatty acids were the 2 most important stool composition parameters. Stool characteristics included stool frequency and stool consistency data derived from the 3-day diary. Stool frequency was expressed as average number of bowel movements per day, whereas stool consistency was expressed in 2 ways: average stool consistency score and percentage of stools in each consistency category, for each of the 3-day diary periods. Differences in stool composition and stool characteristics in the formula groups were evaluated by analysis of variance followed by pairwise comparisons to test for differences between formula groups. Independent t tests were conducted to assess differences between each formula group and the HM group. Differences in stool bifidobacteria concentrations among formula-fed groups were analyzed using analysis of covariance with baseline values and type of delivery (vaginal vs cesarean) included in the model, followed by pairwise comparisons. Differences in IGSQ index scores among formula-fed groups were analyzed using analysis of covariance, followed by pairwise comparisons, including baseline IGSQ index score as the covariate. All tests were 2-sided; α = 0.05 for all tests except those involving individual IGSQ items, which were performed using α = 0.01 to adjust for multiplicity.
RESULTS
Study Population
A total of 300 formula-fed infants were randomly assigned to receive control, sn-2, sn-2+3 g/L OF, or sn-2+5 g/L OF formulas (Fig. 1). Seventy-five HM-fed infants were enrolled. Of the 375 infants enrolled, 369 completed the study. Demographic and clinical characteristics were mostly comparable among the formula groups (Table 2). Several baseline characteristics of the HM group were significantly different from the formula groups, for example, percentage of women reporting homemaker as an occupation was significantly higher in the HM group compared with the formula groups, and a higher proportion of mothers of formula-fed infants had finished college.
FIGURE 1.
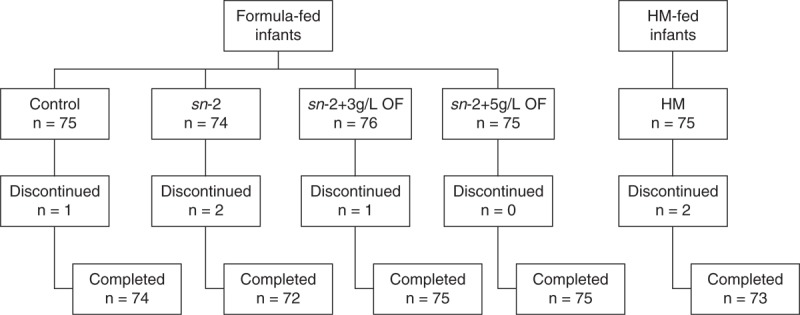
Enrollment and discontinuation of study participants. Control = α-lactalbumin–enriched term infant formula with 100% vegetable fat blend; sn-2 = high sn-2 palmitate formula (control formula modified to contain 60% vegetable fat blend and 40% high sn-2 fat blend); sn-2+3 g/L OF = high sn-2 palmitate formula supplemented with OF at 3 g/L; sn-2+5 g/L OF = high sn-2 palmitate formula supplemented with OF at 5 g/L. OF = oligofructose; sn-2 = sn-2 palmitate.
TABLE 2.
Baseline demographic and clinical characteristics by feeding group
| Control (n = 74) | sn-2 (n = 72) | sn-2+3g/L OF (n = 75) | sn-2+5g/L OF (n = 75) | HM (n = 73) | |
| Infant characteristics | |||||
| Age at enrollment, days* | 11.3 ± 2.0‡ | 11.0 ± 2.0 | 10.9 ± 2.0 | 11.0 ± 2.1‖ | 10.3 ± 2.3 |
| Gestational age, wk* | 38.9 ± 1.0 | 38.9 ± 1.0 | 38.8 ± 1.1 | 39.0 ± 1.1 | 38.8 ± 1.1 |
| Sex, % male | 51 | 47 | 52 | 49 | 48 |
| Maternal characteristics | |||||
| Age, y* | 27.2 ± 6.9 | 26.3 ± 5.9 | 26.9 ± 5.8 | 26.7 ± 6.1 | 26.5 ± 5.9 |
| Marital status, % married¶ | 37 | 49 | 45 | 47 | 40 |
| Education, % completed college§ | 18 | 17|| | 13 | 25‡ | 7 |
| Occupation, % homemaker# | 34† | 33† | 29† | 31† | 74 |
Comparisons between formula groups were conducted using analysis of variance (for continuous variables) or Fisher exact test/chi-square test (for categorical variables); comparisons between the HM group and each formula group were conducted using a series of independent t tests or Fisher exact test. Each formula group versus HM: †P < 0.001, ‡P < 0.01, ||P < 0.05. OF = oligofructose; sn-2 = high sn-2 palmitate infant formula.
*Mean ± standard deviation.
¶Group distribution across 4 marital status categories (eg, married, single, separated, widowed) used for statistical comparisons between groups.
§Group distribution across 6 educational categories (eg, some high school, completed high school, some college) used for statistical comparisons between groups.
#Group distribution across 11 occupational categories (eg, professional, technician/associate professional, service/shop/market sales) used for statistical comparisons between groups.
Stool Soap Fatty Acids and Calcium
At 8 weeks, infants fed high sn-2 palmitate formula with or without OF had significantly less stool soap palmitic acid (C16:0) compared with control (P < 0.001) (Table 3; Fig. 2). There were no significant differences in levels of palmitic acid in fecal soaps between the HM group and any of the 3 experimental formula groups, whereas the level was significantly higher in the control group compared with the HM group (P = 0.0038) (Table 3; Fig. 2). The levels of lauric (C12:0) and myristic (C14:0) acids in fecal soaps in the 3 experimental formula groups were significantly less than the levels in the control (P < 0.001) and HM groups (P < 0.01) (Table 3). At week 8, mean values for total stool soap fatty acids in the sn-2, sn-2+3 g/L OF, and sn-2+5 g/L OF groups were significantly lower compared with control (P < 0.01). A relatively unexpected finding was that mean total stool soap fatty acids were only marginally lower (and not significantly different) in the HM group compared with control (Table 3; Fig. 2). Total soap fatty acids were significantly lower in the sn-2 and sn-2+5 g/L OF groups compared with HM (P < 0.01).
TABLE 3.
Stool soap fatty acids and calcium at week 8 by feeding group
| Control (n = 74) | sn-2 (n = 72) | sn-2+3g/L OF (n = 75) | sn-2+5g/L OF (n = 75) | HM (n = 73) | |
| Soap fatty acids, mean ± SD, mg/g dry stool weight | |||||
| C12:0 (lauric) | 2.0 ± 2.0 | 0.9 ± 1.1*,† | 0.7 ± 0.8*,† | 0.5 ± 0.6*,† | 2.6 ± 3.3 |
| C14:0 (myristic) | 5.3 ± 4.8‡ | 2.2 ± 2.3*,† | 2.3 ± 2.2*,† | 1.7 ± 1.6*,† | 7.8 ± 5.6 |
| C16:0 (palmitic) | 72.7 ± 61.9‡ | 39.4 ± 38.4* | 47.4 ± 41.5* | 37.6 ± 31.6* | 48.3 ± 35.1 |
| C18:0 (stearic) | 15.5 ± 12.5† | 10.2 ± 9.9§,† | 13.6 ± 11.8† | 10.6 ± 8.5§,† | 24.0 ± 15.4 |
| C18:1 (oleic) | 8.8 ± 6.3|| | 9.2 ± 8.2|| | 6.5 ± 4.9¶ | 5.4 ± 4.9§ | 6.3 ± 5.4 |
| C18:2 (linoleic) | 1.9 ± 1.2† | 1.5 ± 0.9¶,|| | 1.0 ± 0.5* | 1.0 ± 1.0* | 1.0 ± 1.7 |
| Total soaps | 108.9 ± 89.4 | 65.3 ± 61.1*,‡ | 74.1 ± 63.2§ | 58.9 ± 47.7*,† | 92.6 ± 61.5 |
| Calcium | 32.4 ± 10.8† | 30.8 ± 12.8† | 28.7 ± 9.5¶,† | 24.3 ± 8.6*,|| | 20.3 ± 10.7 |
Comparisons between formula groups were conducted using analysis of variance followed by pairwise comparisons; comparisons between the HM group and each formula group were conducted using independent t tests. OF = oligofructose; SD = standard deviation; sn-2 = high sn-2 palmitate infant formula. Experimental formula groups vs control: *P < 0.001, §P < 0.01, ¶P < 0.05. Each formula group vs HM: †P < 0.001, ‡P < 0.01, ||P < 0.05.
FIGURE 2.
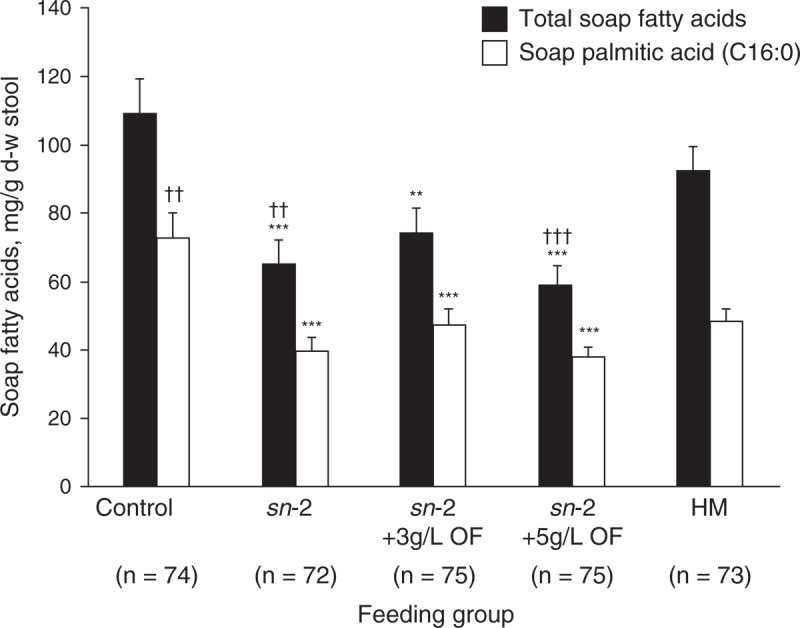
Stool soap palmitic acid (C16:0) and total fatty acids at week 8 for all infants according to feeding group. Analysis of variance followed by pairwise comparisons for formula groups, and independent t tests for each of the formula groups versus the HM group. Mean (±standard error) significantly different from control: ∗∗P < 0.01, ∗∗∗P < 0.001; significantly different from HM: ††P < 0.01, †††P < 0.001. d-w = dry weight; HM = human milk; OF = oligofructose; sn-2 = high sn-2 palmitate infant formula.
Mean stool calcium at 8 weeks was significantly lower in the sn-2+3 g/L OF and sn-2+5 g/L OF groups compared with control (P < 0.05) (Table 3). Stool calcium also differed between the OF formula groups with significantly less stool calcium in the sn-2+5 g/L OF group compared with the sn-2+3 g/L OF group (P = 0.012). The HM group had significantly lower stool calcium content than any of the formula groups (P < 0.05).
Stool Characteristics
Mean percentage of mushy soft stools was significantly higher in the sn-2 group at week 8 compared with control (78% vs 65%, P = 0.026), and the percentage of formed stools was significantly lower (15% vs 29%, P = 0.003) (Fig. 3). Addition of OF to the high sn-2 palmitate formula was associated with further reductions in the percentage of formed stools (10% in sn-2+3 g/L OF, 6% in sn-2+5 g/L OF) compared with control (P < 0.001), and an increase in the percentage of runny stools in the higher OF group. Comparisons of formula groups with the HM group showed a significantly higher percentage of formed stools in all of the formula groups with the exception of sn-2+5 g/L OF, which did not differ significantly versus HM (P = 0.155). Despite significantly softer stools in the sn-2 and sn-2 plus OF groups, mean (SD) daily number of bowel movements in these groups (which ranged from 1.5 [1.1] times/day in the sn-2+3 g/L OF group to 1.9 [1.3] times/day in the sn-2 group) was not significantly different from control (1.9 [1.4] times/day] or HM (1.6 [1.1] times/day).
FIGURE 3.
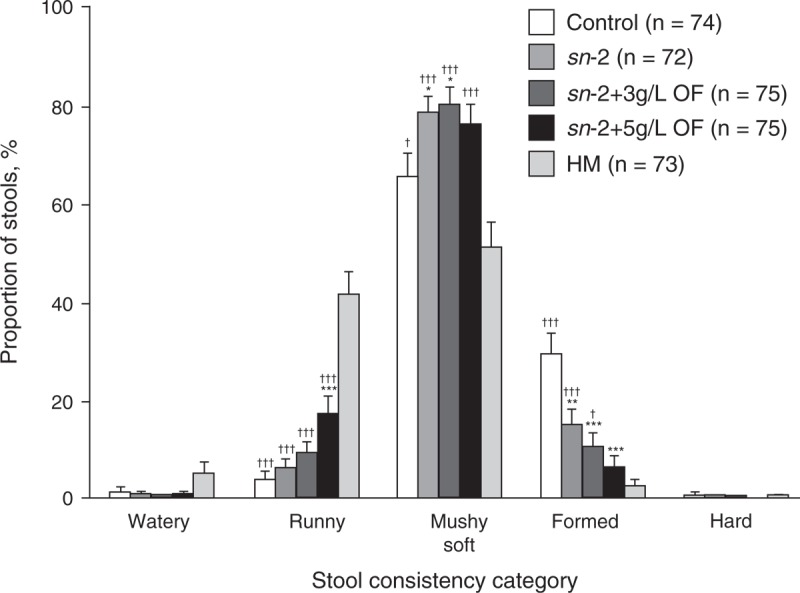
Stool consistency at week 8 for all infants according to feeding group. Analysis of variance followed by pairwise comparisons for formula groups, and independent t tests for each of the formula groups versus the HM group. Mean (±standard error) significantly different from control: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; significantly different from HM: †P < 0.05, ††P < 0.01, †††P < 0.001. HM = human milk; OF = oligofructose; sn-2 = high sn-2 palmitate infant formula.
Fecal Bifidobacteria
Baseline means and unadjusted week 8 means are shown in Figure 4. Statistical comparisons were based on adjusted means, which differed slightly from the unadjusted means shown. At baseline, mean fecal bifidobacteria counts did not differ significantly among formula groups, although mean counts were significantly lower in control and sn-2+3 g/L OF groups compared with the HM group (P = 0.0077 and P = 0.037, respectively). After 8 weeks, infants fed high sn-2 palmitate formulas, both with and without OF, had fecal bifidobacteria concentrations significantly higher than control (P = 0.033 for sn-2, P = 0.0002 for sn-2+3 g/L OF, and P = 0.0022 for sn-2+5 g/L OF, adjusted for baseline values), and did not differ from HM. Similarly, infants fed high sn-2 palmitate formulas with or without OF had a significantly greater increase in fecal bifidobacteria concentrations over an 8-week period compared with control and did not differ from HM.
FIGURE 4.
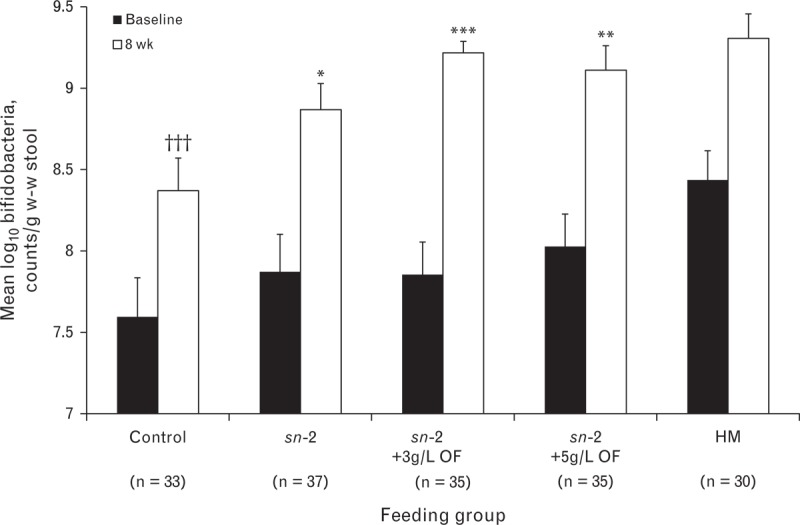
Bifidobacteria counts in w-w stool at baseline and week 8 in a subset according to feeding group. Plotted values are unadjusted mean (±standard error). Data were analyzed using analysis of covariance with baseline values and type of delivery (vaginal vs cesarean section) included in the model, followed by pairwise comparisons for formula groups, as well as for each of the formula groups versus the HM group. LS mean log10 counts/g w-w stool (±standard error) significantly different from control: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; significantly different from HM: †††P < 0.001. HM = human milk; LS = least squares; OF = oligofructose; sn-2 = high sn-2 palmitate infant formula; w-w = wet weight.
GI Tolerance
Mean IGSQ index scores were low in all feeding groups at baseline, week 4, and week 8, indicating an overall low GI symptom burden in the study population. At baseline, mean (SD) index scores were comparable among the formula groups (18.5 [4.0] for control, 18.1 [3.5] for sn-2, 18.5 [3.4] for sn-2+3 g/L OF, 18.5 [3.7] for sn-2+5 g/L OF). Baseline scores in the control and sn-2 as well as OF groups, however, were slightly but significantly higher (P < 0.01) than in the HM group (16.8 [3.14]). At weeks 4 and 8, no differences were observed among any of the feeding groups after adjusting for baseline scores. Assessment of IGSQ individual items and the presence or absence of specific GI symptoms supported the IGSQ index score results and did not reveal notable differences among the feeding groups.
Based on physicians’ reports, the overall incidence of study events ranged from 46.7% to 50.0% among all feeding groups. Pyrexia, cough, and rhinorrhea were the most common study events and were generally comparable among groups. In infants who experienced study events, 93.3% were unrelated to feedings and 83.3% were mild in intensity. GI-related events were reported in 20 infants overall and were slightly more common in formula-fed infants: 4 (5.3%), 6 (8.1%), 7 (9.2%), and 2 (2.7%) in the control, sn-2, sn-2+3 g/L OF, and sn-2+5 g/L OF groups, respectively, compared with 1 (1.3%) in the HM group. A total of 12 infants (3.2%) had GI events considered by the investigators as related to feedings: 3 (4.0%), 3 (4.1%), 4 (5.3%), 2 (2.7%), and 0 (0%) in the control, sn-2, sn-2+3 g/L OF, sn-2+5 g/L OF, and HM groups, respectively.
Anthropometry
Mean z scores for weight-for-age, length-for-age, head circumference-for-age, and weight-for-length were similar across all feeding groups. Moreover, mean z scores for weight-for-age, length-for-age, and head circumference-for-age were above −1.0, and mean weight-for-length z score was above −0.5, for all feeding groups at all time points.
DISCUSSION
Stool characteristics of HM-fed and formula-fed infants are different in many aspects, including levels of fatty acid–calcium soaps (3), stool consistency (3,5), and populations of beneficial bacteria such as bifidobacteria (28,29). Outcomes associated with feeding of infant formulas may be improved by the addition of components with specific functions, such as structured lipids rich in sn-2 palmitic acid (4,5,7,30–32) and also nondigestible oligosaccharides (8–11). These types of ingredients have been evaluated independently in previous studies; however, the effects of their combination have received only limited attention (33,34). HM provides approximately 70% of its palmitic acid in the sn-2 position (14,35), whereas typical formulas contain vegetable oil fat blends, with 8% to 15% palmitic acid in the sn-2 position (15). In this study, the experimental formulas contained approximately 40% of palmitic acid as sn-2 palmitate, an intermediate level between HM and typical formulas. The present study also evaluated the effects of increasing concentrations of the nondigestible carbohydrate OF in a design that permitted assessment of the addition of structured lipid alone or in combination with OF.
Triglycerides contribute approximately 50% of the calories in HM and infant formulas and, therefore, their effective digestion and absorption is critical for meeting the substantial caloric requirements of the rapidly growing infant. Triglyceride digestion occurs predominantly in the small intestine and involves the pancreatic lipase/colipase enzyme complex. Hydrolysis is selective for the sn-1 and sn-3 positions, resulting in the generation of 2 free fatty acids and an sn-2 monoglyceride (17). All sn-2 monoglycerides are well absorbed, as are unsaturated free fatty acids; however, saturated free fatty acids with chain lengths >12 carbons form insoluble, poorly absorbed soaps with intestinal calcium, leading to subsequent loss of both fatty acids and calcium (36). The present study clearly demonstrates that the positional distribution of palmitic acid influences fatty acid absorption in infants receiving formulas containing native oils (low levels of sn-2 palmitic acid) or structured triglycerides (higher levels of sn-2 palmitic acid). Stool soap levels of palmitate are much higher in infants receiving the control formula compared with the groups receiving formulas containing structured lipids. This same pattern is seen for other saturated fatty acids, such as myristic and lauric acids. Not surprisingly, the total fatty acid level in stool soaps followed the same pattern as the individual soap fatty acid levels: the control group had significantly higher levels than did the other formula groups. These results are similar to the stool fatty acid data of previous studies in term infants when similar structured lipid preparations were evaluated (5,6). Although we noted some differences in total fecal fat corresponding to different formulas, sufficient calories were absorbed from each formula to ensure normal growth. Anthropometry data showed infants fed high sn-2 palmitate formula, with or without OF, had growth parameters that were similar to those of infants fed control.
Fecal total fatty acid soaps in the HM-fed group in the present study followed an unexpected pattern. In general, HM-fed infants have lower total fatty acid soaps than infants receiving formulas with fat blends containing high levels of saturated fatty acids in the sn-1 and sn-3 positions (3,5). In the present study, the HM-fed group had lower palmitic acid soaps than the control group, as expected, whereas lauric and myristic acid soaps were at least as high in the HM-fed group as in control. As a consequence, total fatty acid soaps in the HM-fed group were higher than in the groups receiving high sn-2 palmitate formulas and only marginally lower than in the group receiving a standard formula. This is in contrast to other studies, but is not unexpected in light of the mothers enrolled in this study. The study population consisted of women of relatively low socioeconomic status from the Philippines. As previously demonstrated in a similar Filipino population, the breast milk fatty acid composition is high in lauric and myristic acids compared with breast milk collected from other countries (37). For example, the proportion of lauric acid in the Filipino population was >13% of the total fatty acids, which is 2 to 3 times higher than the levels from other countries; myristic acid followed a similar pattern. Levels of linoleic acid were approximately 50% lower in the Filipino group than other groups, indicating that this group was consuming a diet limited in both essential fatty acids and in total fat. Such diets result in increased endogenous mammary gland synthesis of fatty acids, with synthesis terminated at carbon chain lengths of 14 carbons or less by thioesterase II, a mammary gland-specific enzyme as opposed to hepatic (and other tissue) fatty acid synthesis in which synthesis is primarily terminated at a chain length of 16 carbons (38,39). This type of HM composition would result in a substantial increase in lauric and myristic acid intake by the breast-fed infants and the resulting increase in fecal lauric and myristic fatty acid soap output. In addition, stearic fatty acid soaps are elevated in the HM group, which also contributed to their high total fatty acid soap content. HM stearic acid levels from Filipino mothers are relatively low (37), so an elevated level of stool stearic acid soaps is a surprising and unexplained observation.
Stool calcium concentrations were not significantly different in the sn-2 group versus control; however, OF addition to the high sn-2 palmitate formula was associated with a significant reduction in stool calcium at week 8. The amount of calcium in the formulas differed slightly (formula assays revealed 3%, 7%, and 13% less calcium in the sn-2, sn-2+3 g/L OF, and sn-2+5 g/L OF formulas, respectively, compared with the control formula, shown in Table 1), which may have confounded the findings for stool calcium content; however, the magnitude and dose-response reduction of stool calcium in each experimental formula group versus control (mean stool calcium was 5%, 12%, and 25% lower in the sn-2, sn-2+3 g/L OF, and sn-2+5 g/L OF groups, respectively) suggest that the lower amounts of stool calcium in the experimental formula groups (particularly those with added OF) were likely the result of factors beyond differences in calcium intake. Because this was not a metabolic balance study, no firm conclusions can be drawn from these findings. The fact that stool calcium concentration in infants fed the sn-2 and control formulas were not significantly different in our study is consistent with the findings of Carnielli et al (4), who documented significantly lower fecal calcium excretion in infants fed a formula with sn-2 levels similar to HM, but not at levels similar to the sn-2 formula used in the present study. In addition, Lucas et al (7) reported improved calcium absorption in preterm infants but at a much higher sn-2 palmitate level. Two studies (5,30) have reported improved bone strength/quality (bone mineral density determined by dual-energy x-ray absorptiometry or bone speed of sound by quantitative ultrasound) in term infants fed a high sn-2 palmitate formula compared with those fed a standard formula. The possible effect of incremental lowering of fecal calcium content by OF added to the high sn-2 formula in our study is of interest and may be related to the ability of OF to solubilize calcium as well as promote the growth of bifidobacteria and lower colonic pH (40). Indeed, several studies (41–43) have demonstrated improved calcium absorption in pediatric populations as diverse as preterm infants and adolescents receiving supplemental OF. Furthermore, results from a clinical study using formulas with levels of sn-2 palmitate and OF similar to those in the present study, and with similar calcium levels in all study formulas, demonstrated that infants fed sn-2+3 g/L OF excreted 25% less calcium in the stool compared with the control group (44).
Infants receiving high sn-2 palmitate formula had softer, less-formed stools than infants in the control group, which is consistent with other studies (4,5). The addition of OF was associated with further reductions in the proportion of formed stools compared with controls. The sn-2 and sn-2+3 g/L OF groups had improved stool softness intermediate between the control and HM groups, whereas the sn-2+5 g/L OF group had softer stools, similar to those of the HM group. These findings are consistent with those reported by others for infants fed OF-supplemented formula (8–10,45,46). Despite significantly softer stools in the sn-2, sn-2+3 g/L OF, and sn-2+5 g/L OF groups, the frequency of daily bowel movements was not significantly different from that of infants receiving control formula or HM.
Breast milk contains a variety of nondigestible carbohydrates (47), and these may support populations of potentially beneficial colonic bacteria, such as bifidobacteria, which tend to be higher in HM-fed infants than in formula-fed infants (28,29). Previous studies in adults have demonstrated that OF clearly increases the proportion of colonic and fecal bifidobacteria (48), thus meeting the criteria for classification as a prebiotic (20,49). The present study evaluated the effects of a structured lipid in infant formula in combination with the nondigestible prebiotic OF. Surprisingly, infants fed the high sn-2 palmitate formula had significantly higher fecal bifidobacteria concentrations than infants receiving the control formula. Although prebiotic function has been clinically demonstrated for OF (9,10,19,46), the possibility that high sn-2 palmitate formulas may also have a prebiotic function is an important observation and further investigation in this area may prove fruitful. A pilot study (32) demonstrated that infants fed a formula elevated in sn-2 palmitate (similar to the sn-2 palmitate used in the present study) had fecal flora profiles similar to HM-fed infants and different from those fed a control formula. Infants receiving high sn-2 palmitate formula had significantly higher bifidobacteria and lactobacilli levels compared with those receiving control formula. Support of beneficial bacterial populations may result from direct growth promotion of these populations by sn-2-palmitate or from growth inhibition of these populations by palmitate–calcium soaps (32).
The overall tolerability of a new infant formula is an important consideration in its general use. GI tolerance measured by the IGSQ index score in the sn-2 and OF-supplemented formula groups was similar to that of both control and HM-fed infants. OF addition at 3 or 5 g/L did not increase the incidence of watery stools, nor was there evidence of increased gassiness. According to physicians’ reports, there was no increased incidence of study events in infants fed high sn-2 palmitate formula, either with or without OF. Furthermore, the incidence of GI study events in all formula-fed groups was low and similar to that of HM-fed infants.
Our study has several limitations. First, this was a single-center study. Second, the breast-feeding mothers were evidently consuming a relatively lowfat diet, although their diet did not result in production of inadequate breast milk because growth parameters were similar between the HM and formula groups. Finally, we did not collect HM for analysis in the breast-feeding group and were reliant on HM fatty acid data from a previous study in a similar Filipino population (37).
In summary, the present study evaluated alterations to standard infant formula in an effort to improve nutrient absorption, colonic beneficial bacteria levels, and stool consistency in healthy, term infants. The experimental formulas had both a modified lipid system (higher sn-2 palmitate levels) and carbohydrate system (inclusion of OF, a nondigestible carbohydrate). These additions, both moving formulas closer to HM, provided specific benefits in infants, including reduction in fecal loss of fatty acids (specifically palmitate) and calcium, softer stool consistency, and increased bifidobacteria levels. Such improvements to infant formula should not induce parents to use formulas instead of breast-feeding, but will provide a more acceptable option if formula must be used.
Acknowledgment
The authors thank Carol Cooper, PhD, of Caudex Medical (funded by Wyeth Nutrition) for editorial support.
Footnotes
www.clinicaltrials.gov registration number: NCT01861600.
This study was sponsored by Wyeth Nutrition, a Nestlé business.
M.Y., M.F., R.N., and J.L. are current employees of Nestlé Nutrition (M.Y., R.N.) and Wyeth Nutrition (M.F., J.L.). K.R., R.Y., L.W., and P.A.D. were employees of Wyeth Nutrition at the time of the study. The other authors report no conflicts of interest.
REFERENCES
- 1.Forsyth BW, McCarthy PL, Leventhal JM. Problems of early infancy, formula changes, and mothers’ beliefs about their infants. J Pediatr 1985; 106:1012–1017. [DOI] [PubMed] [Google Scholar]
- 2.Weaver LT, Ewing G, Taylor LC. The bowel habit of milk-fed infants. J Pediatr Gastroenterol Nutr 1988; 7:568–571. [DOI] [PubMed] [Google Scholar]
- 3.Quinlan PT, Lockton S, Irwin J, et al. The relationship between stool hardness and stool composition in breast- and formula-fed infants. J Pediatr Gastroenterol Nutr 1995; 20:81–90. [DOI] [PubMed] [Google Scholar]
- 4.Carnielli VP, Luijendijk IH, van Goudoever JB, et al. Structural position and amount of palmitic acid in infant formulas: effects on fat, fatty acid, and mineral balance. J Pediatr Gastroenterol Nutr 1996; 23:553–560. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy K, Fewtrell MS, Morley R, et al. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: effects on stool biochemistry, stool characteristics, and bone mineralization. Am J Clin Nutr 1999; 70:920–927. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Lopez A, Castellote-Bargallo AI, Campoy-Folgoso C, et al. The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces. Early Hum Dev 2001; 65 suppl:S83–S94. [DOI] [PubMed] [Google Scholar]
- 7.Lucas A, Quinlan P, Abrams S, et al. Randomised controlled trial of a synthetic triglyceride milk formula for preterm infants. Arch Dis Child Fetal Neonatal Ed 1997; 77:F178–F184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettler J, Euler AR. An evaluation of the growth of term infants fed formula supplemented with fructo-oligosaccharide. Int J Prebiotics Probiotics 2006; 1:19–26. [Google Scholar]
- 9.Euler AR, Mitchell DK, Kline R, et al. Prebiotic effect of fructo-oligosaccharide supplemented term infant formula at two concentrations compared with unsupplemented formula and human milk. J Pediatr Gastroenterol Nutr 2005; 40:157–164. [DOI] [PubMed] [Google Scholar]
- 10.Kapiki A, Costalos C, Oikonomidou C, et al. The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Hum Dev 2007; 83:335–339. [DOI] [PubMed] [Google Scholar]
- 11.Veereman-Wauters G, Staelens S, Van de Broek H, et al. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J Pediatr Gastroenterol Nutr 2011; 52:763–771. [DOI] [PubMed] [Google Scholar]
- 12.Innis SM. Human milk and formula fatty acids. J Pediatr 1992; 120:S56–S61. [DOI] [PubMed] [Google Scholar]
- 13.Jensen RG, Ferris AM, Lammi-Keefe CJ, et al. Lipids of bovine and human milks: a comparison. J Dairy Sci 1990; 73:223–240. [DOI] [PubMed] [Google Scholar]
- 14.Martin JC, Bougnoux P, Antoine JM, et al. Triacylglycerol structure of human colostrum and mature milk. Lipids 1993; 28:637–643. [DOI] [PubMed] [Google Scholar]
- 15.Straarup EM, Lauritzen L, Faerk J, et al. The stereospecific triacylglycerol structures and fatty acid profiles of human milk and infant formulas. J Pediatr Gastroenterol Nutr 2006; 42:293–299. [DOI] [PubMed] [Google Scholar]
- 16.Small DM. The effects of glyceride structure on absorption and metabolism. Annu Rev Nutr 1991; 11:413–434. [DOI] [PubMed] [Google Scholar]
- 17.Bracco U. Effect of triglyceride structure on fat absorption. Am J Clin Nutr 1994; 60:1002S–1009S. [DOI] [PubMed] [Google Scholar]
- 18.Sabater-Molina M, Larqué E, Torrella F, et al. Dietary fructooligosaccharides and potential benefits on health. J Physiol Biochem 2009; 65:315–328. [DOI] [PubMed] [Google Scholar]
- 19.Van Loo J, Cummings J, Delzenne N, et al. Functional food properties of non-digestible oligosaccharides: a consensus report from the ENDO project (DGXII AIRII-CT94-1095). Br J Nutr 1999; 81:121–132. [DOI] [PubMed] [Google Scholar]
- 20.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995; 125:1401–1412. [DOI] [PubMed] [Google Scholar]
- 21.Hidaka H, Eida T, Takizawa T, et al. Effects of fructo-oligosaccharide on intestinal flora and human health. Bifidobacteria Microflora 1987; 5:37–50. [Google Scholar]
- 22.Closa-Monasterolo R, Gispert-Llaurado M, Luque V, et al. Safety and efficacy of inulin and oligofructose supplementation in infant formula: results from a randomized clinical trial. Clin Nutr 2013; 32:918–927. [DOI] [PubMed] [Google Scholar]
- 23.Standard for infant formula and formulas for special medical purposes intended for infants. Codex Stan 72-1981. www.codexalimentarius.net/download/standards/288/CXS_072e.pdf. Published 2011 Accessed November 25, 2013. [Google Scholar]
- 24.AOAC International. Official Methods of Analysis of AOAC International: Method 968.06. 17th ed.Gaithersburg, MD: AOAC International; 2000. [Google Scholar]
- 25.Dahlquist RL, Knoll JW. Inductively coupled plasma-atomic emission spectrometry of biological materials and soils for major, trace, and ultra-trace elements. Appl Spectrosc 1978; 32:1–29. [Google Scholar]
- 26.Smith SC, Choy R, Johnson SK, et al. Lupin kernel fiber consumption modifies fecal microbiota in healthy men as determined by rRNA gene fluorescent in situ hybridization. Eur J Nutr 2006; 45:335–341. [DOI] [PubMed] [Google Scholar]
- 27.Yao M, Riley A, Trabulsi J, et al. Use of an electronic handheld diary to validate a new parent questionnaire assessing gastrointestinal tolerance in infants. J Pediatr Gastroenterol Nutr 2010; 50 suppl 2:E208. [Google Scholar]
- 28.Fanaro S, Chierici R, Guerrini P, et al. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 2003; 91:48–55. [DOI] [PubMed] [Google Scholar]
- 29.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 2000; 30:61–67. [DOI] [PubMed] [Google Scholar]
- 30.Litmanovitz I, Davidson K, Eliakim A, et al. High beta-palmitate formula and bone strength in term infants: a randomized, double-blind, controlled trial. Calcif Tissue Int 2013; 92:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson CM, Innis SM. Plasma lipoprotein fatty acids are altered by the positional distribution of fatty acids in infant formula triacylglycerols and human milk. Am J Clin Nutr 1999; 70:62–69. [DOI] [PubMed] [Google Scholar]
- 32.Yaron S, Shachar D, Abramas L, et al. Effect of high beta-palmitate content in infant formula on the intestinal microbiota of term infants. J Pediatr Gastroenterol Nutr 2013; 56:376–381. [DOI] [PubMed] [Google Scholar]
- 33.Savino F, Cresi F, Maccario S, et al. Minor’ feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo- and galacto-oligosaccharides. Acta Paediatr Suppl 2003; 91:86–90. [DOI] [PubMed] [Google Scholar]
- 34.Schmelzle H, Wirth S, Skopnik H, et al. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr 2003; 36:343–351. [DOI] [PubMed] [Google Scholar]
- 35.Tomarelli RM, Meyer BJ, Weaber JR, et al. Effect of positional distribution on the absorption of the fatty acids of human milk and infant formulas. J Nutr 1968; 95:583–590. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez M, Amate L, Gil A. Absorption and distribution of dietary fatty acids from different sources. Early Hum Dev 2001; 65 suppl:S95–S101. [DOI] [PubMed] [Google Scholar]
- 37.Yuhas R, Pramuk K, Lien EL. Human milk fatty acid composition from nine countries varies most in DHA. Lipids 2006; 41:851–858. [DOI] [PubMed] [Google Scholar]
- 38.Innis SM. Polyunsaturated fatty acids in human milk: an essential role in infant development. Adv Exp Med Biol 2004; 554:27–43. [DOI] [PubMed] [Google Scholar]
- 39.Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr 1997; 17:159–183. [DOI] [PubMed] [Google Scholar]
- 40.Lee YS, Kang EY, Park MN, et al. Effects of sn-2 palmitic acid-fortified vegetable oil and fructooligosaccharide on calcium metabolism in growing rats fed casein based diet. Nutr Res Pract 2008; 2:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin IJ, Davila PM, Abrams SA. Non-digestible oligosaccharides and calcium absorption in girls with adequate calcium intakes. Br J Nutr 2002; 87 suppl 2:S187–S191. [DOI] [PubMed] [Google Scholar]
- 42.Lidestri M, Agosti M, Marini A, et al. Oligosaccharides might stimulate calcium absorption in formula-fed preterm infants. Acta Paediatr Suppl 2003; 91:91–92. [DOI] [PubMed] [Google Scholar]
- 43.van den Heuvel EG, Muys T, van Dokkum W, et al. Oligofructose stimulates calcium absorption in adolescents. Am J Clin Nutr 1999; 69:544–548. [DOI] [PubMed] [Google Scholar]
- 44.Nowacki J, Lee HC, Lien R, et al. Effects of an alpha-lactalbumin-enriched term infant formula containing high sn-2 palmitate and oligofructose on stool biochemistry, stool characteristics, and gastrointestinal tolerance: a multicenter, randomized, controlled trial in Taiwan (abstract). World Congress of Pediatric Gastroenterology, Hepatology and Nutrition (WCPGHAN); 2012:OP-3-3-4. [Google Scholar]
- 45.Guesry PR, Bodanski H, Tomsit E, et al. Effect of 3 doses of fructooligosaccharides in infants. J Pediatr Gastroenterol Nutr 2000; 31: (Abstr 989). [Google Scholar]
- 46.Waligora-Dupriet AJ, Campeotto F, Nicolis I, et al. Effect of oligofructose supplementation on gut microflora and well-being in young children attending a day care centre. Int J Food Microbiol 2007; 113:108–113. [DOI] [PubMed] [Google Scholar]
- 47.Kunz C, Rudloff S, Baier W, et al. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 2000; 20:699–722. [DOI] [PubMed] [Google Scholar]
- 48.Gibson GR, Beatty ER, Wang X, et al. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995; 108:975–982. [DOI] [PubMed] [Google Scholar]
- 49.Gibson GR, Probert HM, Loo JV, et al. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 2004; 17:259–275. [DOI] [PubMed] [Google Scholar]


