Abstract
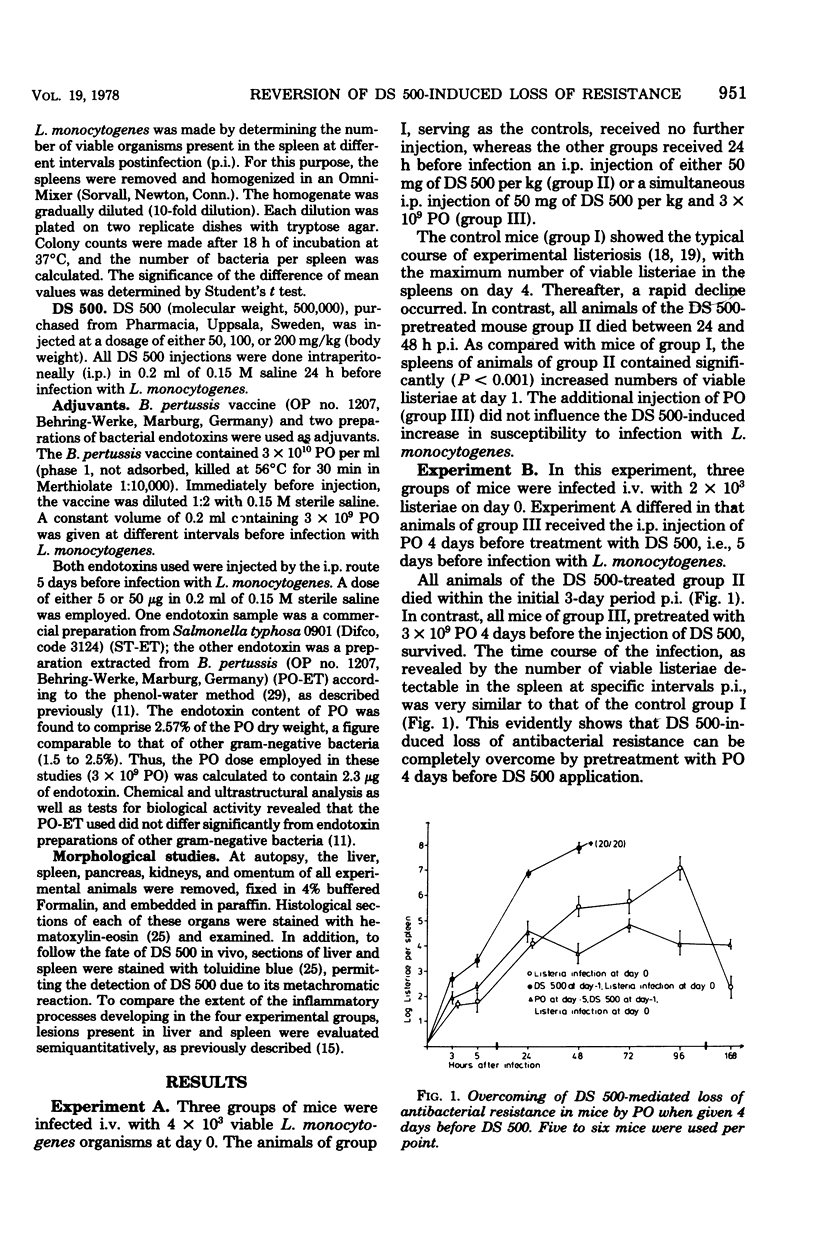
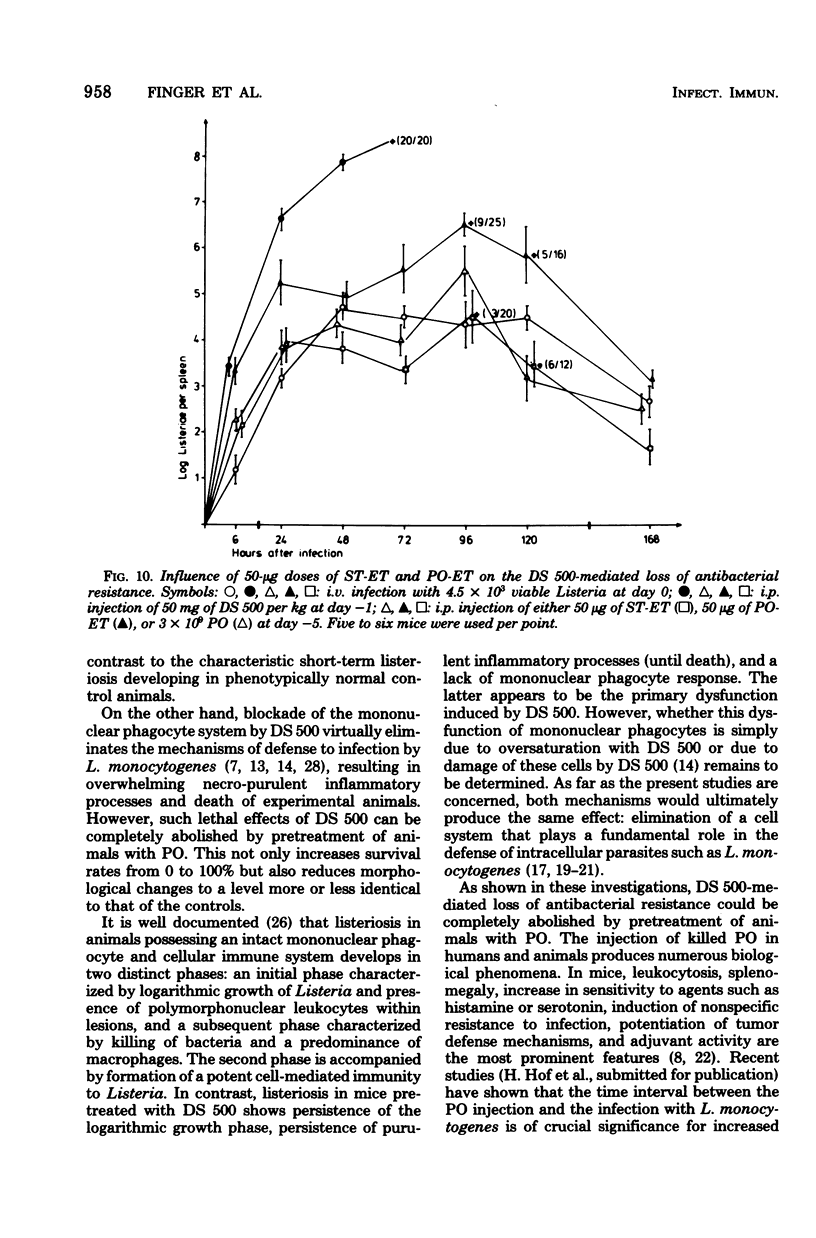
Parenteral injection of dextran sulfate 500 (DS 500; 50 mg/kg of body weight) into mice caused a complete loss of resistance to a sublethal (2 X 10(3) to 5 X 10(3)) infection with Listeria monocytogenes. Such loss could be prevented by pretreatment of animals with 3 X 10(9) heat-killed Bordetella pertussis organisms (PO) 5 to 30 days before the administration of DS 500. The increased phagocytic capcity induced by PO was only exhausted when a fourfold dose of DS 500, effecting complete loss of antibacterial resistance (50 mg/kg ob body weight), was administered. Listeriosis in mice treated with DS 500 is characterized by rapid-progressive necro-purulent inflammation of liver and spleen, lack of mononuclear phagocyte response, and 100% lethality within 72 h after infection. In contrast, the time course, extent, and morphological characteristics of listeriosis in animals pretreated with PO before the DS 500 application were not significantly different from those of nonpretreated controls. Evidence is presented that the protective effect of PO is due to activation of the mononuclear phagocyte system, which without such treatment is blocked by the DS 500 administration. The data presented indicate that the protective effect of PO is due only in part to the endotoxic moiety of these bacteria. Differences in the course and morphology of listeriosis in animals with dysfunction of the mononuclear phagocyte system and in animals with deficiency of the cellular immune system are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beneke G., Finger H., Emmerling P. Einfluss von Bordetella pertussis auf das lymphatische Gewebe von Mäsuen. II. Proliferiernde Zellsysteme in der Milz nach Injektion vonBoredtelle pertussis. Z Med Mikrobiol Immunol. 1968;154(3):178–195. [PubMed] [Google Scholar]
- Chan C., Kongshavn P. A., Skamene E. Enhanced primary resistance to Listeria monocytogenes in T cell-deprived mice. Immunology. 1977 Apr;32(4):529–537. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Dresser D. W., Wortis H. H., Anderson H. R. The effect of pertussis vaccine on the immune response of mice to sheep red blood cells. Clin Exp Immunol. 1970 Dec;7(6):817–831. [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Hof H. Cell-mediated resistance to infection with Listeria monocytogenes in nude mice. Infect Immun. 1977 Feb;15(2):382–385. doi: 10.1128/iai.15.2.382-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger H., Bartoschek M., Emmerling P. Time Relationships Between Injection of Antigen and Adjuvant I. Adjuvancy of Bordetella pertussis Given at Various Times Before the Primary Antigenic Stimulus. Infect Immun. 1970 Nov;2(5):590–600. doi: 10.1128/iai.2.5.590-600.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger H., Emmerling P., Plager L. Time relationships between injection of antigen and adjuvant. 3. Adjuvancy of Bordetella pertussis given at various times after the primary antigenic stimulus. Infect Immun. 1972 May;5(5):783–791. doi: 10.1128/iai.5.5.783-791.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger H., Heymer B., Hof H., Rietschel E., Schleifer K. H. Uber Struktur und biologische Aktivität von Bordetella pertussis-Endotoxin. Zentralbl Bakteriol Orig A. 1976 Aug;235(1-3):56–64. [PubMed] [Google Scholar]
- Finger H., Stein P., Beneke G., Emmerling P., Plager L. Einfluss von Bordetella pertussis auf das lymphatische Gewebe von Mäusen. VII. Untersuchungen über die Beziehungen zwischen den durch Bordetella pertussis ausgelösten biologischen Effekten. Med Microbiol Immunol. 1972;157(4):265–290. doi: 10.1007/BF02121120. [DOI] [PubMed] [Google Scholar]
- Hahn H., Bierther M. Morphological changes induced by dextran sulfate 500 in mononuclear phagocytes of listeria-infected mice. Infect Immun. 1974 Nov;10(5):1110–1119. doi: 10.1128/iai.10.5.1110-1119.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymer B., Hobik H. P., Schäfer H., Bültmann B., Spanel R., Haferkamp O. Animal experimental studies on chronic granulomatous inflammation and T-lymphocyte-system. Beitr Pathol. 1975 Nov;156(2):128–144. doi: 10.1016/s0005-8165(75)80146-6. [DOI] [PubMed] [Google Scholar]
- Heymer B., Hof H., Emmerling P., Finger H. Morphology and time course of experimental listeriosis in nude mice. Infect Immun. 1976 Sep;14(3):832–835. doi: 10.1128/iai.14.3.832-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J., Bergman R. K. Histamine-sensitizing factors from microbial agents, with special reference to Bordetella pertussis. Bacteriol Rev. 1968 Jun;32(2):103–126. doi: 10.1128/br.32.2.103-126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Suppression of cell-mediated immunity to infection by an antimitotic drug. Further evidence that migrant macrophages express immunity. J Exp Med. 1970 Sep 1;132(3):535–545. doi: 10.1084/jem.132.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTMAN M. Effect of Haemophilus pertussis on immunological and physiological reactions. Fed Proc. 1957 Sep;16(3):867–872. [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A., Allison A. C. A role of macrophages in the stimulation of immune responses by adjuvants. J Immunol. 1969 Jul;103(1):71–78. [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V., Langman R. E. Early appearance of sensitized lymphocytes in mice infected with Listeria monocytogenes. J Immunol. 1974 Feb;112(2):496–501. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]







