Abstract
Four different spinal cord injury (SCI) models (hemisection, contusion, transection, and segment resection) were produced in male Sprague–Dawley rats to determine the most suitable animal model of SCI by analyzing the changes in diffusion tensor imaging (DTI) parameters both qualitatively and quantitatively in vivo. Radiological examinations were performed before surgery and weekly within 4 weeks after surgery to obtain DTI tractography, MRI routine images, and DTI data of fractional anisotropy (FA) and apparent diffusion coefficient (ADC). The Basso, Beattie, and Bresnahan scale was used to evaluate the locomotor outcomes. We found that DTI tractography tracked nerve fibers and showed conspicuous changes in the injured spinal cord in all the model groups, which confirmed that our modeling was successful. A decrease in FA values and an increase in ADC were observed in all the model groups after surgery. There were significant differences in FA and ADC between weeks 1 and 4 in both hemisection and contusion groups (P<0.05), whereas the differences in the transection and segment resection groups were not as remarkable (P>0.05). Basso, Beattie, and Bresnahan scores further proved the results because of a significant, positive correlation of the scores with FA (R=0.899, P<0.01) and a significant, negative correlation of the scores with ADC (R=−0.829, P<0.01). Therefore, the transection model, which is more quantified and stable within 4 weeks after injury according to the DTI and behavioral evaluation, should be used as the standard model for SCI animal testing.
Keywords: diffusion tensor imaging, MRI, rat, spinal cord injury
Introduction
Spinal cord injury (SCI) can potentially cause catastrophic damage to the central nervous system 1. Animal models of SCI are a necessary prerequisite for the evaluation of suitable therapeutic regimens clinically 2,3. An ideal animal model should reflect the clinical pathological changing process of SCI and meet the requirements of availability and repeatability 4–6. Currently, the most widely used SCI models in rats include hemisection, contusion, transection, and segment resection. However, each model reproduces the anatomical features and neurological function changes in a certain type of SCI. Not any model can completely simulate the actual SCI in human 7,8.
Diffusion tensor imaging (DTI), as a relatively new method based on MRI that monitors the diffusion of water molecules in tissues and cells, has made up the deficiencies of MRI to some extent and has now become an important means of detective diagnostics in the neuroimaging field. Moreover, the apparent diffusion coefficient (ADC) and fractional anisotropy (FA), two important parameters from DTI data, are usually used to provide quantitative information on inferring microstructural features and the physiological state of tissues 9,10. Many animal studies and preliminary clinical studies have shown that DTI has the ability to detect subtle changes of the injured nerve fibers, even in regions far away from the damage zone of the spinal cord 11–16, and DTI tractography can be used as a qualitative indicator of SCI to track the damaged nerve fibers visually and observe the lesion of axonal bundles clearly 13,15,16.
On the basis of the above considerations, the aim of this study is to determine the standard model for SCI research by comparing four different SCI models in rats in vivo from the perspective of imaging both qualitatively (tractography) and quantitatively (FA and ADC) using DTI, and to show the changes in SCI within four postoperative weeks through DTI-related parameters among the four models and to evaluate the relationship between these changes and the Basso, Beattie, and Bresnahan (BBB) locomotor outcomes.
Materials and methods
Animals
The experiments were approved by the Ethical Committee of Xi’an Jiaotong University. A total of 20 male Sprague–Dawley rats were used, and all the rats were allowed preoperative environmental adaptation for 2 weeks with normal circadian rhythms and had free access to water and food.
The rats (250–300 g) were divided into five groups (n=4): four model groups and a control group. Four different SCI models, namely, hemisection, contusion, transection, and segment resection, were established and the rats with SCI were then subjected to radiological measurements before the surgery and weekly within 4 weeks after the surgery to obtain the tractography showing the axonal integrity and the values of FA and ADC. The four normal rats as controls were also examined to obtain these same data.
Surgical procedures
Rats were anesthetized with an intraperitoneal injection of a 10% chloral hydrate solution (0.35 ml/100 g). A laminectomy was performed at the T9–10 level to expose one spinal cord segment. In the hemisection or the transection model, either a T9 spinal cord hemisection on the left of the spinal cord or a T9 transection was performed with a sharp blade visually. The injury in the contusion model was caused by the NYU weight-drop, (New York University, New York, USA) device with a moderate weight dropping from a height of 25.0 mm 17,18. In the segment resection model, the T9 segment of the spinal cord was taken off. Afterward, the surgical region in all the rats was covered with a piece of fat pad taken from the rat itself to protect the injured spinal cord, and the muscle and skin was then sutured in layers. After these surgical procedures, the rats received intensive care postoperatively, with their urinary bladders emptied manually twice every day.
MRI acquisition and data processing
All MRI data were acquired on a 3 T MR scanner (Signa; GE Medical Systems, Milwaukee, Wisconsin, USA) using a specialized coil designed for scanning rats. The rat was placed in the coil in a supine position to reduce the influence of respiratory movement on magnetic resonance artifacts of the spinal cord. Data acquisition was carried out on the region of interest, namely, the damaged area.
The relevant parameters of T1-weighted and T2-weighted images of the injured rats were as follows: TR=560 and 2600 ms, respectively; TE=11.3 and 120 ms, respectively; matrix size=320×224, FOV=8, and slice thickness=1.5 mm in both. The DTI was obtained in the sagittal view with b value=1000 s/mm2, TR=3500 ms, TE=87.5 ms, 64×64 matrix, FOV=10, and slice thickness 2.4 mm in 15 diffusion gradient directions. When the scan of each rat was completed, the relevant parameters were transferred to a separate workstation (Advantage Windows, version 4.2; GE Healthcare, Waukesha, Wisconsin, USA) and the corresponding photographic images were then synthesized using FuncTool (GE Healthcare), a commercial software.
BBB scores
The hindlimb movements of all the injured rats were recorded weekly through video by an independent researcher and the locomotor BBB scores were evaluated by two other independent researchers. The video-recording was performed at 8:00 p.m. in the evening because of the large difference in the circadian activity of rats between daytime and night-time.
Statistical analysis
A repeated-measures analysis of variance was carried out to examine the change trend in FA and ADC values and BBB scores over time and the differences in these parameters between groups, followed by the Bonferroni adjustment test and the least significant difference test. Then, the Pearson correlation coefficient (R) was calculated for each time point to quantify the linear correlation of the BBB score with FA or ADC. All statistical analyses were carried out using the SPSS 16.0 software (SPSS Inc., Chicago, Illinois, USA).
Results
Conventional MR images and tractography
The conventional magnetic resonance T1-weighted and T2-weighted images of the control group presented an intact spinal cord and no obvious abnormalities were found in the signal intensity (Fig. 1a and b). For the four SCI model groups, the T1-weighted images vaguely showed the injured area whereas the T2-weighted images clearly visualized the cutting trace of the spinal cord and the presence of edema after contusion (Fig. 1c–j).
Fig. 1.
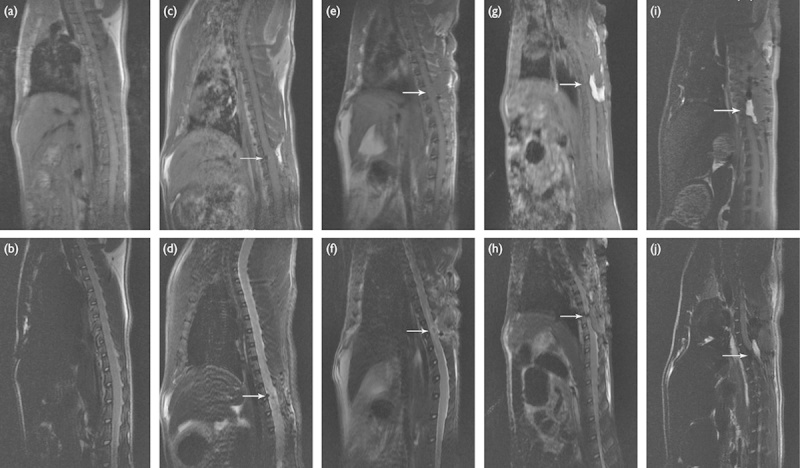
T1-weighted and T2-weighted images of the spinal cord of rats in different groups at week 1 postoperatively. (a, b) Control group; (c, d) hemisection group; (e, f) contusion group; (g, h) transection group; (i, j) segment resection group. The arrow indicates the injured site.
The cross-sections of FA and ADC images showed that the signal intensity decreased gradually in FA and increased in ADC with the scanning layer by layer toward the damaged zone. More critically, the tractography that could track nerve fibers visualized the changes in the injured spinal cord more finely, and even the shape of a single nerve fiber could be distinguished (Fig. 2b–i). During the 4-week postoperative observation, the damage to the spinal cord was the most noticeable in the first week; by the fourth week, the nerve fibers showed different degrees of repair and regeneration (Fig. 2b–i). The signal intensity in the contusion group showed a significant improvement in the damaged nerve fibers in the fourth week compared with that in the first week (Fig. 2d and e), whereas the repair and regeneration of nerve fibers in the transection or segment resection groups appeared disorganized and disordered (Fig. 2f–i).
Fig. 2.
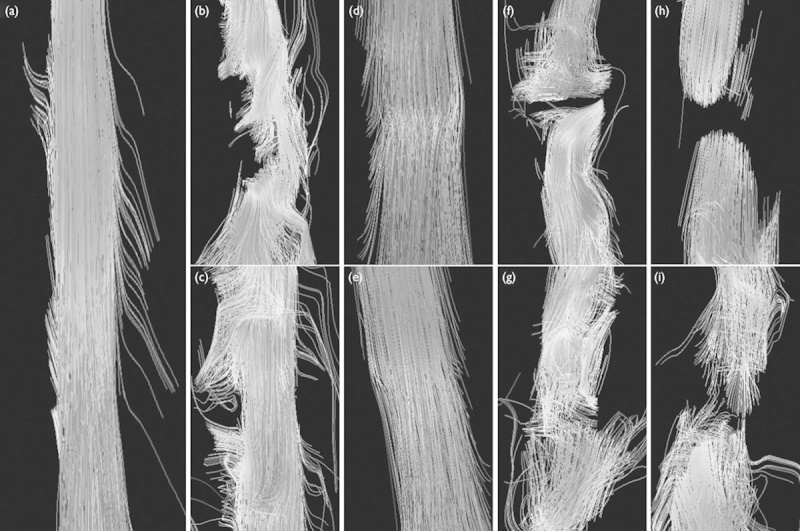
DTI tractography images of the spinal cord of rats in different groups. (a) Control group at postoperative week 1; (b, c) hemisection group; (d, e) contusion group; (f, g) transection group; (h, i) segment resection group; each at postoperative weeks 1 and 4, respectively. DTI, diffusion tensor imaging.
FA and ADC values and BBB score
The FA and ADC values and BBB scores were measured and recorded at five time points, namely, before surgery, and weeks 1, 2, 3, and 4 (Table 1). The FA and ADC values in the control group did not change significantly over the five times of measurement (P=1.00>0.05).
Table 1.
Measurement of FA and ADC values and BBB scores
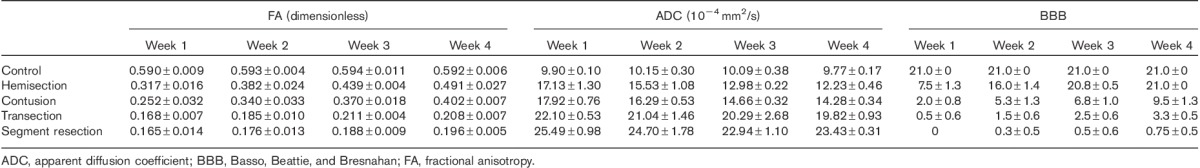
In the hemisection group, the FA value was compared three times: baseline versus the 1-week (P=0.001<0.05), baseline versus the 2-week (P=0.001<0.05), and baseline versus the 3-week (P=0.000<0.05), but there was no notable difference between the value at week 4 and that before surgery (P=0.084>0.05), indicating that the FA value might restore to the normal level after 4 weeks. In the rest of the three model groups, namely, contusion (P=0.000<0.05), transection (P=0.000<0.05), and segment resection (P=0.000<0.05), the FA value changed significantly throughout the 4 weeks after surgery. There was a notable difference between the FA value at week 1 and that at weeks 3 (P=0.009<0.05) and 4 (P=0.017<0.05) in the contusion group, indicating that FA increased again after a decrease upon damage, but did not reach the normal levels by the end of week 4. In the transection and segment resection groups, the FA values did not vary much among the 4 weeks after surgery, namely, the 1- versus the 2-week (P=0.510>0.05), the 2- versus the 3-week (P=0.373>0.05), and the 3- versus the 4-week (P=1.000>0.05) in the transection group, the 1- versus the 2-week (P=1.000>0.05), the 2- versus the 3-week (P=0.228>0.05), and the 3- versus the 4-week (P=0.695>0.05) in the segment resection group, indicating that the SCI in rats caused by transection or segment resection was stable within 4 weeks postoperatively. Before surgery, the FA values were almost the same in the five groups, respectively, normal versus hemisection (P=0.233>0.05), normal versus contusion (P=0.963>0.05), normal versus transection (P=0.607>0.05), and normal versus segment resection (P=0.403>0.05), as were the ADC values and BBB scores. At all the postoperative time points, the FA values were significantly different between groups (P=0.000<0.05), except at weeks 1 (P=0.830>0.05), 2 (P=0.541>0.05), and 4 (P=0.219>0.05) between the transection group and the segment resection group, which indicated a similar injury in the two models.
In either the hemisection or the contusion group, a significant difference in the ADC value existed between the baseline and week 4 (P=0.033<0.05 in the hemisection group; P=0.004<0.05 in the contusion group), and between weeks 1 and 4 (P=0.042<0.05 in the hemisection group; P=0.048<0.05 in the contusion group). However, we compared the ADC values between different postoperative time points in the transection and segment resection groups, respectively, the 1- versus the 2-week (P=1.000>0.05), the 2- versus the 3-week (P=1.000>0.05), and the 3- versus the 4-week (P=1.000>0.05) in the transection group; the 1- versus the 2-week (P=1.000>0.05), the 2- versus the 3-week (P=0.352>0.05), and the 3- versus the 4-week (P=1.000>0.05) in the segment resection group; thus, no significant difference was found both in the transection and in the segment resection group. The ADC values of different groups at each time point were also compared and there were only notable differences at weeks 1 (P=0.206>0.05), 2 (P=0.373>0.05), and 3 (P=0.091>0.05) between the hemisection and contusion groups, which showed a heavier injury in segment resection than in hemisection.
The BBB scores in the hemisection group were increasingly significantly at weeks 3 (P=0.006<0.05) and 4 (P=0.002<0.05) compared with week 1, respectively, and the scores at weeks 3 (P=1.000>0.05) and 4 (P=1.000>0.05) were almost the same with the baseline. The trend of the change in BBB scores in the contusion group was similar to that in the hemisection group, but the significant difference between week 4 and the baseline remained after week 4 (P=0.000<0.05). For the transection and segment resection groups, respectively, there were no significant differences between two postoperative time points, namely, the 1- versus the 2-week (P=0.917>0.05), the 2- versus the 3-week (P=0.917>0.05), and the 3- versus the 4-week (P=0.577>0.05) in the transection group; the 1- versus the 2-week (P=1.000>0.05), the 2- versus the 3-week (P=1.000>0.05), and the 3- versus the 4-week (P=1.000>0.05) in the segment resection group. At the same time point, the BBB scores in the hemisection group were equal to those in the control group at weeks 3 (P=0.568>0.05) and 4 (P=1.000>0.05), respectively, indicating that the injury caused by hemisection restored. However, the BBB scores in the transection group were different from those in either the segment resection group (P=0.000<0.05 either at weeks 3 or 4) or the contusion group (P=0.000<0.05 either at weeks 3 or 4) at weeks 3 and 4.
Correlation analysis showed a significant positive correlation (R=0.899) between FA values and BBB scores and a significant negative correlation (R=−0.829) between the ADC values and BBB scores.
Discussion
Our results show that DTI is a good predictor for SCI in rats. DTI tractography images can visually indicate the injury of the spinal cord tract; FA and ADC values from DTI data are roughly in line with locomotor outcomes and can be used to assess the severity of the injury. On the basis of the results of this study, the transection model should be the most appropriate animal model for the study of SCI.
Studies have suggested that a residue of a minimum of 5% nerve fibers could cause the joint movement of the paraplegic hindlimbs of rats to restore partially or completely; when the residual rate is over 40%, the locomotor function may return to normal 19,20. Considering all the above, we assume that a good animal model of SCI must meet both of the following two conditions. First, the nerve fiber bundles are completed transected or the residual fibers can be quantified before any treatment is administered; second, the model must be stable. There should not be a significant difference in kinematic evaluation or any signs of recovery through a period of time after SCI without any treatment. The difference and signs can make the experimentally derived treatment effect controversial. Besides this, availability and repeatability of the animal model should also be taken into account. In the present study, the transection model was found to be able to fulfill the above conditions and we recommend it to be used as the standard model of SCI animal testing.
DTI is developed on the basis of detecting the diffusion anisotropy and diffusion intensity of free water molecules. DTI tractography is a novel method to distinguish the orientation of nerve fibers and axon damage after SCI as the injured nerve fibers are associated closely with the diffusion of water molecules inside and outside the damaged cytomembrane. Through the positioning of microstructures that are orderly arranged, such as white matter fiber bundles, DTI tractography can clearly display the shapes of the structures. In our study, DTI tractography detected that the never fibers at the site of SCI had partial or complete robust disruption at week 1 after injury, which represented the success of the modeling. It was especially evident in the transection and segment resection models where the spinal cord was completely severed. The injuries are further confirmed by conventional T1-weighted and T2-weighted images. In the subsequent observations, DTI tractography showed different degrees of regrowth in the damaged nerve fibers whereas the MRI results remained the same. Although the regeneration of nerve fibers appeared disorganized and actually no signs of recovery were identified in the behavioral outcome evaluation in transection and segment resection groups, DTI is still more perspicuous than conventional MRI in showing the dynamic changes of this process. In addition, our tractography results are more distinct compared with those in other studies 13,15,16,21.
On the basis of the DTI tractography images, transection should be the best choice of the standard animal model for SCI. The nerve fiber bundles in the transection model are completely amputated, which meets the first condition of a good animal model for SCI. There are only signal intensity changes in the contusion model and the nerve fibers are partially transected in the hemisection model, in which the quantity of residual fibers could not be assessed accurately. As for the segment resection model, the injury is too severe and extensively different from real SCI in human. In our experiments, we also observed that the spinal cord of some rats in the transection or segment resection groups appeared to have very small amounts of fiber bundles connected between the broken ends. We speculate that the broken ends might be in the same cross-section resulting from rotation or mismatching of the spinal cord because of the manipulation in operation, even though the spinal cord was really transected. In the postsynthesis of tractography by tracking nerve fibers in different cross-sections, the water molecules diffused across the injured site as the interspace between the broken ends could not be identified, resulting in the illusion on the radiograph that the spinal cord was still conjoint. This made it unclear whether the regeneration of nerve fibers after 4 weeks was purely regeneration or caused by certain subtle movements resulted from the loss of spine stability after spinal laminectomy. The above phenomena did not occur in the contusion model as contusion did not cause rupture of the spinal cord.
We have also found a decrease in FA and an increase in ADC after SCI, similar to other studies 21,22,23. However, there have existed controversies on the use of ADC or FA as biomarkers of SCI. For example, Shanmuganathan et al. 24 consider ADC as more sensitive than FA, whereas Mondragon-Lozano et al. 22 recommend the use of FA instead of ADC. In this study, we took both ADC and FA into consideration. The significant difference in FA and ADC between postoperative weeks 1 and 4 in the hemisection and contusion models indicated that SCI caused in these two ways recovered with time. It is especially the case in the hemisection model, where the FA values restored to almost the normal level 4 weeks after injury. The FA and ADC values in the transection and segment resection models did not alter significantly during the 4 weeks after surgery, suggesting that SCI caused by these two methods is stable. However, the segment resection model is still not a good choice because the injury is too severe and extensively different from the real SCI situation. Therefore, the transection model is the most suitable among the four SCI rat models for SCI animal testing.
Correlation analysis showed an association of BBB scores with the FA and ADC values; it is therefore feasible to use behavioral evaluation to verify injury assessment. Studies have found that, because of the compensatory effect of contralateral normal nerve fibers, the locomotor function of the paralyzed hindlimbs in rats after hemisection of the spinal cord can restore to normal after 3 weeks and that in macaques can gradually restore to basic motor function after 1 month 25,26. Our results are consistent with these findings.
The DTI data in this study were collected from only the damaged area of the spinal cord and none were obtained from the uninjured area from the same animal to be set as self-control. Studies have shown that the DTI data from different sections of the spinal cord can be slightly different, but this might be because of an extension of edema and inflammation 22. Therefore, lack of self-control would not influence our results. The imageological parameters in our study were different from others 16,21,22,23. The parameters were specifically debugged before the experiments to obtain images with the highest quality and to obtain precision data in measurement. We believe that our parameters may serve as a reference for other researchers.
Conclusion
DTI can provide qualitatively and quantitatively different information about SCI in rats. The transection model, which is more quantified and stable than other animal models within 4 weeks after injury, should be used as the standard model for SCI animal testing. In addition, we suggest that DTI should be used in combination with routine MRI, which will be more effective in identifying the severity and extent of SCI.
Acknowledgements
This study was supported by the research grants from the National Natural Science Foundation of China (No. 81271340) and the Project of Science and Technology Development in Shaanxi Province in 2013 (No. 2013K12-20-08).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Van Goethem JW, Maes M, Ozsarlak O, van den Hauwe L, Parizel PM. Imaging in spinal trauma. J Eur Radiol 2005; 15:582–590. [DOI] [PubMed] [Google Scholar]
- 2.Constantini S, Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg 1994; 80:97–111. [DOI] [PubMed] [Google Scholar]
- 3.Wrathall JR, Choiniere D, Teng YD. Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. J Neurosci 1994; 14:6598–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrmann DL, Bresnahan JC, Beattie MS. Modeling of acute spinal cord injury in the rat: neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Exp Neurol 1994; 126:61–75. [DOI] [PubMed] [Google Scholar]
- 5.Behrmann DL, Bresnahan JC, Beattle MS, Shah BR. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histological analysis. J Neurotrauma 1992; 9:197–217. [DOI] [PubMed] [Google Scholar]
- 6.Bresnahan JC, Beattie MS, Stokes BT, Conway KM. Three-dimensional computer-assisted analysis of graded contusion lesions in the spinal cord of the rat. J Neurotrauma 1991; 8:91–101. [DOI] [PubMed] [Google Scholar]
- 7.Sedý J, Urdzíková L, Jendelová P, Syková E. Methods for behavioral testing of spinal cord injured rats. Neurosci Biobehav Rev 2008; 32:550–580. [DOI] [PubMed] [Google Scholar]
- 8.Li XF, Dai LY. Three-dimensional finite element model of the cervical spinal cord: preliminary results of injury mechanism analysis. Spine (Phila Pa 1976) 2009; 34:1140–1147. [DOI] [PubMed] [Google Scholar]
- 9.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 1995; 8:333–344. [DOI] [PubMed] [Google Scholar]
- 10.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111:209–219. [DOI] [PubMed] [Google Scholar]
- 11.Deo AA, Grill RJ, Hasan KM, Narayana PA. In vivo serial diffusion tensor imaging of experimental spinal cord injury. J Neurosci Res 2006; 83:801–810. [DOI] [PubMed] [Google Scholar]
- 12.Herrera JJ, Chacko T, Narayana PA. Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J Neurosci Res 2008; 86:443–447. [DOI] [PubMed] [Google Scholar]
- 13.Hobert MK, Stein VM, Dziallas P, Ludwig DC, Tipold A. Evaluation of normal appearing spinal cord by diffusion tensor imaging, fiber tracking, fractional anisotropy, and apparent diffusion coefficient measurement in 13 dogs. Acta Vet Scand 2013; 55:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo WK, Kim TH, Hai DM, Sundaram S, Yang YM, Park MS, et al. Correlation of magnetic resonance diffusion tensor imaging and clinical findings of cervical myelopathy. Spine J 2013; 13:867–876. [DOI] [PubMed] [Google Scholar]
- 15.Rao JS, Zhao C, Yang ZY, Li SY, Jiang T, Fan YB, Li XG. Diffusion tensor tractography of residual fibers in traumatic spinal cord injury: a pilot study. J Neuroradiol 2013; 40:181–186. [DOI] [PubMed] [Google Scholar]
- 16.Kelley B, Harel N, Kim CY, Papademetris X, Coman D, Wang X, et al. Diffusion tensor imaging as a predictor of locomotor function following experimental spinal cord injury and recovery. J Neurotrauma 2014; 29[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12:1–21. [DOI] [PubMed] [Google Scholar]
- 18.Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. J Am Med Assoc 1911; 57:878–880. [Google Scholar]
- 19.Basso DM, Beattle MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996; 139:244–256. [DOI] [PubMed] [Google Scholar]
- 20.Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol 1995; 132:220–228. [DOI] [PubMed] [Google Scholar]
- 21.Pease A, Miller R. The use of diffusion tensor imaging to evaluate the spinal cord in normal and abnormal dogs. Vet Radiol Ultrasound 2011; 52:492–497. [DOI] [PubMed] [Google Scholar]
- 22.Mondragon-Lozano R, Diaz-Ruiz A, Ríos C, Olayo Gonzalez R, Favila R, Salgado-Ceballos H, Roldan-Valadez E. Feasibility of in vivo quantitative magnetic resonance imaging with diffusion weighted imaging, T2-weighted relaxometry, and diffusion tensor imaging in a clinical 3 tesla magnetic resonance scanner for the acute traumatic spinal cord injury of rats: technical note. Spine (Phila Pa 1976) 2013; 38:E1242–E1249. [DOI] [PubMed] [Google Scholar]
- 23.Griffin JF, 4th, Cohen ND, Young BD, Eichelberger BM, Padua A, Jr, Purdy D, Levine JM. Thoracic and lumbar spinal cord diffusion tensor imaging in dogs. J Magn Reson Imaging 2013; 37:632–641. [DOI] [PubMed] [Google Scholar]
- 24.Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. Am J Neuroradiol 2008; 29:655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saruhashi Y, Young W. Effect of mianserin on locomotory function after thoracic spinal cord hemisection in rats. Exp Neurol 1994; 129:207–216. [DOI] [PubMed] [Google Scholar]
- 26.Darian-Smith I, Burman K, Darian-Smith C. Parallel pathways mediating manual dexterity in the macaque. Exp Brain Res 1999; 1281–2101–108. [DOI] [PubMed] [Google Scholar]


