Lumbar magnetic resonance images and low back pain (LBP) questionnaires of 188 hospital staffs were reviewed to investigate the relationship between radiological signs of Scheuermann disease (SD) and LBP. A total of 18.1% of the participants met SD diagnostic criteria and having “SD-like” spine was associated with the severity and progressive nature of LBP.
Keywords: Scheuermann disease, atypical Scheuermann disease, lumbar Scheuermann disease, low back pain, lumbar spine, magnetic resonance imaging, Schmorl node, irregular endplate, wedged vertebra, hospital
Abstract
Study Design.
Retrospective cohort study.
Objective.
To investigate the relationship between radiological signs of Scheuermann disease (SD) and low back pain (LBP) in a local population using lumbar magnetic resonance (MR) images.
Summary of Background Data.
SD is a spinal disorder, and both its classic and atypical (lumbar) forms are associated with LBP. However, radiological signs of SD are present in 18% to 40% of the general population, in whom the clinical significance of “SD-like” spine remains largely unknown.
Methods.
This retrospective cohort study included 188 staff members from a single hospital. Participants' lumbar MR images and self-administered questionnaires concerning demographic information, LBP status, consequences, and functional limitations were collected. Participants were classified into 2 groups according to whether lumbar MR images met SD diagnostic criteria, and LBP status, consequences, and functional limitation were compared. Follow-up interviews were conducted after 6 years to compare LBP progression.
Results.
Thirty-four participants (18.1%) had SD-like spine. Rates of lifetime, previous 1-year, and point LBP did not significantly differ between groups. However, among participants who had ever had LBP, SD-like spine was associated with higher rates of work absence (42.1% vs. 9.5%, χ2 = 9.620, P = 0.002) and seeking medical care (68.4% vs. 39.2%, χ2 = 5.216, P = 0.022) due to LBP, as well as significantly greater intensity of the most severe LBP episode in the past 2 years (6.4 ± 2.5 vs. 4.1 ± 2.5, t = 3.564, P = 0.001). Among the 159 participants who completed the 6-year follow-up, a significantly higher proportion of people with SD-like spine reported aggravated LBP during the follow-up.
Conclusion.
Our results suggest that in the general population, lumbar MR images of many people meet SD diagnostic criteria, and having SD-like spine seemed to be associated with the severity and progressive nature of LBP. Our findings should inspire further research in this field.
Level of Evidence: 3
Low back pain (LBP) is a leading debilitating disorder worldwide,1 affecting up to 84% of the general population at some point in life.2 It not only impacts patients' quality of life and financial well-being, but also the financial health of the entire health care system.3 The majority of LBP is nonspecific, with no sign of a specific spinal disorder or definite underlying condition such as cancer or infection, posing difficulty for its prevention and treatment.4
Potential LBP risk factors include physiological factors (female sex,5,6 obesity,2,7 and poor health8), socioeconomic factors (smoking,2,9 heavy workload,2,6,8 and low income10), and psychological factors (work dissatisfaction10 and depression11). However, the majority of these risk factors concern outside influences that are inherently difficult to assess in an individual clinical encounter. Among intrinsic factors, some are too subtle (e.g., sex) for use in the spinal community, whereas others are too subtle (e.g., interleukin-1 gene cluster polymorphism2) for accessibility in typical clinical settings. Magnetic resonance (MR) of the spine can provide straightforward information on disc degeneration (DD). However, current evidence on the association between DD and LBP in adult populations is generally not strong and has been controversial.2,12,13 Therefore, identifying new intrinsic risk factors of LBP that are both straightforward and acquirable in daily practice is significant to LBP investigation and management.
Scheuermann disease (SD) is a spinal disorder named after Dr. Holger Werfel Scheuermann, who, in 1921, first described a structural thoracic kyphosis mainly affecting adolescents.14 Its best-known manifestations are multiple wedged vertebrae (WV) and thoracic kyphosis known as Scheuermann kyphosis. Its classic diagnostic criterion was “3 or more consecutive wedged thoracic vertebrae,” proposed by Sorensen in 1964.15 However, SD pathological changes also include disc and endplate lesions, primarily Schmorl node (SN) and irregular vertebral endplate (IE).14,15 Therefore, the diagnosis of “atypical SD” was proposed for patients with only one or 2 WV and no notable kyphosis, but characteristic disc/endplate lesions, including SN and IE.16–20 Because atypical SD tends to affect the lumbar or thoracolumbar junction region instead of the thoracic spine, it is also called “lumbar SD.”16,17,19,20 Thus, SD represents a broader concept than Scheuermann kyphosis (classic SD) because it also includes lumbar SD (atypical SD) (Figure 1; Table 1).
Figure 1.
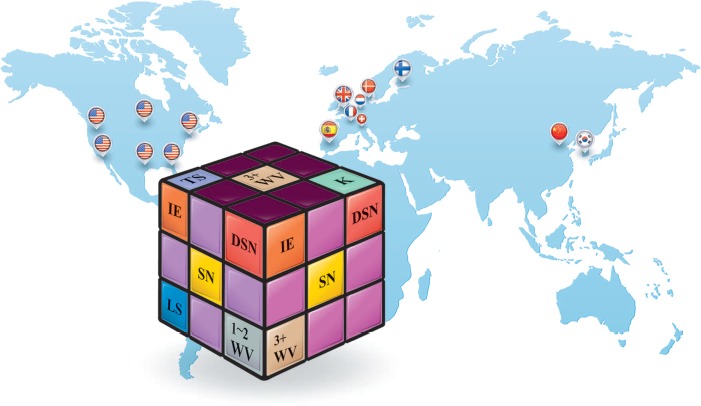
What makes up “Scheuermann disease”? The definition of SD is not uniform or fixed. Instead, it depends on the form being referred to and a corresponding combination of pathological changes. Classic SD (the upper surface of the cube) is characterized by K and 3 or more WV occurring in the TS. Atypical SD (the left surface) tends to occur in the LS, and patients typically have 1 or 2 WV and lack notable kyphosis, but have characteristic disc/endplate lesions, including SN, IE, and DSN. The 2 forms often overlap in the same patient (the right surface). This comprehensive definition of SD has been accepted by many authors: as of December 31, 2013, a literature search revealed 15 studies (15 national flags representing the nationalities and locations of the primary authors, Table 1) that included specific criteria for diagnosing atypical or lumbar SD. We searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) for articles published in English, with the terms “atypical” and “lumbar” in successive combination with the terms “Scheuermann” and “Scheuermann's” in the title/abstract. This search strategy revealed 115 articles, from which these 15 were identified on the basis of the criterion mentioned in the earlier text. SD indicates Scheuermann disease; K, kyphosis; WV, wedged vertebrae; TS, thoracic spine; LS, lumbar spine; SN, Schmorl node; IE, irregular endplate; DSN, disc space narrowing.
TABLE 1. Description of 15 Reports on the Diagnostic Criteria of Atypical (Lumbar) SD.
| Year | Country | Authors | Criteria of Atypical (Lumbar) SD |
|---|---|---|---|
| 1976 | The Netherlands | Rogge and Nieman | Paradiscal defects and the development of vertebral stenosis36 |
| 1981 | United States | Cleveland and Delong | Disc space narrowing, Schmorl node28 |
| 1987 | United States | Blumenthal et al | Only 1 or 2 (wedged) vertebral bodies, vertebral endplate changes, disc space narrowing, and anterior Schmorl nodes16 |
| 1993 | United States | Mandell et al | One or 2 (wedged) vertebral bodies, anterior Schmorl node herniations, and disc space narrowing26 |
| 1994 | United States | Heithoff et al | “… manifested by disc space narrowing, disc dehydration, endplate irregularity, wedging of anterior vertebral body margins, and the presence of Schmorl nodes. Three of these criteria were necessary to diagnose thoracolumbar Scheuermann disease.”17 |
| 1994 | France | Linthoudt and Revel | Adopted Blumenthal criteria34 |
| 1995 | Denmark | Harreby et al | One or more vertebrae wedged at least 5° and irregularity of the superior and inferior endplate, or narrowing of the disc space in connection with the wedged vertebra37 |
| 2002 | United States | Gustavel and Beals | “…endplate irregularities in only 1 or 2 vertebrae, anterior Schmorl nodes, and disk-space narrowing, but no anterior wedging of the vertebral bodies.”38 |
| 2003 | Finland | Karppinen et al | “Scheuermann disease was diagnosed if either endplate irregularities or Schmorl nodules and 2 of the other 3 criteria (disk space narrowing, disk dehydration, and wedging of anterior vertebral body margins) were present at 3 or more adjacent disk levels from T10–T11 to L3–L4.”20 |
| 2008 | United Kingdom | Summers et al | “…endplate irregularities and disc narrowing in the lumbar spine or thoracolumbar junction, but with no abnormal kyphosis.”39 |
| 2009 | United States | Kruse and Lemmen | Anterior vertebral body wedging, endplate irregularity, disc space narrowing, and Schmorl nodes32 |
| 2011 | Korea | Song and Yang | “…characterized by the significance of SNs and endplate irregularity at the thoracolumbar junction without severe clinical kyphosis.”40 |
| 2013 | Switzerland | Hasler | Disc herniation into the vertebral body, anterior endplate lesions, and disk space narrowing41 |
| 2013 | Spain | Lucas-García et al | Schmorl hernias in 1 or 2 vertebral bodies, narrowing of disc space, and changes in vertebral endplates42 |
| 2014 | China | Liu et al | “The fulfillment of 4 signs that must include SN, IE, and WV of the aforementioned 5 signs (SN, IE, WV, disc-space narrowing and disc dehydration) is necessary to diagnose (atypical) SD.”25 |
WV indicates wedged vertebrae; SN, Schmorl node; IE, irregular endplate; SD, Scheuermann disease.
Notably, both classic SD and atypical SD are associated with back pain.14,16,17,19–22 Although Scheuermann kyphosis is uncommon, SD radiological signs have been observed in 18% to 40% of the general population,23 suggesting that SD, or more precisely, “SD-like” spine, may be a variant of normal spine morphology rather than a disease. A genetic role in the cause of SD has been proposed, with a suspected autosomal dominant pattern of inheritance.20,24 The Trp3 allele, a variant of the COL9A3 gene, has been associated with SD.20
We speculated whether an SD-like spine is associated with LBP in the general population. However, previous studies on the relationship between SD and LBP have primarily focused on patients with Scheuermann kyphosis, back pain, or sciatica. Therefore, we conducted this study to investigate the relationship between SD-like spines and LBP in a local population of hospital staff members.
MATERIALS AND METHODS
Overview
This retrospective cohort study involved 188 staff members from Peking University Third Hospital. A database was established in 2007 with the original purpose to investigate potential LBP risk factors among hospital employees, which contained lumbar MR images and self-administered questionnaires concerning participants' LBP issues. This primary information formed the basis of this study 6 years later. Participants were classified into 2 groups, SD-like and “non–SD-like,” according to whether their lumbar MR images met SD diagnostic criteria, and LBP rates were compared between the groups based on questionnaire data. Among LBP sufferers (participants who ever had LBP), LBP consequences, and functional limitations were compared between SD-like participants and non–SD-like participants. We also conducted follow-up interviews to compare LBP progression during a 6-year period between the 2 groups. The study protocols were approved by the hospital ethic committee. All participants provided written informed consent.
Participants' Enrolment
The database inclusion criterion was full-time employment at our hospital. Exclusion criteria included a history of spinal fracture or violent back trauma, spinal surgery, ankylosing spondylitis, spinal tuberculosis, and tumor. Pregnant females and employees committed to long-term analgesic use for reasons other than back pain were also excluded. Participants were sampled from all 66 departments of our hospital using stratified cluster sampling with unequal probabilities. The departments were stratified according to function: clinical services (36) and administrative and logistical affairs (30). In each stratum, we randomly sampled 2 departments using the method of sampling with unequal probabilities, according to the number of staff members in each department. The corresponding author of this study visited the directors of the sampled departments and invited all departmental staff members to participate. Qualified participants were offered a free lumbar MR examination and multiple clinical visits at the spine service. Initially, 198 participants from the departments of orthopedics (29 physicians, 51 nurses), respiratory disorders (13 physicians, 30 nurses), finance (30 administrative/clerical/accounting staffs), and department of general services (30 drivers, 15 engineers/plumbers) were enrolled. Each department had more than 85% enrolment. After reviewing the data, 1 case of ankylosing spondylitis, 1 previous spinal fracture, and 8 participants who did not take the scheduled lumbar MR examinations were excluded. Finally, 188 participants were included in this study.
The LBP Questionnaire
At the time of enrolment, each participant completed a questionnaire on their demographic information, LBP status, consequences, and functional limitations. An indicative anatomical drawing showing the low back area (between the lowest ribs and lower gluteal folds) was printed on the questionnaire, and 9 questions (Table 2) were used to evaluate LBP status and consequences. Functional limitations were assessed with the Oswestry Disability Index (version 2.0). Although participants completed the questionnaire, a spine surgeon was present for instruction and technical checking of missing data and logical errors. Then, the retrieved questionnaires were relayed to the medical assistants' office, and the data were double entered and corrected for entry errors using EpiData software version 3.0 (EpiData Association, Odense, Denmark). The questionnaires were completed, and all data entered and verified, during 2 months from June to August 2007.
TABLE 2. The LBP Questionnaire and the Follow-up Questionnaire.
| Questions | Answer Options | |
|---|---|---|
| The LBP questionnaire | ||
| 1 | Have you ever had LBP? If yes, go through the remaining questions. If no, stop here. | Yes No |
| 2 | Have you ever had work absence due to LBP? | Yes No |
| 3 | Have you ever sought medical care (consulted a physician or had a radiological examination) due to LBP? | Yes No |
| 4 | If you choose “Yes” in question 3, what was your diagnosis or suspected diagnosis? | (1) Lumbar disc herniation. (2) Lumbar disc degeneration. (3) Back myofascitis. (4) Lumbar muscle strain. (5) Other____ |
| 5 | Which of the following most closely describes your LBP? | Chronic pain |
| Acute pain | ||
| 6 | What were the causes of your LBP? | (1) Heavy workload. (2) Awkward working posture. (3) Back sprain, (4) Minor body movement. (5) Gaining weight. (6) Pregnancy. (7) Cold environment. (8) Smoking. (9) Bad mood. (10) Unable to define. (11) Other causes. |
| 7 | Please designate the pain intensity of your most severe episode of LBP in the past 2 yr in a number chosen from 0 to 10, if 0 refers to no pain and 10 refers to intolerable pain. | |
| 8 | Have you had LBP within the last year? | Yes No |
| 9 | Do you have LBP today? Current pain intensity (0–10)? | Yes No |
| In questions 7 and 9, pain intensity was evaluated by a linear VAS, with choices ranging from 0 (no pain) to 10 (intolerable pain). For participants’ ease, we did not print the word “VAS” on the actual questionnaire. | ||
| The follow-up questionnaire | ||
| 1 | Compared with the status in 2007 when you completed the last questionnaire, what is your current LBP status? | Better/same/worse |
| 2 | Compared with the status in 2007 when you completed the last questionnaire, what is the current frequency of your LBP episodes? | More/same/less |
| 3 | Compared with the status in 2007 when you completed the last questionnaire, what is the current overall intensity of your LBP? | More severe/same/reduced |
LBP indicates low back pain.
Lumbar MR Classification
The participants underwent lumbar MR examination within 6 months after completing the questionnaire. Lumbar MR image covered the T10–T11 disc level in 156 of 188 participants (83.0%). Participants were classified as SD-like or non–SD-like according to lumbar MR image manifestations. SD-like participants were identified using SD diagnostic criteria, either by the classical Sorenson criterion of at least 3 consecutive wedge-shaped vertebrae showing more than 5° of anterior wedging,15 or by a modified Heithoff criterion for SD. In the original study by Heithoff et al,17 3 of the 5 signs on MR image, including WV, SN, IE, disc space narrowing, and disc dehydration, were necessary for SD diagnosis. However, because disc space narrowing and disc dehydration are highly nonspecific in adults, we modified Heithoff criterion to “simultaneous presence of WV, SN, and IE” to identify SD-like participants (Figure 2). Two spine surgeons blinded to participants' LBP status independently reviewed and classified the MR images. The inter-rater consistency between these evaluators demonstrated good consistency (κ= 0.931, P < 0.0001). The classification results presented in this study are from the senior physician of the 2 evaluators.
Figure 2.
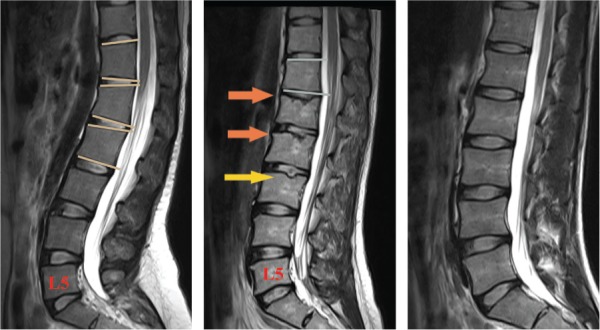
Lumbar MR classification of “Scheuermann (SD)-like” and non–SD-like participants. Left image. This participant has 3 consecutive more than 5° WV (T12–L2) and was identified as “SD-like” according to Sorensen criterion. Middle image. This participant has SN (yellow arrow) and IEs (orange arrows). Although only 2 WV (T12 and L2) were observed, WV, SN, and IE were simultaneously present in lumbar MR images. Therefore, she was also identified as SD-like, according to the modified Heithoff criteria. Right image. This participant has no Scheuermann signs and was classified as non–SD-like. WV indicates wedged vertebrae; SN, Schmorl node; IE, irregular endplate; SD, Scheuermann disease; LS, lumbar spine; MR, magnetic resonance.
LBP Follow-up
Follow-up interviews were conducted to compare LBP progression during a 6-year period between SD-like participants and non–SD-like participants. Participants completed a 3-question follow-up questionnaire concerning change in LBP status during the follow-up period (Table 2). A surgical resident who was blinded to the study protocol and had completed an orthopedics rotation conducted the follow-up. We prepared gifts for each interviewee (hand mirror for females and mobile phone portable charger for males) to encourage their participation.
Study Items
The objective of this study was to investigate the relationship between SD-like spine and LBP in this series of hospital employees. The study items and outcome measures are listed in Table 3.
TABLE 3. Study Items.
| Study Items | Outcome Measures |
|---|---|
| Classification of SD-like participants’ and non–SD-like participants’ demographic information | Comparison of sex, age, height, weight, BMI, occupation, and smoking status between the 2 groups* |
| Radiological features of SD-like spine | The level distribution of WV, SN, and IE from T10–T11 to L5–S1 in SD-like participants |
| Comparison of average thoracolumbar kyphotic angle (angle between the extension lines of the superior endplate of T10 vertebra and the inferior endplate of L2 vertebra) between the 2 groups | |
| Comparison of LBP issues | Comparison of lifetime LBP, previous 1-year LBP, and point LBP rates between the 2 groups |
| Comparison of rates of work absence due to LBP, seeking medical care due to LBP, intensity (VAS score) of the most severe LBP episode in the past 2 yr, point LBP intensity (VAS score), point low back function (ODI score with a range between 0% [normal] and 100% [totally disabled]), and causes of LBP between patients with LBP in the 2 groups | |
| Comparison of LBP progression | Comparison of the proportion of participants with LBP aggravated during the follow-up period and the manner of progression between the 2 groups. Rate of LBP aggravation is defined as the number of participants whose LBP progressed (in overall status, pain intensity, or frequency of LBP episode, which correspond with 3 questions in the LBP follow-up questionnaire) during the follow-up period divided by the number of participants in that group who were successfully followed up. |
*We did not compare educational background because of the incorrect credential evaluation system used in the original database.
VAS indicates visual analogue scale; WV, wedged vertebrae; SN, Schmorl node; IE, irregular endplate; BMI, body mass index; ODI, Oswestry Disability Index; LBP, low back pain.
Statistics
The independent-samples t test was used to compare normally distributed data. For data that were not normally distributed, the rank sum test was used. The χ2 test was used to compare rates. SPSS version 21.0 (SPSS, Chicago, IL) was used for statistical analysis. The α value was set at 0.05.
RESULTS
SD-Like and Non–SD-Like Participants' Demographic Information
Among the 188 study participants, 34 had SD-like spine, and the remaining 154 had non–SD-like spine. Among the 34 SD-like participants, 24 met Sorensen criterion, 21 met modified Heithoff criteria, and 11 met both. A higher proportion of males were included in the SD-like group (67.6% vs. 39.6%; χ2 = 8.857, P = 0.003). Therefore, the majority of the following study items were examined overall and according to sex. No significant differences in age, occupation, height, weight, body mass index, or smoking status were observed between groups (Table 4).
TABLE 4. Demographic Data, Radiological Data, and General LBP Rates for SD-Like Participants and Non–SD-Like Participants.
| SD-Like | Non–SD-Like | t | Z | χ2 | P | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 23 (69.7%) | 61 (39.6%) | 8.857 | 0.003* | ||
| Female | 11 (30.3%) | 93 (60.4%) | ||||
| Age, yr | ||||||
| All | 41.5 (28.8–51.0) | 34.5 (27.0–44.0) | −1.459 | 0.135 | ||
| Males | 47.0 (11.2) | 42.0 (11.3) | 1.787 | 0.078 | ||
| Females | 26.0 (25.0–30.0) | 29.0 (25.0–38.0) | −1.774 | 0.076 | ||
| Height, cm | ||||||
| Males | 172.8 (6.4) | 172.5 (5.6) | 0.224 | 0.832 | ||
| Females | 162.1 (5.7) | 162.1 (4.1) | 0.004 | 0.997 | ||
| Weight, kg | ||||||
| Males | 76.0 (9.5) | 73.3 (8.5) | 1.243 | 0.218 | ||
| Females | 56.5 (7.5) | 56.7 (7.7) | 0.082 | 0.935 | ||
| BMI, kg/m2 | ||||||
| Males | 25.4 (2.7) | 24.6 (2.7) | 1.201 | 0.233 | ||
| Females | 21.5 (2.4) | 21.6 (2.9) | 0.127 | 0.899 | ||
| Occupation | ||||||
| a† | 14 (41.2%) | 31 (20.1%) | 6.902 | 0.075 | ||
| b‡ | 4 (11.8%) | 26 (16.9%) | ||||
| c§ | 5 (14.7%) | 35 (22.7%) | ||||
| d¶ | 11 (32.4%) | 62 (40.3%) | ||||
| Smoking status | ||||||
| Males | 17 (73.9%) | 35 (57.4%) | 1.937 | 0.164 | ||
| Females | 1 (9.1%) | 1 (1.8%) | 0.201║ | |||
| Thoracolumbar kyphotic angle, ° | ||||||
| All | 12.1 (10.8–12.8) | 7.3 (5.7–8.5) | −7.635 | <0.0001* | ||
| Males | 11.9 (10.4–12.4) | 6.8 (5.7–8.1) | −6.233 | <0.0001* | ||
| Females | 12.7 (1.7) | 7.6 (2.3) | 6.436 | <0.0001* | ||
| Lifetime LBP ratio | ||||||
| All | 19 (55.9%) | 74 (48.1%) | 1.479 | 0.224 | ||
| Males | 13 (56.5%) | 20 (32.8%) | 3.945 | 0.047* | ||
| Females | 6 (54.5%) | 54 (58.1%) | 0.000 | 1.000 | ||
| Past 1-year LBP ratio | ||||||
| All | 15 (44.1%) | 51 (33.1) | 0.683 | 0.409 | ||
| Males | 10 (43.5%) | 11 (18.0%) | 5.768 | 0.016* | ||
| Females | 5 (45.5%) | 40 (43.0%) | 0.266 | 0.606 | ||
| Point LBP ratio | ||||||
| All | 10 (29.4%) | 36 (23.4%) | 0.549 | 0.459 | ||
| Males | 6 (26.1%) | 9 (14.8%) | 0.792 | 0.374 | ||
| Females | 4 (36.4%) | 27 (29.0%) | 0.024 | 0.877 |
Data are presented as number (%), mean (standard deviation), or median (IQR).
*Statistically significant.
†Drivers, engineers, and plumbers.
‡Financial department administrative/clerical/accounting staffs.
§Physicians.
¶Nurses.
║Fisher exact test.
IQR indicates interquartile range; BMI, body mass index; LBP, low back pain.
Radiological Features of SD-Like Participants
Scheuermann signs, including WV, SN, and IE, were clustered in the thoracolumbar region between T11–T12 and L2–L3 (Figure 3). The median thoracolumbar kyphotic angle of SD-like participants (12.1°; interquartile range, 10.8°–12.8°) was significantly higher than that of non–SD-like participants (7.3°; interquartile range, 5.7°–8.5°; Z =−7.635; P < 0.0001).
Figure 3.
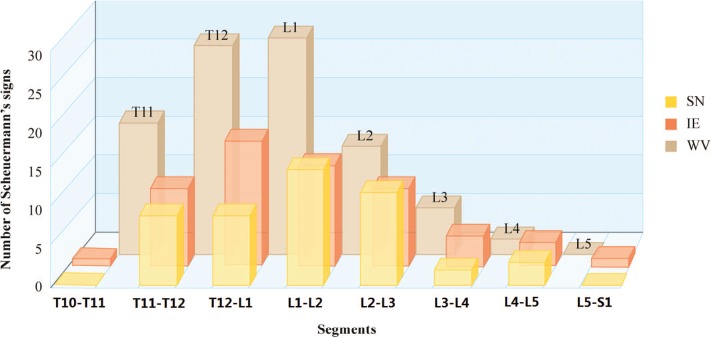
The level distribution of Scheuermann signs in SD-like spine. These signs were clustered in the thoracolumbar junction area between T10–T11 and L2–L3 and sparse in the lower lumbar region. SD indicates Scheuermann disease; SN, Schmorl node; IE, irregular endplate; WV, wedged vertebra.
LBP Issues in SD-Like and Non–SD-Like Participants
Rates of lifetime LBP, previous 1-year LBP, and point LBP did not significantly differ between the 2 groups (Table 4). When stratified by sex, SD-like males had a higher rate of lifetime LBP and previous 1-year LBP than non–SD-like males (Table 4).
SD-like and non-SD-like LBP sufferers demonstrated no significant difference in pain type (chronic vs. acute), point VAS score, or Oswestry Disability Index score. However, SD-like LBP sufferers had higher rates of work absence and sought medical care due to LBP than non-SD-like LBP sufferers (Table 5). The intensity (VAS score) of the most severe LBP episode in the past 2 years was also significantly higher among SD-like LBP sufferers.
TABLE 5. Data From SD-Like LBP Sufferers and Non-SD-Like LBP Sufferers.
| SD-Like | Non–SD-Like | t | Z | χ2 | P | |
|---|---|---|---|---|---|---|
| LBP type | ||||||
| All | ||||||
| Chronic | 8 (42.1%) | 21(28.4%) | 1.327 | 0.249 | ||
| Acute | 11 (57.9%) | 53 (71.6%) | ||||
| Males | ||||||
| Chronic | 6 (46.2%) | 7 (35%) | 0.411 | 0.522 | ||
| Acute | 7 (53.8%) | 13 (65.0%) | ||||
| Females | ||||||
| Chronic | 2 (33.3%) | 14 (25.9%) | 0.000 | 1.000 | ||
| Acute | 4 (66.7%) | 40 (74.1%) | ||||
| ODI score, % | ||||||
| All | 8 (2–18) | 4 (2–10) | 1.330 | 0.184 | ||
| Males | 6 (2–19) | 4 (0–5.5) | 1.273 | 0.221 | ||
| Females | 10.4 (8.4) | 7.0 (6.9) | 1.085 | 0.282 | ||
| Point LBP VAS score | ||||||
| All | 2.0 (0–3) | 0 (0–2) | 1.334 | 0.179 | ||
| Males | 0 (0–2) | 0 (0–2) | 0.485 | 0.676 | ||
| Females | 3.5 (1.5–5.3) | 0 (0–2) | 2.483 | 0.020* | ||
| Highest LBP VAS score in the past 2 yr | ||||||
| All | 6.4 (2.5) | 4.1 (2.5) | 3.564 | 0.001* | ||
| Males | 6.2 (2.6) | 4.0 (2.6) | 2.408 | 0.022* | ||
| Females | 6.8 (2.6) | 4.1 (2.5) | 2.510 | 0.015* | ||
| Work absence | ||||||
| All | 8 (42.1%) | 7 (9.5%) | 9.620 | 0.002* | ||
| Males | 6 (46.2%) | 2 (10%) | 0.035*† | |||
| Females | 2 (33.3%) | 4 (7.4%) | 0.105† | |||
| Seeking medical care | ||||||
| All | 13 (68.4%) | 29 (39.2%) | 5.216 | 0.022* | ||
| Males | 9 (69.2%) | 7 (35%) | 0.080† | |||
| Females | 4 (66.7%) | 22 (40.7%) | 0.611 | 0.434 |
Data are in number (%) or mean (standard deviation), or median (IQR).
*Statistically significant.
†Fisher exact test.
VAS indicates visual analogue scale; IQR, interquartile range; ODI, Oswestry Disability Index; LBP, low back pain.
The most frequent causes of LBP were heavy workload, awkward working postures, and cold environment in both groups (Figure 4), with the exception of SD-like females, in whom the third most frequent cause was minor body movement. A significantly higher proportion of SD-like participants than non–SD-like participants listed “heavy workload” (94.7% [18/19] vs. 61.4% [48/74], χ2 = 6.548, P = 0.011).
Figure 4.
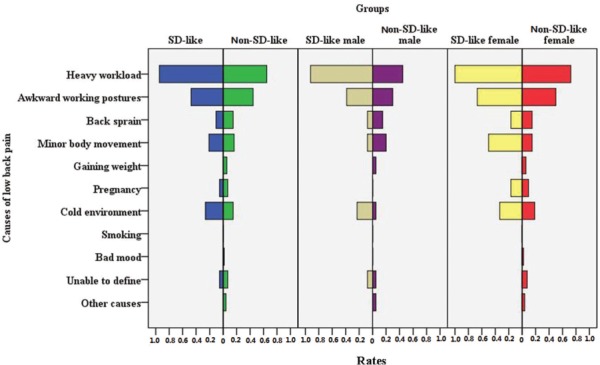
Causes of LBP in SD-like LBP sufferers and in non-SD-like LBP sufferers. SD indicates Scheuermann disease; LBP, low back pain.
LBP Progression in SD-Like and Non–SD-Like Participants
We successfully followed 159 of the 188 participants (84.6%), including 29 SD-like participants and 130 non–SD-like participants. The follow-up rates did not significantly differ between groups (SD-like: 85.3% [29/34], non–SD-like: 84.4% [130/154]; χ2 = 0.016, P = 0.898). The reasons for loss to follow-up were death in 3 participants, resignation in 23 participants, and retirement in 3 participants. The follow-up period was 6 years (from August 2007 to August 2013). During the follow-up period, 21 new cases of LBP occurred in 6 SD-like participants and 15 non–SD-like participants.
LBP incidence during the follow-up period did not significantly differ between SD-like participants and non–SD-like participants (20.7% [6/29] vs. 11.5% [15/130], χ2 = 1.026, P = 0.311). However, the rate of LBP aggravation during the follow-up period was significantly higher among SD-like participants than non–SD-like participants (55.2% [16/29] vs. 26.9% [35/130], χ2 = 8.685, P = 0.003). The difference remained significant for both sexes (males, 47.4% [9/19] vs. 18.8% [9/48], χ2 = 5.647, P = 0.017; females, 70.0% [7/10] vs. 31.7% [26/82], χ2 = 4.139, P = 0.042). Aggravation in pain intensity was noted by 93.7% (15/16) of SD-like participants whose LBP progressed, with or without an increase in frequency, whereas 6.3% (1/16) only reported increased frequency. In non–SD-like participants whose LBP progressed, the corresponding rates were 68.6% (24/35) and 31.4% (9/35) (χ2 = 2.596, P = 0.107).
DISCUSSION
In this series of hospital employees, 18.1% of the participants met radiological criteria of SD, and 55.9% of these SD-like participants had a history of LBP. SD-like LBP sufferers were more likely to experience work absence and seek medical care due to LBP than non-SD-like LBP sufferers, and a higher proportion of SD-like participants demonstrated LBP progression over time. Moreover, when sex was considered, SD-like males were more likely to experience LBP than non–SD-like males. However, LBP incidence during the follow-up period was not significantly different between groups, which does not support that an SD-like spine is a risk factor for LBP.
Nearly one-fifth of these hospital staff members had lumbar MR image manifestations that met SD diagnostic criteria. This rate is comparable with the reported 18% to 40% prevalence of SD radiological signs in the general population.23 They did share some traits with patients with SD. First, more males were classified as SD-like, consistent with previously reported sex ratios in patients with SD.16–19,21,22,24 Second, Scheuermann signs in SD-like participants were mainly aggregated at the thoracolumbar junction area between T11–T12 and L2–L3 and sparse in the lower lumbar region, consistent with previous radiological descriptions of “lumbar” or “atypical SD.”18,19,25,26 Third, thoracolumbar kyphosis of SD-like participants was significantly greater than that of non–SD-like participants, which, we postulate, was indicative of a mild form of Scheuermann kyphosis.
Although SD has been associated with back pain,14,16–22 our results indicate 3 characteristics of LBP in SD-like spine. First, LBP was more painful. Although point LBP intensity did not significantly differ between LBP sufferers in the 2 groups, the most severe episode of LBP in the past 2 years was significantly more painful among SD-like LBP sufferers. Follow-up assessment also indicated that aggravation of pain intensity was present in most SD-like participants whose LBP progressed. Second, LBP was more disabling. Significantly higher proportions of SD-like LBP sufferers required work absence or medical care, and selected “heavy workload” as a causative factor of LBP. Third, LBP was more progressive, with a significantly higher proportion of SD-like participants reporting LBP progression at follow-up. Statistically, these characteristics were more robust in males than in females, which may be because of the greater prevalence of males than females in the SD-like group. In contrast, female sex itself is a potential risk factor for LBP, which may weaken the difference in LBP status between SD-like females and non–SD-like females.5,6
These observed characteristics of LBP associated with SD-like spine may originate from a synergism of multiple pathologies, including DD, endplate lesions, and abnormal spinal curvature. SD has been shown to promote DD.17,18,25 Histological studies revealed developmental structural weakness in the disc and endplate of patients with SD,27 which may favor the development of both DD and endplate lesions. Endplate lesions, especially SN,28 are well associated with SD and may irritate local nerve endings that supply it, resulting in LBP.29 As for increased thoracolumbar kyphosis, as observed in SD-like participants, it may cause LBP via altered stresses or mechanisms of referred pain due to irritation of the T12 or L1 spinal nerve.30 Thus, LBP in people with SD-like spine seems more related to specific pathologies with a possible genetic background than to nonspecific functional disturbances.
Several possible confounding factors should be discussed. DD has long been suspected of playing a role in LBP development,2,12 but we did not evaluate DD or stratify results by the degree of DD in this study, because current evidence has not verified that DD is associated with LBP, and SD itself can promote DD.17,18,25 In addition, participants were enrolled and classified regardless of their DD status. Another possible confounder is occupation because the percentage of manual workers (drivers, engineers, and plumbers) was twice as high in the SD-like group (P = 0.075). However, several systematic reviews suggested that work-related mechanical factors were unlikely to independently cause LBP,2 and among athletes who typically handled heavy physical loads, those with SD were more likely to present back symptoms and radiological degeneration aggravated over time than those without SD.31 Therefore, in this study, occupation was unlikely independently causative of LBP. Nevertheless, mechanical factors were notably associated with SD development.31–34 Thus, we postulate that occupation-related mechanical factors and SD-related intrinsic factors may work together in causing LBP in some study participants with SD-like spines.
CONCLUSION
It is important to note that this study is more related to a preliminary investigation in an attempt to establish a justifiable hypothesis than late-stage research with results that could change current spinal practice. The limitations of this study include its retrospective design, single-hospital setting, and small sample size. Furthermore, the definition of atypical SD remains controversial. However, our results suggest a possible association between SD-like spine and LBP in the general adult population. This phenomenon definitely warrants further research, because additional verification may bring some social effects. For example, people with SD-like spine may want to know the risks if they develop LBP and make career and recreational choices accordingly. Future investigations in this field should involve lumbar MR in larger, prospective population-based cohorts.12,35
Key Points
In the general population, lumbar MR images of many people would meet the diagnostic criteria of SD.
Among LBP sufferers, SD-like spine is associated with higher rates of work absence and seeking medical care due to LBP, as well as greater intensity of the most severe LBP episode in the past 2 years.
When sex is considered, SD-like males are more likely to experience LBP than non–SD-like males.
Having SD-like spine is likely to be associated with the severity and progressive nature of LBP in the general population.
Footnotes
Ning Liu and Xinhu Guo contributed equally to this article.
Acknowledgment date: April 2, 2014.
The manuscript submitted does not contain information about medical device(s)/drug(s).
2013 AOSpine China Research Grant was received to support this work.
No relevant financial activities outside the submitted work.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balagué F, Mannion AF, Pellisé F, et al. Nonspecific low back pain. Lancet 2012;379:482–91. [DOI] [PubMed] [Google Scholar]
- 3.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 2008;8:8–20. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007;147:478–91. [DOI] [PubMed] [Google Scholar]
- 5.Chenot JF, Becker A, Leonhardt C, et al. Sex differences in presentation, course, and management of low back pain in primary care. Clin J Pain 2008;24:578–84. [DOI] [PubMed] [Google Scholar]
- 6.Oksuz E. Prevalence, risk factors, and preference-based health states of low back pain in a Turkish population. Spine 2006;31:968–72. [DOI] [PubMed] [Google Scholar]
- 7.Shiri R, Karppinen J, Leino-Arjas P, et al. The association between obesity and low back pain: a meta-analysis. Am J Epidemiol 2010;171:135–54. [DOI] [PubMed] [Google Scholar]
- 8.Landry MD, Raman SR, Sulway C, et al. Prevalence and risk factors associated with low back pain among health care providers in a Kuwait hospital. Spine 2008;33:539–45. [DOI] [PubMed] [Google Scholar]
- 9.Shiri R, Karppinen J, Leino-Arjas P, et al. The association between smoking and low back pain: a meta-analysis. Am J Med 2010;123:87.e7–35. [DOI] [PubMed] [Google Scholar]
- 10.Clays E, De Bacquer D, Leynen F, et al. The impact of psychosocial factors on low back pain: longitudinal results from the Belstress study. Spine 2007;32:262–8. [DOI] [PubMed] [Google Scholar]
- 11.Bener A, Verjee M, Dafeeah EE, et al. Psychological factors: anxiety, depression, and somatization symptoms in low back pain patients. J Pain Res 2013;6:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takatalo J, Karppinen J, Niinimäki J, et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults? Spine 2011;36:2180–9. [DOI] [PubMed] [Google Scholar]
- 13.Waris E, Eskelin M, Hermunen H, et al. Disc degeneration in low back pain: a 17-year follow-up study using magnetic resonance imaging. Spine 2007;32:681–4. [DOI] [PubMed] [Google Scholar]
- 14.Lowe TG. Scheuermann disease. J Bone Joint Surg Am 1990;72:940–5. [PubMed] [Google Scholar]
- 15.Sorensen KH. Scheuermann's Juvenile Kyphosis: Clinical Appearances, Radiography, Aetiology, and Prognosis. Copenhagen, Denmark: Ejnar Munksgaard Forlag; 1964. [Google Scholar]
- 16.Blumenthal SL, Roach J, Herring JA. Lumbar Scheuermann's. A clinical series and classification. Spine 1987;12:929–32. [PubMed] [Google Scholar]
- 17.Heithoff KB, Gundry CR, Burton CV, et al. Juvenile discogenic disease. Spine 1994;19:335–40. [DOI] [PubMed] [Google Scholar]
- 18.Paajanen H, Alanen A, Erkintalo M, et al. Disc degeneration in Scheuermann disease. Skeletal Radiol 1989;18:523–6. [DOI] [PubMed] [Google Scholar]
- 19.Green TL, Hensinger RN, Hunter LY. Back pain and vertebral changes simulating Scheuermann's disease. J Pediatr Orthop 1985;5:1–7. [DOI] [PubMed] [Google Scholar]
- 20.Karppinen J, Pääkkö E, Paassilta P, et al. Radiologic phenotypes in lumbar MR imaging for a gene defect in the COL9A3 gene of type IX collagen. Radiology 2003;227:143–8. [DOI] [PubMed] [Google Scholar]
- 21.Ristolainen L, Kettunen JA, Heliövaara M, et al. Untreated Scheuermann's disease: a 37-year follow-up study. Eur Spine J 2012;21:819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray PM, Weinstein SL, Spratt LF. The natural history and long-term follow-up of Scheuermann kyphosis. J Bone Joint Surg Am 1993;75:236–48. [DOI] [PubMed] [Google Scholar]
- 23.Lesoin F, Leys D, Rousseaux M, et al. Thoracic disk herniation and Scheuermann's disease. Eur Neurol 1987;26:145–52. [DOI] [PubMed] [Google Scholar]
- 24.Damborg F, Engell V, Andersen M, et al. Prevalence, concordance, and heritability of Scheuermann kyphosis based on a study of twins. J Bone Joint Surg Am 2006;88:2133–6. [DOI] [PubMed] [Google Scholar]
- 25.Liu N, Chen Z, Qi Q, et al. The relationship of symptomatic thoracolumbar disc herniation and Scheuermann's disease [published online ahead of print November 17, 2013]. Eur Spine J. 10.1007/s00586-013-3108-7. [DOI] [PubMed] [Google Scholar]
- 26.Mandell GA, Morales RW, Harcke HT, et al. Bone scintigraphy in patients with atypical lumbar Scheuermann disease. J Pediatr Orthop 1993;13:622–7. [PubMed] [Google Scholar]
- 27.Aufdermaur M. Juvenile kyphosis (Scheuermann's disease): radiography, histology, and pathogenesis. Clin Orthop Relat Res 1981;154:166–74. [PubMed] [Google Scholar]
- 28.Cleveland RH, Delong GR. The relationship of juvenile lumbar disc disease and Scheuermann's disease. Pediatr Radiol 1981;10:161–4. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Videman T, Battié MC. ISSLS prize winner: lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine 2012;37:1490–6. [DOI] [PubMed] [Google Scholar]
- 30.Sebastian D. Thoracolumbar junction syndrome: a case report. Physiother Theory Pract 2006;22:53–60. [DOI] [PubMed] [Google Scholar]
- 31.Blazek O, Streda A, Cermák V, et al. The incidence of morbus Scheuermann in sportsmen. J Sports Med Phys Fitness 1986;26:55–9. [PubMed] [Google Scholar]
- 32.Kruse D, Lemmen B. Spine injuries in the sport of gymnastics. Curr Sports Med Rep 2009;8:20–8. [DOI] [PubMed] [Google Scholar]
- 33.Palazzo C, Sailhan F, Revel M. Scheuermann's disease: an update [published online ahead of print January 24, 2014]. Joint Bone Spine 10.1016/j.jbspin.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Linthoudt DV, Revel M. Similar radiologic lesions of localized Scheuermann's disease of the lumbar spine in twin sisters. Spine 1994;19:987–9. [DOI] [PubMed] [Google Scholar]
- 35.Mok FP, Samartzis D, Karppinen J, et al. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine 2010;35:1944–52. [DOI] [PubMed] [Google Scholar]
- 36.Rogge CW, Nieman A. Isolated and atypical manifestation of Scheuermann's disease. Arch Chir Neerl 1976;28:149–60. [PubMed] [Google Scholar]
- 37.Harreby M, Neergaard K, Hesselsøe G, et al. Are radiologic changes in the thoracic and lumbar spine of adolescents risk factors for low back pain in adults? A 25-year prospective cohort study of 640 school children. Spine 1995;10:2298–302. [DOI] [PubMed] [Google Scholar]
- 38.Gustavel M, Beals RK. Scheuermann's disease of the lumbar spine in identical twins. AJR Am J Roentgenol 2002;179:1078–9. [DOI] [PubMed] [Google Scholar]
- 39.Summers BN, Singh JP, Manns RA. The radiological reporting of lumbar Scheuermann's disease: an unnecessary source of confusion amongst clinicians and patients. Br J Radiol 2008;81:383–5. [DOI] [PubMed] [Google Scholar]
- 40.Song KS, Yang JJ. Acutely progressing paraplegia caused by traumatic disc herniation through posterior Schmorl's node opening into the spinal canal in lumbar Scheuermann's disease. Spine 2011;36:E1588–91. [DOI] [PubMed] [Google Scholar]
- 41.Hasler CC. Back pain during growth. Swiss Med Wkly 2013;143:w13714. [DOI] [PubMed] [Google Scholar]
- 42.Lucas-García FJ, Vicent-Carsí V, Sánchez-González M. Atypical lumbar Schuermann's disease: a presentation of 6 cases. Rev Esp Cir Ortop Traumatol 2013;57:135–9. [DOI] [PubMed] [Google Scholar]


