Supplemental digital content is available in the text.
Key Words: solid tumors, antiangiogenic treatments, functional imaging, DCE-US
Abstract
Objectives
Dynamic contrast-enhanced ultrasound (DCE-US) has been used in single-center studies to evaluate tumor response to antiangiogenic treatments: the change of area under the perfusion curve (AUC), a criterion linked to blood volume, was consistently correlated with the Response Evaluation Criteria in Solid Tumors response. The main objective here was to do a multicentric validation of the use of DCE-US to evaluate tumor response in different solid tumor types treated by several antiangiogenic agents. A secondary objective was to evaluate the costs of the procedure.
Materials and Methods
This prospective study included patients from 2007 to 2010 in 19 centers (8 teaching hospitals and 11 comprehensive cancer centers). All patients treated with antiangiogenic therapy were eligible. Dynamic contrast-enhanced ultrasound examinations were performed at baseline as well as on days 7, 15, 30, and 60. For each examination, a perfusion curve was recorded during 3 minutes after injection of a contrast agent. Change from baseline at each time point was estimated for each of 7 fitted criteria. The main end point was freedom from progression (FFP). Criterion/time-point combinations with the strongest correlation with FFP were analyzed further to estimate an optimal cutoff point.
Results
A total of 1968 DCE-US examinations in 539 patients were analyzed. The median follow-up was 1.65 years. Variations from baseline were significant at day 30 for several criteria, with AUC having the most significant association with FFP (P = 0.00002). Patients with a greater than 40% decrease in AUC at day 30 had better FFP (P = 0.005) and overall survival (P = 0.05). The mean cost of each DCE-US was 180€, which corresponds to $250 using the current exchange rate.
Conclusions
Dynamic contrast-enhanced ultrasound is a new functional imaging technique that provides a validated criterion, namely, the change of AUC from baseline to day 30, which is predictive of tumor progression in a large multicenter cohort. Because of its low cost, it should be considered in the routine evaluation of solid tumors treated with antiangiogenic therapy.
In recent years, targeted antitumor agents have significantly improved outcomes across a wide range of solid tumors.1,2 Progression-free survival and overall survival are the preferred end points for assessing response to treatment with these agents. However, because of the high variability in postprogression survival and the possibility of multiple subsequent treatment lines, the impact of treatment is increasingly difficult to evaluate on the basis of overall survival. In addition, tumor response measures such as Response Evaluation Criteria in Solid Tumors (RECIST)3 have proved to be of limited value in assessing response to antiangiogenic agents because early necrosis is often observed before reduction in tumor size.4
Dynamic contrast-enhanced ultrasound (DCE-US) is a new functional technique enabling a quantitative assessment of solid tumor perfusion using a mathematical model to analyze raw linear ultrasound data.5 Reduction in tumor vascularization in responders is detectable earlier than what is possible with RECIST,6 and DCE-US has therefore been proposed as an alternative method of measuring early response to treatment. Moreover, DCE-US has been shown to be predictive of long-term survival.7 Previously, we conducted several single-center studies that demonstrated the utility of DCE-US for predicting early treatment efficacy with a number of antiangiogenic therapies in patients with solid tumors: sunitinib (SUTENT; Pfizer Inc, New York, NY) in patients with metastatic renal cell carcinoma (RCC),7 bevacizumab (Avastin; Roche, Basel, Switzerland) in patients with hepatocellular carcinoma (HCC),8 and masatinib (AB1010; AB Science, Paris, France) in patients with gastrointestinal stromal tumor (GIST).9 Correlations were observed between several DCE-US criteria and the RECIST response.7–9 Among these criteria, the change from baseline of the area under the perfusion curve (AUC) was consistently found to be related to the RECIST response. However, multicenter studies with larger sample sizes are warranted to improve the evidence-based medicine level of these findings.
The prospective multicenter French National Program for the Evaluation of DCE-US was established to evaluate DCE-US in patients with a variety of solid tumors. Our objectives were to validate previous findings and to evaluate the cost of the technique.
MATERIALS AND METHODS
Patients and Study Design
Details of the study methods and patients enrolled have been reported previously.10 In brief, 19 centers across France (11 comprehensive cancer centers and 8 university hospitals) participated in this prospective study, which included patients with metastatic breast cancer, those with metastatic melanoma, those with metastatic colon cancer, those with metastatic GIST, those with metastatic RCC, or those with primary HCC enrolled in a clinical trial of antiangiogenic-based therapy or are otherwise eligible to be started on therapy with an approved antiangiogenic treatment.
All patients provided written informed consent, either specific to this study or in the context of a clinical trial. The study was approved by the ethics committee of each institution and was declared to the French Commission Nationale Informatique et Liberté (CNIL declaration No. 912346).
DCE-US Technique and Quantification
Dynamic contrast-enhanced ultrasound was conducted using an Aplio sonograph (Toshiba, Puteaux, France) in accordance with a standardized procedure published recently.10 Ultrasonography was performed in 2 steps. First, a morphologic study was conducted, which allowed the target tumor to be identified.10 The DCE-US step of the examination started with an intravenous bolus injection of 4.8 mL of SonoVue (Bracco S.P.A., Milan, Italy), which was immediately flushed with 5 mL of isotonic sodium chloride solution. For each examination, a perfusion curve was recorded during 3 minutes after the injection of the contrast agent. The DCE-US criteria were quantified using CHI-Q software (Toshiba, Puteaux, France). A quantitative analysis of the time-intensity curve was performed with a mathematical model (patent PCT/IB2006/003742)11 on the basis of indicator-dilution theory 12 to determine 7 functional criteria: 4 related to blood volume (peak intensity, AUC, AUC during wash-in, and AUC during wash-out), 2 related to blood flow (time to peak intensity and slope of the wash-in), and mean transit time.4 The quality of DCE-US was determined according to 6 criteria, expressed as a score of 0 to 5.10 The DCE-US examinations with a score of 0, corresponding to very poor quality, were excluded (3% of all examinations; 48 of 1122 liver metastases and 11 of 936 nonliver metastases).
Assessments
Dynamic contrast-enhanced ultrasound was performed at baseline and at 4 postbaseline time points after treatment (days 7, 15, 30, and 60). For each DCE-US examination, we modeled the tumor perfusion curve with the 7 DCE-US criteria described previously. Changes from baseline in each criterion were calculated for each patient at each postbaseline time point. The change was expressed as the ratio of the postbaseline value over the baseline value.
Patients were to be followed up with a computed tomographic (CT) scan every 2 months for 1 year or until death. Measurements of RECIST were thus performed every 2 months during the first year. Follow-up data were available after the first year. Duration of follow-up was estimated in accordance with the Schemper method.13
The main end point was freedom from progression (FFP), defined as the time between the baseline DCE-US examination and the date of progression or the date of death of patients who died because of their malignancy without a documented progression. Progression was assessed in accordance with RECIST. Patients who stopped the treatment because of toxicity were censored when the treatment stopped and patients who died without progression were censored at the date of death.
Analyses
Statistical analyses were performed using SAS software (SAS, Cary, NC). All statistical tests were 2-sided and the significance level was 0.05.
We performed classic survival analyses to assess the relationship between each criterion and FFP. Each analysis tested the relation between the value of the criterion used as a continuous covariate and FFP with a log-rank test. Separate analyses were performed with the criterion values at baseline and at each of the 4 postbaseline time points; we also used this strategy to test the relationship between changes from baseline in each criterion at each time point. Thus, 9 tests were done for each of the 7 criteria, with a total of 63 tests. To identify criteria/time-point combinations with the strongest correlation with FFP, we focused on those where the association with FFP had a P value less than 0.001 to account for multiple testing.
Criteria/time points with the strongest association with FFP were analyzed further through a systematic search to identify the best cutoff point for each. The best single cutoff point was that with the lowest P value for association with FFP. Correlation between the criteria and the overall survival was studied after the best cutoff point had been estimated. The impact on FFP of the best combination cutoff point/criteria was tested in a multivariate analysis controlling for sex, age, tumor type, and treatment type.
Subgroup Analyses
Given the heterogeneity of the population, results obtained at the overall population level might not be applicable to specific groups of patients. Subgroup analyses according to the treatment and tumor type were therefore performed to identify groups of patients who might contribute most to the heterogeneity. For any such subgroups identified, a new search for the best combination criteria/time point and the corresponding best cutoff point was performed.
Economic Evaluation
The total (direct and indirect) cost of DCE-US was assessed from the hospital’s perspective. Resource use was evaluated from data collected prospectively for each procedure. It included staff inputs (procedure duration including bolus injection, quantification of criteria, and medical reporting) and the number of vials of contrast medium used. Unit costs applied included wages (radiologist, nurse, engineer, and secretary), machine acquisition and maintenance logistics of an Aplio ultrasound machine, as well as the cost of SonoVue contrast medium (80€, approximately $110 per vial). Indirect costs were also included (overheads for medical logistics, general logistics, and capital costs). Sensitivity analyses on unit costs were performed using a variation of plus or minus 10%.
RESULTS
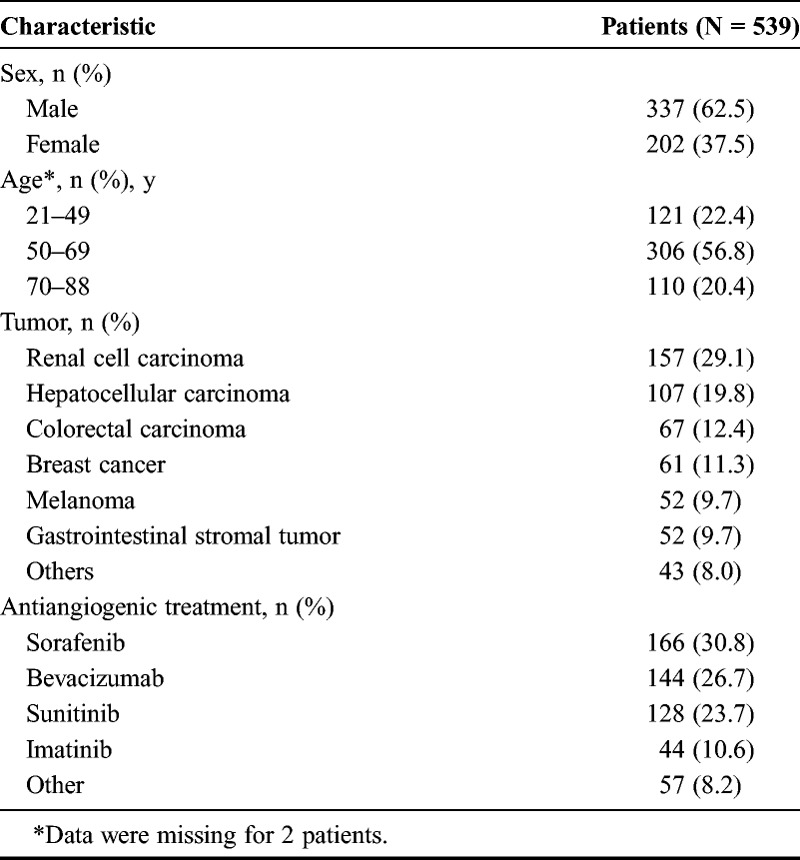
A total of 539 patients from 19 centers were enrolled in the study between October 2007 and March 2010. Patient characteristics, including tumor types and antiangiogenic treatments received, are briefly described in Table 1. A total of 2339 DCE-US examinations were performed, of which 371 were excluded from the analysis because of the following: raw data were not quantified because of technical problems (total or partial loss of data, n = 277) and the quality of examination was poor (n = 59). Finally, examinations performed at day 1 (n = 35) were too rare to be included in the present analyses. Thus, 1968 DCE-US examinations were evaluated: 463, 401, 412, 392, and 300 at baseline as well as on days 7, 15, 30, and 60, respectively (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/RLI/A163). Examinations with a corresponding baseline were available for 360 patients at day 7, for 370 patients at day 15, for 353 patients at day 30, and for 266 patients at day 60. The median follow-up was 1.65 years (95% confidence interval [CI], 0.02–3.58). A total of 190 patients (35%) were still alive after 12 months of follow-up.
TABLE 1.
Characteristics of the Patients
At baseline, there was no significant association between any of the DCE-US criteria values and FFP.
Analysis of changes from baseline revealed significant associations with FFP at day 30 for several criteria (Table 2). Change in AUC at day 30 showed the strongest association (P = 0.00002), and a systematic search for the best cutoff point for this criterion identified an AUCday30/AUCbaseline value of 0.6 (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/RLI/A164). This corresponds to a decrease of 40% from baseline in AUC at day 30. The difference between the groups defined by this cutoff point was significant for both FFP (P = 0.005) and overall survival (P = 0.05; Fig. 1). In the multivariate analysis controlling for age, sex, tumor type, and treatment type, the cutoff point remained significant (Table 3). Subgroup analyses according to treatment suggested heterogeneity, which could be attributed either to patients treated with sunitinib or to the group of patients treated with “other molecules.” We performed a separate analysis of patients treated with sunitinib. Most of these patients (81/128) had RCC. In this subgroup, the best cutoff for change in AUC at 30 days was 0.1, corresponding to a decrease of 90% in the AUC. Twenty-seven patients of 81 had an AUC less than 0.1. Curves of FFP for this cutoff point are shown for patients with RCC in Figure 2.
TABLE 2.
Significance Level (P Value) of the Association Between Criteria Values and Freedom From Progression at Different Time Points
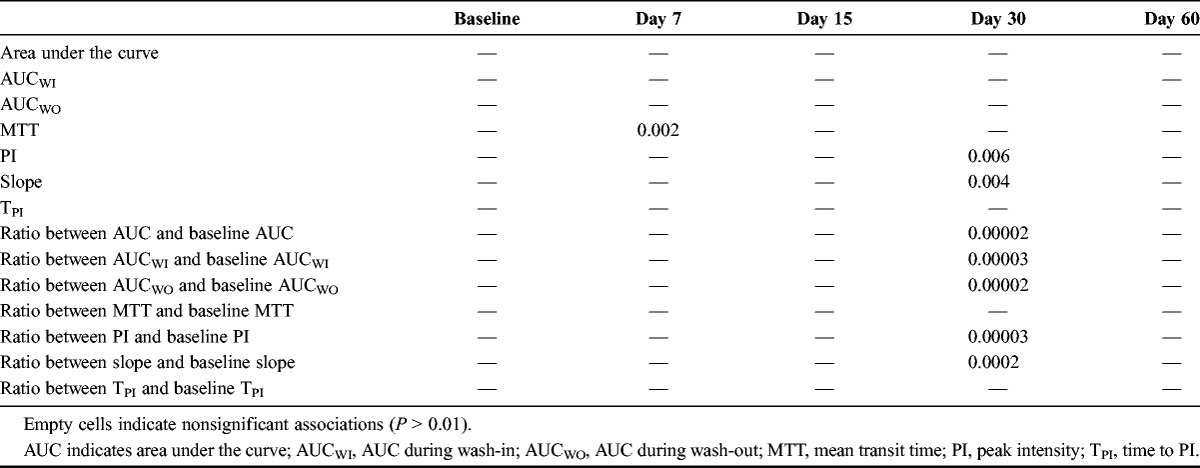
FIGURE 1.
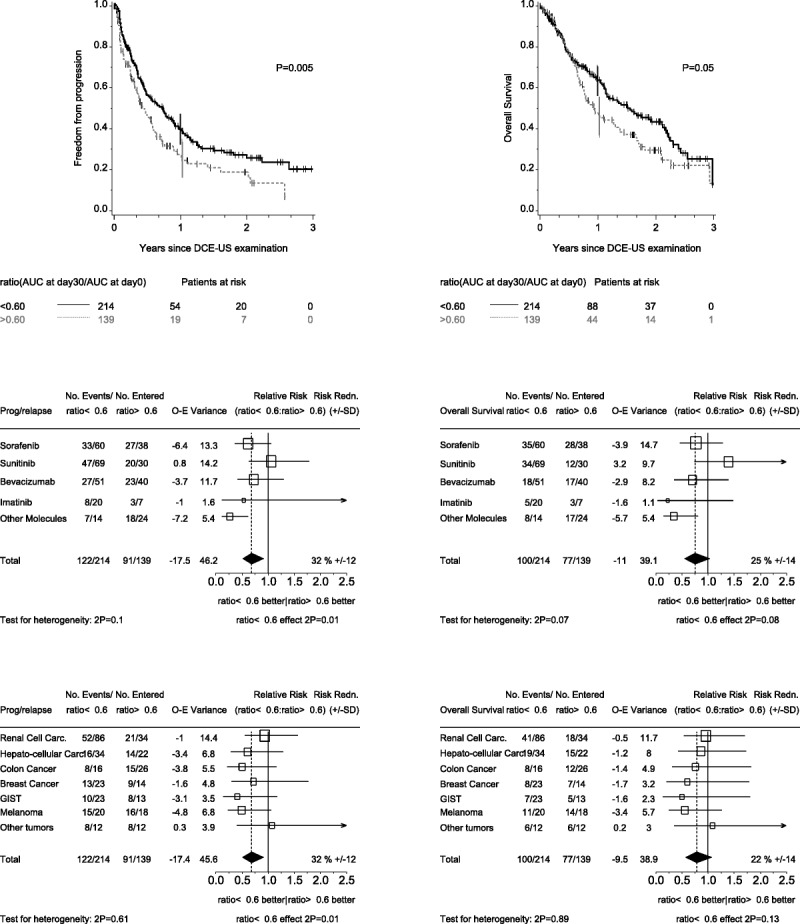
Impact of change in AUC from baseline to day 30 on FFP (left-hand panels) and on overall survival (right-hand panels). A ratio AUCday30/AUCbaseline smaller than 0.60 indicates a decrease in AUC greater than 40%. The upper panels display the results for the overall population. The middle panels show forest plots of relative risks according to specific antiangiogenic agents. The lower panels show forest plots of relative risks according to tumor type.
TABLE 3.
Multivariate Analyses of Freedom From Progression
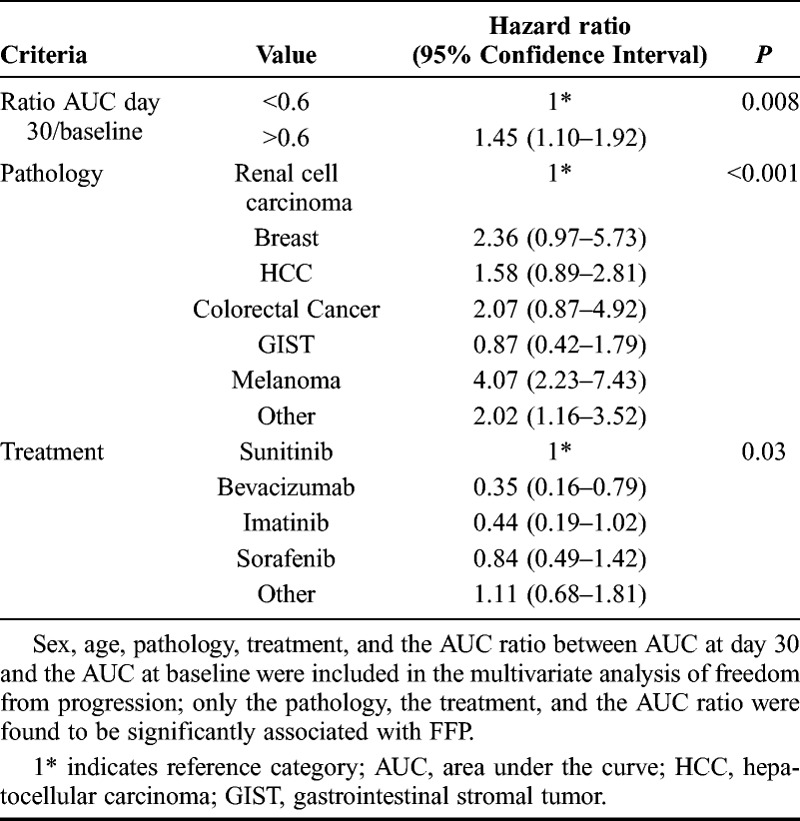
FIGURE 2.
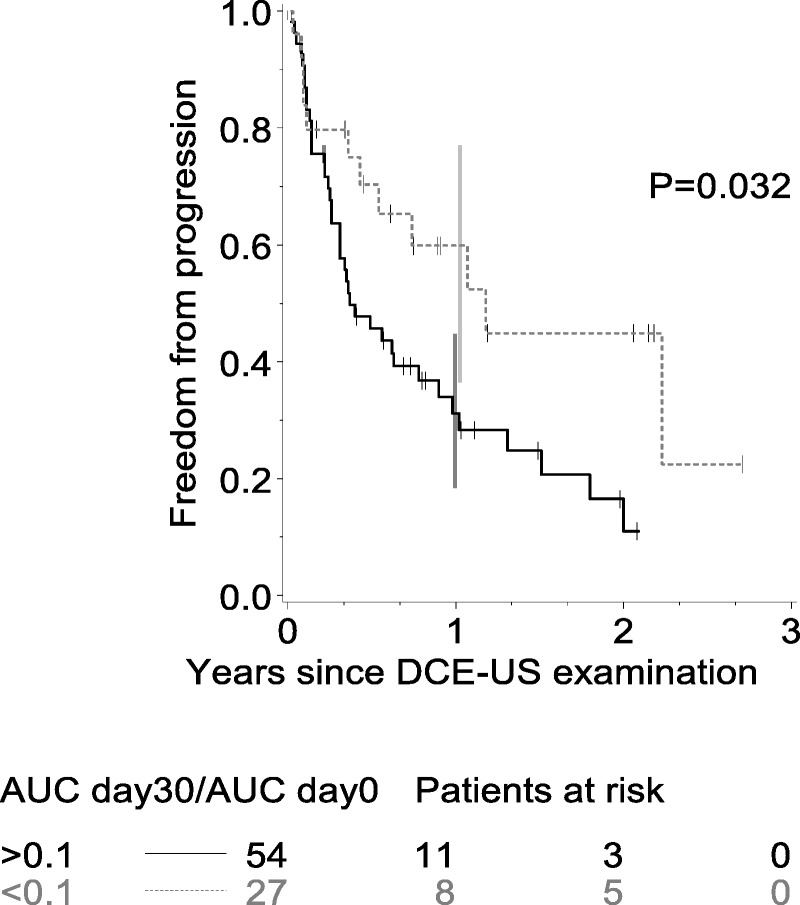
Time to progression (in years) according to AUCday30/AUCbaseline less than 0.1 in RCC.
Economic Evaluation
The mean duration of the DCE-US procedure was estimated to be 28 minutes (95% CI, 13–50). The radiologist’s intervention, including the medical report, lasted 23 minutes (95% CI, 8–45). Where reinjection was required, the procedure took an additional 7 minutes. The overall reinjection rate was 10%, but this was highest in baseline procedures (17%). The mean cost of a DCE-US procedure was estimated at 180€ corresponding to 250$ because of the current exchange rate (95% CI, 145€ [approximately $200] to 278€ [approximately $381]). The main cost drivers were the cost of Sonovue (49% of total cost) and the reinjection rate. Staff costs accounted for 29% of the total cost of the procedure. In the sensitivity analyses, the cost of DCE-US ranged from 177€ (approximately $243) to 223€ (approximately $306), being most sensitive to the manufacturer’s price of the contrast agent and to the reinjection rate.
DISCUSSION
Currently, there is a need to improve the evaluation of patients treated with antiangiogenic treatments. Imaging biomarkers for the evaluation of tumor vascularization have been developed using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) or dynamic contrast-enhanced CT (DCE-CT). Concerning the DCE-US, the monitoring of antiangiogenic treatments was added to the European Federation of Societies for Ultrasound in Medicine and Biology guidelines on the clinical practice of contrast-enhanced ultrasound in 201114 and to the World Federation for Ultrasound in Medicine and Biology guidelines in 2012.15
Compared with other imaging techniques, the advantages of DCE-US are its suitability for use in patients with renal failure and its availability because of the pulmonary elimination of the contrast agent. However, DCE-US cannot be used for metastasis in the lung or brain because ultrasound waves are stopped by gas and bone structures.
For CT scan, a perfusion acquisition adds approximately 10% to 20% of radiation dose to a standard abdomen and pelvis CT (ie, approximately 20 mSv), and this must be taken into account when considering longitudinal follow-up.16
With DCE-US and DCE-CT, the relationship between the concentration and the intensity of the signal is linear. In DCE-US, the contrast medium remains solely intravascular compared with DCE-CT and DCE-MRI.
Dynamic contrast-enhanced magnetic resonance imaging is the most complex technique with respect to data interpretation because results depend on the relationship between concentration and intensity as well as on the method of data analysis.17 To date, more than 100 clinical trials have been performed using DCE-MRI to evaluate antiangiogenic or antivascular drugs.18 The latest consensus statement for DCE-MRI19 recommends that lesions smaller than 3 cm be avoided, that acquisition be performed for at least 10 minutes, and that the Tofts model be used to evaluate Ktrans, the recommended volume transfer criteria.
We used the standardized DCE-US methodology in 19 oncology centers in France.10 Only 3% of the tests had to be excluded because they were not interpretable. We validated the change in AUC from baseline to day 30 as a criterion correlated with FFP.
The AUC is calculated by integration of the signal longer than 3 minutes and is indicative of blood volume.20 In vitro and in vivo studies have shown that the variability in the AUC is smaller than the variability in other criteria associated with blood flow, such as the slope and time to peak.21
Since 2006, published studies using qualitative analysis of tumor perfusion22,23 have suggested that DCE-US may be useful in evaluating early tumor response. More recently, 5 studies, including a total of 145 patients, used quantitative DCE-US to monitor response to antiangiogenic therapies in solid tumors.7–9 In these studies, AUC was associated with response according to RECIST in patients with HCC treated with bevacizumab9 or sorafenib,24 in patients with RCC treated with sunitinib,8 in patients with GIST treated with masatinib,10 and in patients with phase 1 with sorafenib.25 The current large multicenter study extends this association to FFP and overall survival.
Until now, the majority of studies that used functional imaging as biomarkers26,27 were conducted on patients with RCC, HCC, and glioma.
In RCC, significant correlations with overall survival were found in 3 studies: 2 with CT (size and density in Hounsfield units)28,29 and one with DCE-US.7 In all of these studies, the population was small (<70 patients) compared with the 141 patients with RCC included in the present study. Another small study (<50 patients) showed a significant association with progression-free survival using DCE-MRI.30
In HCC, only 2 studies identified imaging criteria correlated with overall survival. One study using DCE-MRI31 included 38 patients and found a relationship between Ktrans at day 15 and survival. The other study on 42 patients used DCE-US8 and found that AUC at day 3 was a significantly associated criterion. Several other studies, all including fewer than 32 patients, showed correlations between time to progression and Ktrans (for DCE-MRI)31,32 or between time to progression and modified Choi criteria (for CT scan).33 The present study is consistent with the DCE-US study, with a population more than twice as large (107 patients).
We have confirmed that the AUC may be used to predict progression and survival after treatment with tyrosine kinase inhibitors. These inhibitors block neoangiogenesis, inducing the destruction of neovessels and resulting in a decrease in blood volume within tumors. In our parameterization of the perfusion curve, 4 criteria were linked to tumor blood volume. We have shown in mice that AUC is the criterion with the smallest intraoperator variability (<15%). The good precision in the estimation of this criterion could explain why AUC was always significant in previous studies and in this multicenter study.
To increase the robustness of the technique, in the future, several further directions should be followed: the first is the use of an arterial input,11 as is done with DCE-CT, to improve the precision of the measurements. This process takes into account the hemodynamics of the patient and has been implemented with DCE-US in research work in mice.11 The second direction involves 3-dimensional quantification of the tumor34,35 because, in 2-dimensional, it is difficult to ensure the same area of the tumor measured during follow-up. Furthermore, a new utilization of DCE-US demonstrated on mice36 could be proposed to evaluate patients with osteolytic bone metastasis. To finish, a very promising new approach described by Burns should be adopted to study vascular heterogeneity as a prognostic factor.37
CONCLUSIONS
Dynamic contrast-enhanced ultrasound is a new functional imaging technique that provides a validated criterion, namely, the area under the time-intensity curve, which predicts tumor progression in a large multicenter cohort. Because of its low cost, it should be considered for the routine evaluation of solid tumors treated with antiangiogenic therapy.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Muriel Ducourtieux, Stana Agnes, Julien Pellier, Louis Chapotot, Julie Chevalier, and Florence Journeau for data collection and quality control as well as Dr Jean-Pierre Armand for his valuable advice on the preparation of the protocol.
Medical editing support was provided by Joanne Fitz-Gerald, BPharm, MRPharmS, a freelance medical writer, and Rachel Mason at ACUMED (Tytherington, United Kingdom).
Final medical editing support was provided by S. Randall Thomas (IR4M UMR8081 CNRS University Paris Sud).
Footnotes
Supported by a grant from the French National Cancer Institute. Additional funding was provided by Toshiba and Bracco.
Conflicts of interest and sources of funding: All the authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Dr Lassau received a grant from the Institut National de Cancer for another ongoing project as well as honoraria and lectures fees from Pfizer, Novartis, Hoffmann-La Roche, Bracco, and Toshiba. Dr Kind received a grant from Toshiba for a past project. Dr Lucidarme received payment from Bracco for a past project to write educational material about contrast-enhanced ultrasound imaging of the liver. Dr Vilgrain received grants from the Cancer Institute as an associate investigator for an ongoing project and from SIRTeX as a principal investigator for a study on liver radioembolization. No other disclosures are reported.
Author contributions: Drs Lassau and Koscielny had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Lassau, Bonastre, and Koscielny. Acquisition of data: Lassau, Kind, Vilgrain, Lacroix, Cuinet, Taieb, Aziza, Sarran, Labbe-Devilliers, Gallix, Lucidarme, Ptak, Rocher, Caquot, Chagnon, Marion, Luciani, Feutray, Uzan-Augui, Coiffier, and Benatsou. Analysis and interpretation of data: Lassau, Bonastre, Coiffier, Benatsou, and Koscielny. Drafting of the manuscript: Lassau, Bonastre, and Koscielny. Critical review of the manuscript for important intellectual content: Lassau, Bonastre, Kind, Vilgrain, Lacroix, Cuinet, Taieb, Aziza, Sarran, Labbe-Devilliers, Gallix, Lucidarme, Ptak, Rocher, Caquot, Chagnon, Marion, Luciani, Feutray, Uzan-Augui, and Koscielny. Statistical analysis: Lassau, Bonastre, and Koscielny. Obtained funding: Lassau. Administrative, technical, or material support: Lassau, Bonastre, Kind, Vilgrain, Lacroix, Cuinet, Taieb, Aziza, Sarran, Labbe-Devilliers, Gallix, Lucidarme, Ptak, Rocher, Caquot, Chagnon, Marion, Luciani, Feutray, Uzan-Augui, Coiffier, Benatsou, and Koscielny. Study supervision: Lassau, Bonastre, Kind, Vilgrain, Lacroix, Cuinet, Taieb, Aziza, Sarran, Labbe-Devilliers, Gallix, Lucidarme, Ptak, Rocher, Caquot, Chagnon, Marion, Luciani, Feutray, Uzan-Augui, and Koscielny.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.investigativeradiology.com)
REFERENCES
- 1. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007; 356: 125– 134. [DOI] [PubMed] [Google Scholar]
- 2. Demetri GD, Reichardt P, Kang YK, et al. GRID study investigators. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381: 295– 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228– 247. [DOI] [PubMed] [Google Scholar]
- 4. Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol. 2010; 194: 157– 165. [DOI] [PubMed] [Google Scholar]
- 5. Peronneau P, Lassau N, Leguerney I, et al. Contrast ultrasonography: necessity of linear data processing for the quantification of tumor vascularization. Ultraschall Med. 2010; 31: 370– 378. [DOI] [PubMed] [Google Scholar]
- 6. Lassau N, Chami L, Benatsou B, et al. Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumour perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol. 2007; 6: 89– 98. [DOI] [PubMed] [Google Scholar]
- 7. Lassau N, Koscielny S, Albiges L, et al. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res. 2010; 16: 1216– 1225. [DOI] [PubMed] [Google Scholar]
- 8. Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification—preliminary results. Radiology. 2011; 258: 291– 300. [DOI] [PubMed] [Google Scholar]
- 9. Lassau N, Chami L, Koscielny S, et al. Quantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in GIST patients treated with masatinib. Invest New Drugs. 2012; 30: 765– 771. [DOI] [PubMed] [Google Scholar]
- 10. Lassau N, Chapotot L, Benatsou B, et al. Standardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies. The French Multicenter Support for Innovative and Expensive Techniques Study. Invest Radiol. 2012; 47: 711– 716. [DOI] [PubMed] [Google Scholar]
- 11. Gauthier M, Tabarout F, Leguerney I, et al. Assessment of quantitative perfusion parameters by dynamic contrast enhanced-ultrasonography using a deconvolution method: an in vitro and in vivo study. J Ultrasound Med. 2012; 31: 595– 608. [DOI] [PubMed] [Google Scholar]
- 12. Strouthos C, Lampaskis M, Sboros V, et al. Indicator dilution models for the quantification of microvascular blood flow with bolus administration of ultrasound contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 2010; 57: 1296– 1310. [DOI] [PubMed] [Google Scholar]
- 13. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trial. 1996; 17: 343– 346. [DOI] [PubMed] [Google Scholar]
- 14. Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012; 33: 33– 59. [DOI] [PubMed] [Google Scholar]
- 15. Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013; 39: 187– 210. [DOI] [PubMed] [Google Scholar]
- 16. Miles KA, Lee TY, Goh V, et al. Experimental Cancer Medicine Centre Imaging Network Group. Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. Eur Radiol. 2012; 22: 1430– 1441. [DOI] [PubMed] [Google Scholar]
- 17. Van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al. Targeted therapies in renal cell cancer: recent developments in imaging. Target Oncol. 2010; 5: 95– 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leach MO, Morgan B, Tofts PS, et al. Experimental Cancer Medicine Centres Imaging Network Steering Committee. Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur Radiol. 2012; 22: 1451– 1464. [DOI] [PubMed] [Google Scholar]
- 19. O’Connor JB, Jackson A, Parker GJ, et al. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012; 9: 167– 177. [DOI] [PubMed] [Google Scholar]
- 20. Dietrich CF, Averkiou MA, Correas JM, et al. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012; 33: 344– 351. [DOI] [PubMed] [Google Scholar]
- 21. Gauthier M, Leguerney I, Thalmensi J, et al. Estimation of intra-operator variability in perfusion parameter measurements using DCE-US. World J Radiol. 2011; 3: 70– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lamuraglia M, Escudier B, Chami L, et al. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer. 2006; 42: 2472– 2479. [DOI] [PubMed] [Google Scholar]
- 23. Lassau N, Lamuraglia M, Chami L, et al. Gastrointestinal stromal tumours treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol. 2006; 187: 1267– 1273. [DOI] [PubMed] [Google Scholar]
- 24. Zocco MA, Garcovich M, Lupascu A, et al. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013; 5: 1014– 1021. [DOI] [PubMed] [Google Scholar]
- 25. Lazar V, Lassau N, Meurice G, et al. Sorafenib plus dacarbazine in solid tumors: a phase I study with dynamic contrast-enhanced ultrasonography and genomic analysis of sequential tumor biopsy samples. Invest New Drugs. 2014; 32: 312– 322. [DOI] [PubMed] [Google Scholar]
- 26. La Thangue NB, Kerr DJ. Predictive biomarkers: a paradigm shift towards personalized cancer medicine. Nat Rev Clin Oncol. 2011; 8: 587– 596. [DOI] [PubMed] [Google Scholar]
- 27. O’Connor JP, Jayson GC. Do imaging biomarkers relayed to outcome in patients treated with VEGF inhibitors? Clin Cancer Res. 2012; 18: 6588– 6598. [DOI] [PubMed] [Google Scholar]
- 28. Van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer. 2010; 102: 803– 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krajewski KM, Guo M, Van den Abbeele AD, et al. Comparison of four early posttherapy imaging changes (EPTIC; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor-targeted therapy in patients with advanced renal cell carcinoma. Eur Urol. 2011; 59: 856– 862. [DOI] [PubMed] [Google Scholar]
- 30. Flaherty KT, Rosen MA, Heitjan DF, et al. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther. 2008; 7: 496– 501. [DOI] [PubMed] [Google Scholar]
- 31. Hsu CY, Shen YC, Yu CW, et al. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011; 55: 858– 865. [DOI] [PubMed] [Google Scholar]
- 32. Yopp AC, Schwartz LH, Kemeny N, et al. Antiangiogenic therapy for primary liver cancer: correlation of changes in dynamic contrast-enhanced magnetic resonance imaging with tissue hypoxia markers and clinical response. Ann Surg Oncol. 2011; 18: 2192– 2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Faivre S, Zappa M, Vilgrain V, et al. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin Cancer Res. 2011; 17: 4504– 4512. [DOI] [PubMed] [Google Scholar]
- 34. Williams R, Hudson JM, Lloyd BA, et al. Dynamic microbubble contrast-enhanced ultrasound to measure tumor response to targeted therapy: A proposed clinical protocol with results from renal cell carcinoma patients receiving anti-angiogenic therapy. Radiology. 2011; 260: 581– 590. [DOI] [PubMed] [Google Scholar]
- 35. Hoyt K, Sorace A, Saini R. Quantitative mapping of tumor vascularity using volumetric contrast-enhanced ultrasound. Invest Radiol. 2012; 47: 167– 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Merz M, Komljenovic D, Semmler W, et al. Quantitative contrast-enhanced ultrasound for imaging antiangiogenic treatment response in experimental osteolytic breast cancer bone metastases. Invest Radiol. 2012; 47: 422– 429. [DOI] [PubMed] [Google Scholar]
- 37. Hudson JM, Williams R, Karshafian R, et al. Quantifying vascular heterogeneity using microbubble disruption-replenishment kinetics in patients with renal cell cancer. Invest Radiol. 2014; 49: 116– 123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


