Abstract
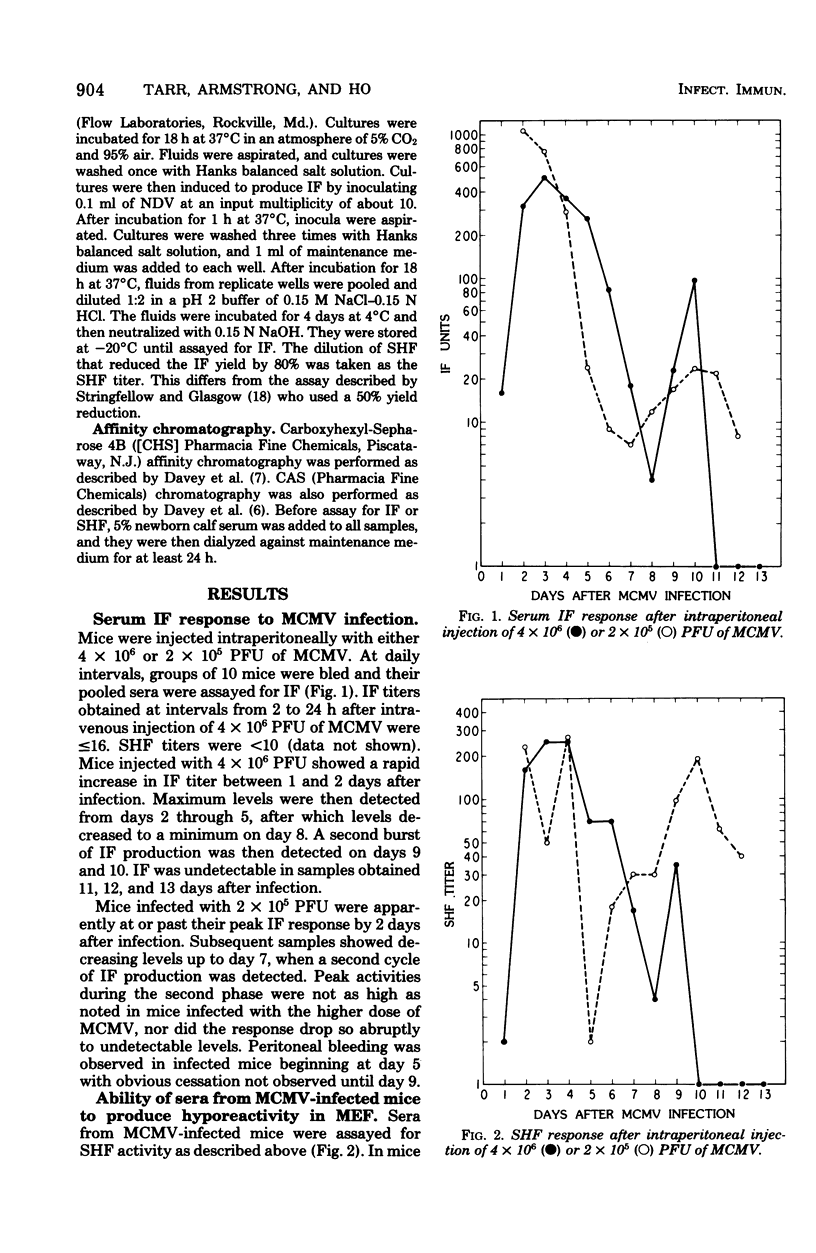
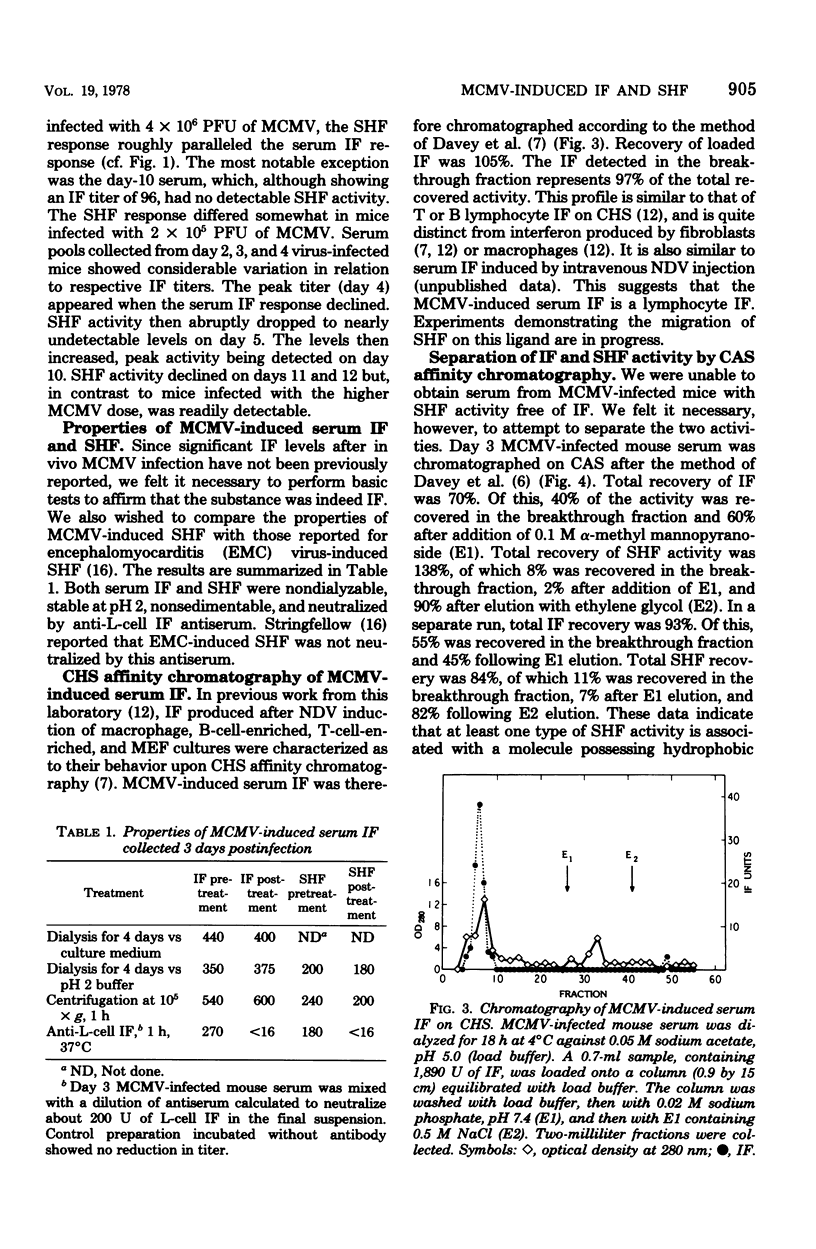
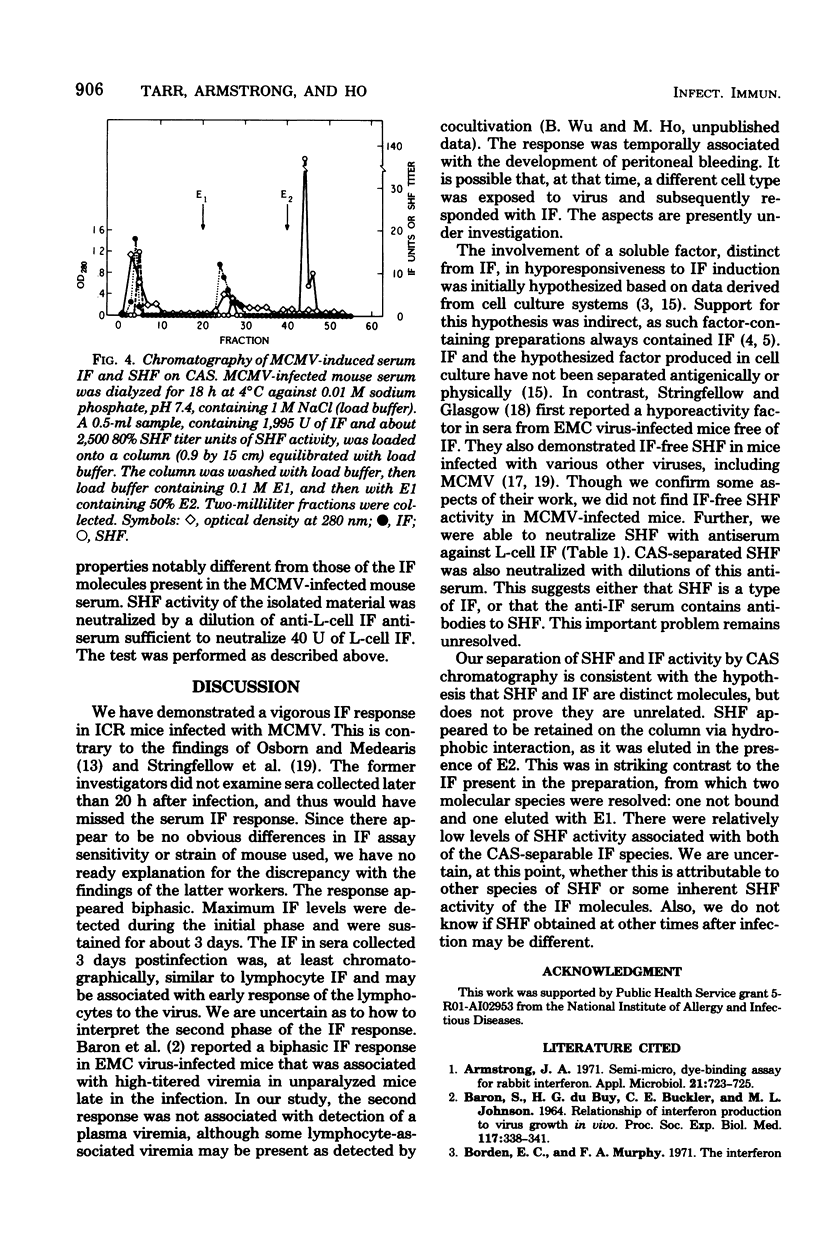
Mice injected intraperitoneally with murine cytomegalovirus produced as many as 1,000 U of serum interferon. The response appeared biphasic, with maximum titers in the first phase detectable from 2 through 4 days after infection. A second phase peaked 10 days after infection. By carboxyhexyl-Sepharose affinity chromatography, the serum interferon behaved like lymphocyte interferon. The infected mice also produced substantial quantities of serum hyporeactivity factor (D.A. Stringfellow, E.R. Kern, D.K. Kelsey, and L.A. Glasgow, J. Infect. Dis. 135:540-551, 1977), although always in the presence of interferon. This factor was separated from the serum interferon by concanavalin A-Sepharose affinity chromatography.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARON S., DUBUY H. G., BUCKLER C. E., JOHNSON M. L. RELATIONSHIP OF INTERFERON PRODUCTION TO VIRUS GROWTH IN VIVO. Proc Soc Exp Biol Med. 1964 Nov;117:338–341. doi: 10.3181/00379727-117-29575. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Murphy F. A. The interferon refractory state: in vivo and in vitro studies of its mechanism. J Immunol. 1971 Jan;106(1):134–142. [PubMed] [Google Scholar]
- Borden E. C., Prochownik E. V., Carter W. A. The interferon refractory state. II. Biological characterization of a refractoriness-inducing protein. J Immunol. 1975 Feb;114(2 Pt 2):752–756. [PubMed] [Google Scholar]
- Chadha K. C., Davey M. W., Byrd D. M., Carter W. A. Differential production of interferon and refractoriness inducing principle in L cells. Infect Immun. 1974 Nov;10(5):1057–1061. doi: 10.1128/iai.10.5.1057-1061.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. W., Huang J. W., Sulkowski E., Carter W. A. Hydrophobic interaction of human interferon with concanavalin A-agarose. J Biol Chem. 1974 Oct 10;249(19):6354–6355. [PubMed] [Google Scholar]
- Davey M. W., Sulkowski E., Carter W. A. Purification and characterization of mouse interferon with novel affinity sorbents. J Virol. 1976 Feb;17(2):439–445. doi: 10.1128/jvi.17.2.439-445.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO M., BREINIG M. K. METABOLIC DETERMINANTS OF INTERFERON FORMATION. Virology. 1965 Mar;25:331–339. doi: 10.1016/0042-6822(65)90052-8. [DOI] [PubMed] [Google Scholar]
- Ho M., Breinig M. C., Maehara N. Cellular basis of interferon formation and hyporeactivity after exposure to bacterial lipopolysaccharide. J Infect Dis. 1976 Jun;133 (Suppl):A30–A36. doi: 10.1093/infdis/133.supplement_2.a30. [DOI] [PubMed] [Google Scholar]
- Maehara N., Ho M., Armstrong J. A. Differences in mouse interferons according to cell source and mode of induction. Infect Immun. 1977 Sep;17(3):572–579. doi: 10.1128/iai.17.3.572-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara N., Ho M. Cellular origin of interferon induced by bacterial lipopolysaccharide. Infect Immun. 1977 Jan;15(1):78–83. doi: 10.1128/iai.15.1.78-83.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Studies of relationship between mouse cytomegalovirus and interferon. Proc Soc Exp Biol Med. 1966 Mar;121(3):819–824. doi: 10.3181/00379727-121-30897. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Suppression of interferon and antibody and multiplication of Newcastle disease virus in cytomegalovirus infected mice. Proc Soc Exp Biol Med. 1967 Feb;124(2):347–353. doi: 10.3181/00379727-124-31740. [DOI] [PubMed] [Google Scholar]
- Paucker K., Boxaca M. Cellular resistance to induction of interferon. Bacteriol Rev. 1967 Jun;31(2):145–156. doi: 10.1128/br.31.2.145-156.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A., Glasgow L. A. Hyporeactivity due to infection: recognition of a transferable hyporeactive factor in the serum of encephalomyocarditis virus-infected mice. Infect Immun. 1974 Dec;10(6):1337–1342. doi: 10.1128/iai.10.6.1337-1342.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A. Hyporeactivity to interferon induction: characterization of a hyporeactive factor in the serum of encephalomyocarditis virus-infected mice. Infect Immun. 1975 Feb;11(2):294–302. doi: 10.1128/iai.11.2.294-302.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A., Kern E. R., Kelsey D. K., Glasgow L. A. Suppressed response to interferon inducation in mice infected with encephalomyocarditis virus, Semliki forest virus, influenza A2 virus, Herpesvirus hominis type 2, or murine cytomegalovirus. J Infect Dis. 1977 Apr;135(4):540–551. doi: 10.1093/infdis/135.4.540. [DOI] [PubMed] [Google Scholar]
- Stringfellow D. A. Murine leukemia: depressed response to interferon induction correlated with a serum hyporeactive factor. Infect Immun. 1976 Feb;13(2):392–398. doi: 10.1128/iai.13.2.392-398.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


