Abstract
Objective:
To determine in a population-based study whether long sleep duration was associated with increased risk of dementia mortality.
Methods:
In this prospective, population-based study of 3,857 people without dementia aged 65 years and older (NEDICES [Neurological Disorders in Central Spain]), participants reported their daily sleep duration. The average daily total sleep duration was grouped into 3 categories: ≤5 hours (short sleepers), 6–8 hours (reference category), and ≥9 hours (long sleepers). Community-dwelling elders were followed for a median of 12.5 years, after which the death certificates of those who died were examined.
Results:
A total of 1,822 (47.2%) of 3,857 participants died, including 201 (11.0%) deaths among short sleepers, 832 (45.7%) among long sleepers, and 789 (43.3%) among those participants in the reference category. Of 1,822 deceased participants, 92 (5.1%) had a dementia condition reported on the death certificate (49 [53.3%] were long sleepers, 36 [39.1%] reported sleeping between 6 and 8 hours, and 7 [7.6%] were short sleepers). In an unadjusted Cox model, risk of dementia-specific mortality was increased in long sleepers (hazard ratio for dementia mortality in long sleepers = 1.58, p = 0.04) when compared with the reference group. In a Cox model that adjusted for numerous demographic factors and comorbidities, the hazard ratio for dementia mortality in long sleepers was 1.63 (p = 0.03).
Conclusions:
Self-reported long sleep duration was associated with 58% increased risk of dementia-specific mortality in this cohort of elders without dementia. Future studies are required to confirm these findings.
Sleep disorders are common in modern society, and especially in the elderly.1 Chronic insomnia, prolonged daytime sleepiness, and long sleeping have been associated with poorer cognitive function.2–6 However, the contribution of sleep problems to the risk of dementia is poorly understood. Only 2 groups have examined this association.7,8 In these 2 separate prospective studies, reduced8 and prolonged sleep7 were associated with increased risk of incident dementia. Understanding the link between dementing disorders and sleep is not a trivial clinical issue. Because sleep duration is potentially modifiable, this has practical implications for the primary prevention of dementing disorders.9 Another currently unexplored method to assess this possible relationship is to study sleep patterns among deaths for which dementia has been assigned as a contributory cause (hereafter referred to as “dementia mortality”). The goal of the present study is to expand our knowledge about this association, examining whether dementia mortality is increased in long sleepers compared to short sleepers or to participants with usual sleep duration (6–8 hours). To address this question, we utilized data from the Neurological Disorders in Central Spain (NEDICES) Study, in which participants were followed for a median of 12.5 years, after which the death certificates of those who died were examined.10–20
METHODS
Study population.
Data were derived from the NEDICES Study, a longitudinal, population-based survey of the epidemiology of major age-associated conditions of the elderly, including Parkinson disease, essential tremor, stroke, and dementia.10–20 A detailed account of the NEDICES study population and sampling methods has been published.21–23 The survey area consisted of 3 communities: (1) Las Margaritas (approximately 14,800 inhabitants), a working-class neighborhood in Getafe (Greater Madrid); (2) Lista (approximately 150,000 inhabitants), a professional-class neighborhood in the Salamanca district (Central Madrid); and (3) Arévalo (approximately 9,000 inhabitants), an agricultural zone of Arévalo County (located 125 km northwest of Madrid). Updated lists of residents were generated from population registers. In each community, eligibility was restricted to residents aged 65 years or older who were present on December 31, 1993, or during 6 or more months of 1993.
Study evaluation.
At the baseline assessment (1994–1995), 5,278 elderly subjects were interviewed using a 500-item screening questionnaire that assessed demographic factors and medical conditions. This face-to-face interview included data collection on demographics, including all medications with CNS effects (anxiolytics, stimulants, antipsychotics, antidepressants, antihistamines, and antiepileptics drugs), and medical conditions. We assessed depressive symptoms by self-report, using the screening question, “Do you suffer from depression?” The same screening approach has been used in previous population studies of depression.24–26 To assess sleep duration, each participant was asked to indicate their “total hours of actual sleep in a 24-hour period.” Participants indicated their typical total daily sleep duration as the sum of nighttime sleep and daytime napping.
We mailed a short form of the questionnaire to subjects who either refused or were unavailable for face-to-face or telephone screening. This form assessed demographic characteristics, neurologic disorders (stroke, essential tremor, dementia, and parkinsonism), medications, and the name of the family doctor.
Follow-up data on death were collected until May 1, 2007. The date that the subject died was obtained from the National Population Register of Spain (Instituto Nacional de Estadística). In Spain, a doctor completes a death certificate for all deceased individuals at the time of death. This certificate is sent to the local authority in the municipality where the person had resided, and the information is then added to the National Register. The cause of death (using ICD-9 for deaths that occurred before 1999 [http://www.cdc.gov/nchs/icd/icd9.htm] and ICD-10 [http://www.cdc.gov/nchs/icd/icd10.htm] for deaths occurring thereafter) was classified by NEDICES investigators into 1 of 6 primary categories: dementia, cerebrovascular disorders, respiratory diseases, cardiovascular disorders (pulmonary embolism, congestive heart failure, myocardial infarction, heart or aortic rupture, and asystole), cancer, and other causes (infections, trauma, and genitourinary or gastrointestinal disorders). As recommended by the World Health Organization, the classification of causes of death had been tabulated by the doctors who completed the death certificates, depending on the basic cause of death (http://www.who.int/topics/mortality/en/). This was defined as the illness or injury that started the chain of pathologic events, which directly led to death (http://www.who.int/topics/mortality/en/).
Final selection of participants.
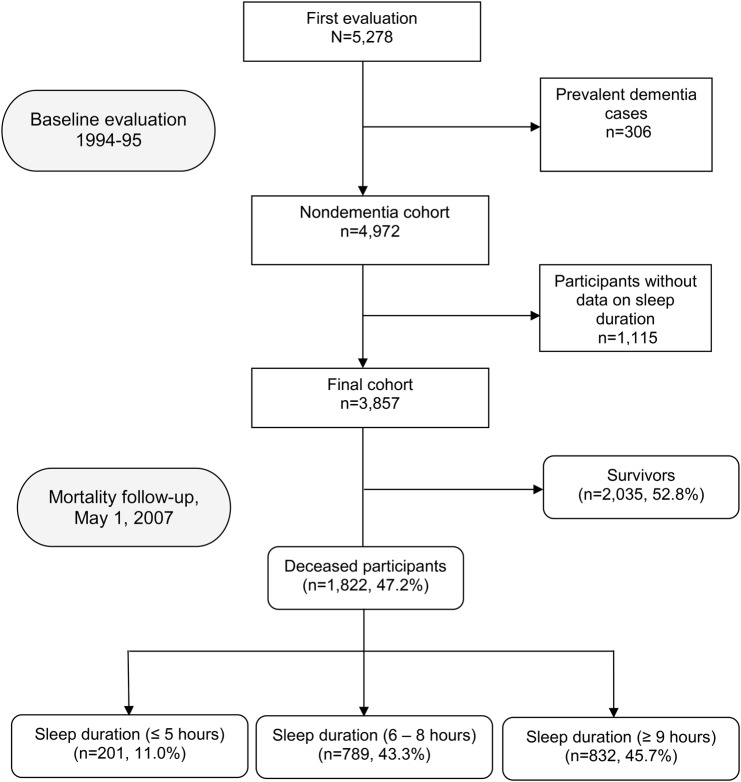
Of the 5,278 participants evaluated at baseline, we excluded 306 with dementia diagnosed at baseline evaluation (1994–1995). We further excluded 1,115 subjects without available data on daily sleep duration, which left 3,857 remaining participants who were included in our analyses (figure 1).
Figure 1. Flowchart of the study.
The final sample of 3,857 was similar to the base sample of 5,278 participants regarding sex (2,177 [56.4%] vs 3,040 [57.6%] women, χ2 = 1.21, p = 0.27) and education (482 [12.5%] vs 711 [13.6%] were illiterate, χ2 = 6.40, p = 0.09), and on average, were only 0.7 years younger (73.6 ± 6.4 vs 74.3 ± 7.0 years, t = 5.09, p < 0.001).
Standard protocol approvals, registrations, and patient consents.
The ethical standards committees on human experimentation at the University Hospitals 12 de Octubre (Madrid) and La Princesa (Madrid) approved all procedures. All enrollees provided written (signed) informed consent.
Statistical analyses.
Analyses were performed in SPSS (version 21.0; IBM Corp., Armonk, NY). All tests were 2-sided, and significance was set at the 5% level (α = 0.05). Age was not distributed normally (1-sample Kolmogorov–Smirnov test). Therefore, while the mean and median values were reported, differences were compared using a nonparametric (Mann–Whitney U and Kruskal–Wallis tests). The χ2 or Fisher p test was used to analyze categorical variables. Participants were divided in short sleepers (≤5 hours daily) and long sleepers (≥9 hours daily) according to the categories used in previous studies.7,27,28 Short and long sleep duration categories were compared with the reference category (6–8 hours daily).
We used Cox proportional-hazards models to estimate hazard ratios (HRs) for dementia-specific mortality; this produced 95% confidence intervals (CIs). The time variable was the years from the date of the first evaluation (1994–1995) to the date of death in subjects who had died. The dependent (outcome) variable was presence of a dementia condition on the death certificate, with the remaining causes of death serving as the reference group; meanwhile, the independent variable was the sleep duration category (long sleep duration vs short sleep duration vs sleeping 6–8 hours daily [reference category]). We began with an unadjusted model. Then, in adjusted models, we first considered baseline variables that in bivariate analyses were associated at the p < 0.05 level with both the exposure (long sleep duration vs short sleep duration vs 6–8 hours daily [the reference category]) and the outcome (dementia-related mortality) (model 1 [more restrictive criteria for confounding]), and then considered baseline variables that in bivariate analyses were associated at the p < 0.05 level with either the exposure or the outcome (model 2 [less restrictive criteria for confounding]). Finally, for completeness, we adjusted for all the potential confounders, independent of their statistical significance (i.e., even if they were not associated with either the exposure or the outcome) (model 3).
Kaplan-Meier survival curves for long sleepers vs short sleepers vs those who slept between 6 and 8 hours daily were assessed; the log-rank test was used to compare the differences among the 3 curves.
RESULTS
The 3,857 participants had a mean duration of follow-up of 10.1 years (median = 12.5 years; range = 0.02–13.5 years). Of the 3,857 participants, 1,822 (47.2%) died over a median follow-up of 7.0 years (range = 0.02–13.3 years), including 201 (11.0%) deaths among short sleepers, 832 (45.7%) among long sleepers, and 789 (43.3%) among those participants who reported sleeping between 6 and 8 hours (figure 1). Of 1,822 deceased participants, 92 (5.1%) had a dementia condition reported on the death certificate. Of these 92, 49 (53.3%) were long sleepers, 36 (39.1%) reported sleeping between 6 and 8 hours, and 7 (7.6%) were short sleepers.
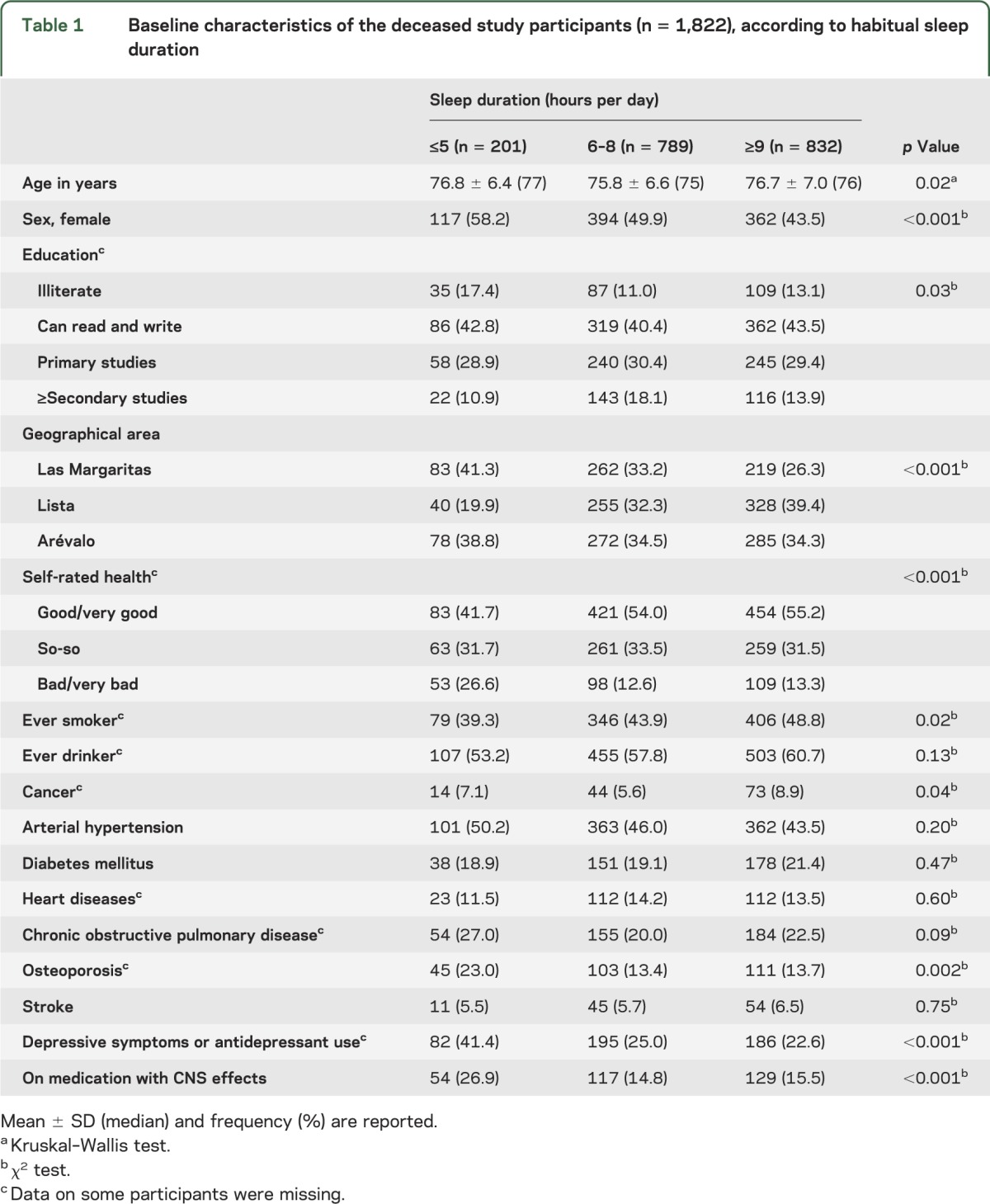
Baseline characteristics of the deceased participants in the 3 sleep duration categories are shown in table 1. Short sleep was associated with female sex, older age, and lower educational category. In addition, short sleepers were more likely to have had osteoporosis and depressive symptoms or antidepressant use; a higher proportion used medication with CNS effects, rated their health as bad or very bad, and were living in Las Margaritas (blue-collar area) (table 1). Furthermore, longer sleep duration was associated with smoking and cancer (table 1).
Table 1.
Baseline characteristics of the deceased study participants (n = 1,822), according to habitual sleep duration
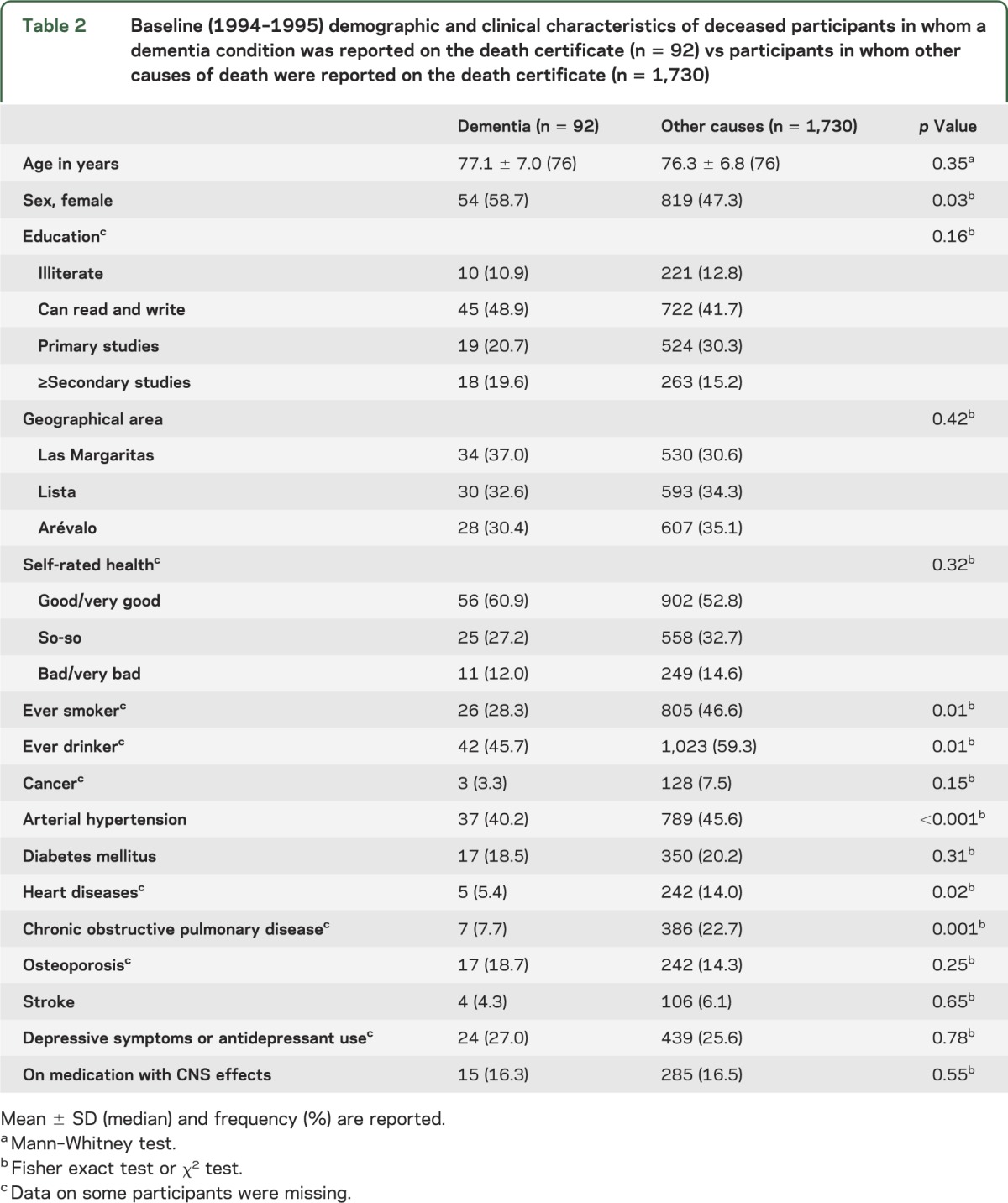
Baseline characteristics of the participants who died of dementia vs those who died of other causes are shown in table 2. Those who died of other causes were more likely to have been male and to have been smokers and drinkers. In addition, they were more likely to have had hypertension, heart diseases, and chronic obstructive pulmonary disease.
Table 2.
Baseline (1994–1995) demographic and clinical characteristics of deceased participants in whom a dementia condition was reported on the death certificate (n = 92) vs participants in whom other causes of death were reported on the death certificate (n = 1,730)
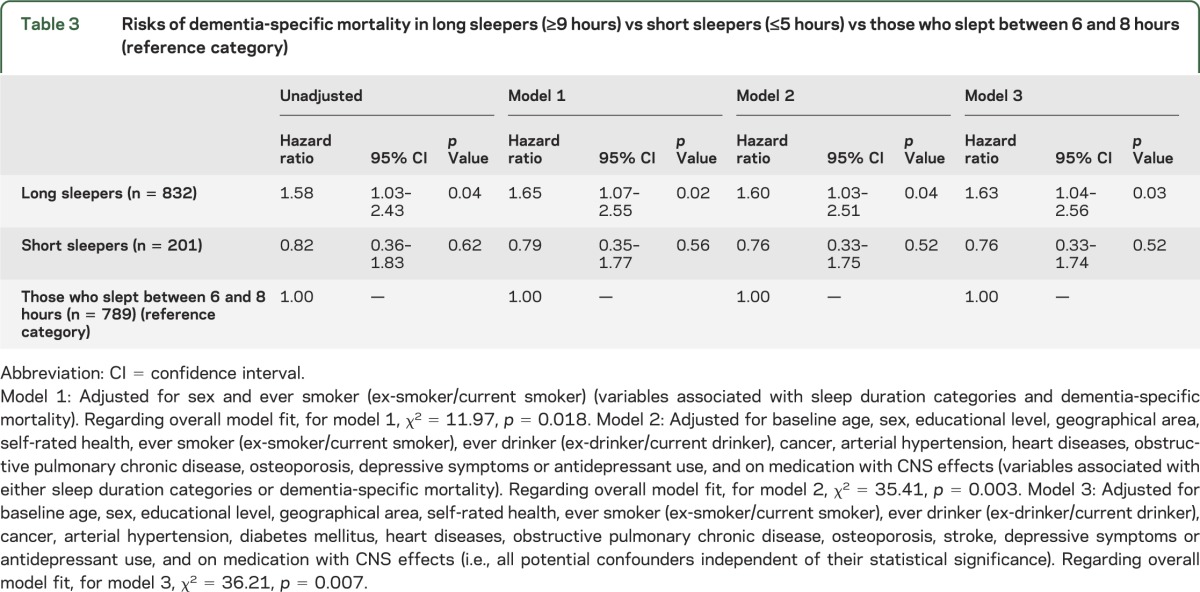
In an unadjusted Cox model, risk of dementia-specific mortality was increased in long sleepers vs the reference group (table 3). In a Cox model that adjusted for sex and ever smoking (i.e., variables associated with sleep duration categories and dementia-specific mortality), the risk of mortality remained increased in long sleepers (model 1, table 3). The results did not change in a Cox model that adjusted for variables associated with either sleep duration categories or dementia-specific mortality (i.e., baseline age, sex, educational level, geographical area, self-rated health, ever smoker [ex-smoker/current smoker], ever drinker [ex-drinker/current drinker], cancer, arterial hypertension, heart diseases, obstructive pulmonary chronic disease, osteoporosis, depressive symptoms or antidepressant use, and CNS medication use) (model 2, table 3). Furthermore, in a model that adjusted for baseline age, sex, educational level, geographical area, self-rated health, ever smoker (ex-smoker/current smoker), ever drinker (ex-drinker/current drinker), cancer, arterial hypertension, diabetes mellitus, heart diseases, obstructive pulmonary chronic disease, osteoporosis, stroke, depressive symptoms or antidepressant use, and CNS medication use (i.e., all potential confounders independent of their statistical significance) (model 3, table 3), the results remained unchanged.
Table 3.
Risks of dementia-specific mortality in long sleepers (≥9 hours) vs short sleepers (≤5 hours) vs those who slept between 6 and 8 hours (reference category)
In a final analysis, we excluded all participants (n = 53) in whom a diagnosis of non-Alzheimer disease dementia was listed on the death certificate. In these analyses, the HR for dementia mortality in long sleepers remained elevated (HR = 2.09, 95% CI = 1.05–4.17, p = 0.04, model 1; HR = 2.13, 95% CI = 1.02–4.42, p = 0.04, model 2; and HR = 2.13, 95% CI = 1.02–4.42, p = 0.04, model 3). For short sleepers, HR = 0.95, 95% CI = 0.27–3.34, p = 0.94, model 1; HR = 1.0, 95% CI = 0.27–3.65, p = 1.0, model 2; and HR = 0.99, 95% CI = 0.27–3.66, p = 0.99, model 3.
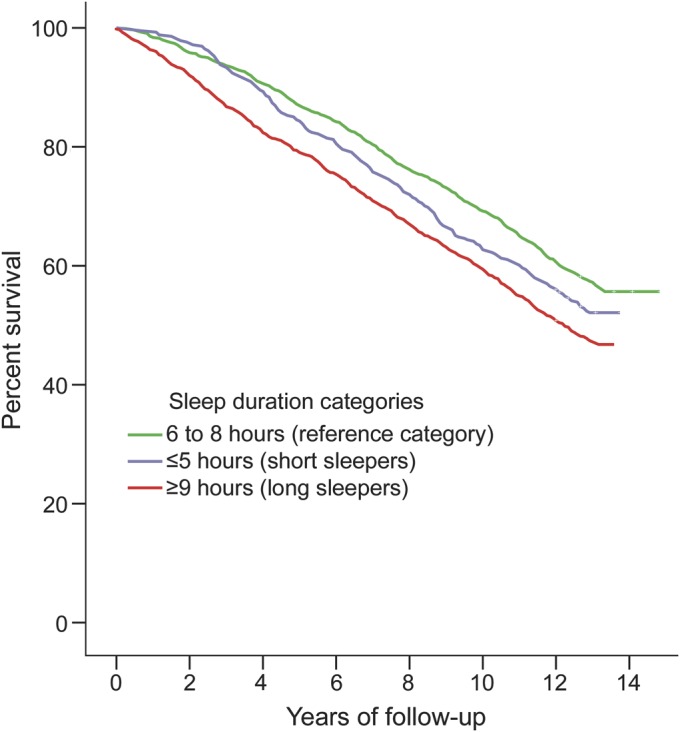
The Kaplan-Meier curves (figure 2) showed that long sleepers were at increased risk of death compared with those in the reference category (log-rank p < 0.001).
Figure 2. Sleep duration categories.
DISCUSSION
The results of the current study suggest that elderly long sleepers are at increased risk of mortality from dementia. Relative to those who reported sleeping 6 to 8 hours daily, the HR for dementia as the underlying cause of death was 58% higher in long sleepers. The association persisted even after controlling for a variety of combinations of covariates in adjusted models. For short sleepers, the risk was unchanged relative to the reference group. The current research further supports the previously reported association between baseline long sleep duration, but not short sleep duration, and increased risk of incident dementia in the NEDICES Study.7
We could only identify 2 previous population-based studies that prospectively analyzed the contribution of sleep problems to the risk of incident dementia. In the NEDICES Study, 3,286 participants without dementia were evaluated at baseline (1994–1995) and 3 years later.7 There were 140 cases of incident dementia.7 The HR for dementia for short sleepers was 2.36 (95% CI = 1.07–5.21, p = 0.03) and for long sleepers it was 2.40 (95% CI = 1.20–4.81, p = 0.01).7 After adjusting for potential confounders, the HR was only marginally increased for short sleepers (HR = 1.87, 95% CI = 0.85–4.15, p = 0.12), but it remained increased for long sleepers (HR = 2.18, 95% CI = 1.09–4.37, p = 0.03).7 In this sense, in the NEDICES Study, during a 3-year follow-up period, the scores of the 37-item version of the Mini-Mental State Examination declined by 0.5 ± 4.0 points in short sleepers, 0.6 ± 4.3 points in long sleepers, and 0.2 ± 3.8 points in the reference category (6–8 hours).27 The difference between short sleepers and the reference category was not significant (p = 0.142); however, the difference between long sleepers and the reference category was significant (p = 0.04).27 Finally, in a population-based study of 214 Swedish elderly people who were dementia-free both at baseline and at first follow-up (3 years later), reduced sleep was associated with a 75% increased all-cause dementia risk (HR = 1.75, 95% CI = 1.04–2.93, p = 0.03) after adjusting for age, sex, and education.8 The results remained similar after adjusting for lifestyle and vascular factors, but not after adjusting for depressive symptoms.8
Although the current findings suggest that longer sleeping may pose a significant risk of dementia mortality, the mechanisms underlying this association remain unknown. We can speculate on potential mechanisms. First, an unrecognized confounder (e.g., sleep-disordered breathing) may lead both to an increased need for sleep and dementia.29 Second, longer sleep duration could be an early symptom of dementia. Third, increased sleep duration heralding dementia might be explained, at least partly, by pathologic changes related to dementing conditions. Specifically, patients with severe dementia tend to sleep significantly longer than patients with less severe dementia.30 Fourth, the unequal distribution of traditional cardiovascular risk factors may be of significance. Specifically, in the current cohort, subjects with longer sleep duration were more likely to have been smokers. This is in line with a large cohort study in which a higher incidence of dementia among smokers was reported.31 Finally, excessive sleeping might lead to an increased risk of dementia per se.
Our study had several limitations. First, data on sleep duration was self-reported and may be subject to bias, including the existence of a change in the typical sleep duration anticipating the onset of dementia. Such bias, however, is equally likely for all of the 3 study groups and, given the large number of participants, is likely to be negligible. Second, we did not distinguish between nighttime sleep and daytime napping. There are many examples in the literature on the association of each of these aspects of sleep and different health outcomes.32,33 Third, subjects with mild cognitive impairment at baseline may have perceived their sleep differently from those without such impairment, so that the observed association may be attributable to misreporting of actual sleep patterns by participants who were impaired. Although possible, there is not enough evidence to think that mild cognitive impairment would result in participants consistently underreporting their sleep duration or overreporting difficulties sleeping. Fourth, we did not collect data on who signed the death certificate (a general physician, a neurologist, or a geriatrician). It is logical to assume that the level of expertise of the physician signing the death certificate would predict the level of accuracy of that certificate. In addition, dementia may be omitted as a cause of death from the death certificates of patients with known dementia in life.34,35 Such omissions, however, are equally likely for all sleep categories. Finally, our evaluation of depressive symptoms was not complete and we may have underestimated depression, resulting in residual confounding. However, a previous study showed a high level of agreement between the data generated from the screening question we used for depression and a detailed in-person psychiatric assessment.26
The strengths of the study included the large number of subjects who were assessed prospectively in a standardized manner, and that we were able to adjust for the potential confounding effects of a number of important factors.
GLOSSARY
- CI
confidence interval
- HR
hazard ratio
- ICD
International Classification of Diseases
- NEDICES
Neurological Disorders in Central Spain
AUTHOR CONTRIBUTIONS
Dr. Benito-León collaborated in: (1) the conception, organization, and execution of the research project; (2) the statistical analysis design; and (3) the writing of the manuscript first draft and the review and critique of the manuscript. Dr. Louis collaborated in: (1) the conception, organization of the research project; and (2) the review and critique of the manuscript. Dr. Villarejo-Galende collaborated in: (1) the conception, organization of the research project; and (2) the review and critique of the manuscript. Dr. Romero collaborated in: (1) the conception, organization of the research project; and (2) the review and critique of the manuscript. Dr. Bermejo-Pareja collaborated in: (1) the conception, organization, and execution of the research project; and (2) the review and critique of the manuscript.
STUDY FUNDING
Additional information about collaborators and detailed funding of the NEDICES Study can be found at http://www.ciberned.es/estudio-nedices. The Spanish Health Research Agency and the Spanish Office of Science and Technology supported NEDICES. Dr. Benito-León is supported by the NIH, Bethesda, MD (NINDS R01 NS039422), the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR), and the Spanish Health Research Agency (grant FIS PI12/01602). Dr. Elan D. Louis has received research support from the NIH, Bethesda, MD: NINDS R01 NS042859 (principal investigator), NINDS R01 NS39422 (principal investigator), R01 NS085136 (principal investigator), NINDS R01 NS073872 (principal investigator), and NINDS T32 NS07153-24 (principal investigator), as well as the Parkinson's Disease Foundation (principal investigator). Dr. Bermejo-Pareja is supported by the NIH, Bethesda, MD (NINDS R01 NS039422) and the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions. Neurosci Biobehav Rev 1995;19:553–571. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med 2002;162:201–208. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep 2011;34:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep 2011;34:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virta JJ, Heikkila K, Perola M, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep 2013;36:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos AR, Dong C, Elkind MS, et al. Association between sleep duration and the Mini-Mental score: the Northern Manhattan Study. J Clin Sleep Med 2013;9:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benito-León J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol 2009;16:990–997. [DOI] [PubMed] [Google Scholar]
- 8.Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry Epub 2013 Aug 13. [DOI] [PubMed]
- 9.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev 2004;8:159–174. [DOI] [PubMed] [Google Scholar]
- 10.Benito-León J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord 2003;18:389–394. [DOI] [PubMed] [Google Scholar]
- 11.Benito-León J, Bermejo-Pareja F, Louis ED; Neurological Disorders in Central Spain (NEDICES) Study Group. Incidence of essential tremor in three elderly populations of central Spain. Neurology 2005;64:1721–1725. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, Benito-León J, Ottman R, Bermejo-Pareja F; Neurological Disorders in Central Spain (NEDICES) Study Group. A population-based study of mortality in essential tremor. Neurology 2007;69:1982–1989. [DOI] [PubMed] [Google Scholar]
- 13.Benito-León J, Bermejo-Pareja F, Rodríguez J, et al. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord 2003;18:267–274. [DOI] [PubMed] [Google Scholar]
- 14.Benito-León J, Bermejo-Pareja F, Morales-Gonzalez JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology 2004;62:734–741. [DOI] [PubMed] [Google Scholar]
- 15.Posada IJ, Benito-León J, Louis ED, et al. Mortality from Parkinson's disease: a population-based prospective study (NEDICES). Mov Disord 2011;26:2522–2529. [DOI] [PubMed] [Google Scholar]
- 16.Díaz-Guzmán J, Bermejo-Pareja F, Benito-León J, et al. Prevalence of stroke and transient ischemic attack in three elderly populations of central Spain. Neuroepidemiology 2008;30:247–253. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Salio A, Benito-León J, Díaz-Guzmán J, Bermejo-Pareja F. Cerebrovascular disease incidence in central Spain (NEDICES): a population-based prospective study. J Neurol Sci 2010;298:85–90. [DOI] [PubMed] [Google Scholar]
- 18.Bermejo-Pareja F, Benito-León J, Vega S, et al. Consistency of clinical diagnosis of dementia in NEDICES: a population-based longitudinal study in Spain. J Geriatr Psychiatry Neurol 2009;22:246–255. [DOI] [PubMed] [Google Scholar]
- 19.Bermejo-Pareja F, Benito-León J, Vega S, Medrano MJ, Roman GC; Neurological Disorders in Central Spain (NEDICES) Study Group. Incidence and subtypes of dementia in three elderly populations of central Spain. J Neurol Sci 2008;264:63–72. [DOI] [PubMed] [Google Scholar]
- 20.Villarejo A, Benito-León J, Trincado R, et al. Dementia-associated mortality at thirteen years in the NEDICES Cohort Study. J Alzheimers Dis 2011;26:543–551. [DOI] [PubMed] [Google Scholar]
- 21.Morales JM, Bermejo FP, Benito-León J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from central Spain. Public Health 2004;118:426–433. [DOI] [PubMed] [Google Scholar]
- 22.Bermejo-Pareja F, Benito-León J, Vega QS, et al. The NEDICES cohort of the elderly: methodology and main neurological findings [in Spanish]. Rev Neurol 2008;46:416–423. [PubMed] [Google Scholar]
- 23.Vega S, Benito-León J, Bermejo-Pareja F, et al. Several factors influenced attrition in a population-based elderly cohort: Neurological Disorders in Central Spain Study. J Clin Epidemiol 2010;63:215–222. [DOI] [PubMed] [Google Scholar]
- 24.Benito-León J, Louis ED, Rivera-Navarro J, Medrano MJ, Vega S, Bermejo-Pareja F. Low morale is associated with increased risk of mortality in the elderly: a population-based prospective study (NEDICES). Age Ageing 2010;39:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benito-León J, Louis ED, Bermejo-Pareja F; Neurological Disorders in Central Spain Study Group. Population-based case-control study of morale in Parkinson's disease. Eur J Neurol 2009;16:330–336. [DOI] [PubMed] [Google Scholar]
- 26.Louis ED, Benito-León J, Bermejo-Pareja F; Neurological Disorders in Central Spain (NEDICES) Study Group. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol 2007;14:1138–1146. [DOI] [PubMed] [Google Scholar]
- 27.Benito-León J, Louis ED, Bermejo-Pareja F. Cognitive decline in short and long sleepers: a prospective population-based study (NEDICES). J Psychiatr Res 2013;47:1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benito-León J, Louis ED, Bermejo-Pareja F. Short sleep duration heralds essential tremor: a prospective, population-based study. Mov Disord 2013;28:1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fetveit A, Bjorvatn B. Sleep duration during the 24-hour day is associated with the severity of dementia in nursing home patients. Int J Geriatr Psychiatry 2006;21:945–950. [DOI] [PubMed] [Google Scholar]
- 31.Reitz C, den Heijer T, van Duijn C, Hofman A, Breteler MM. Relation between smoking and risk of dementia and Alzheimer disease: the Rotterdam Study. Neurology 2007;69:998–1005. [DOI] [PubMed] [Google Scholar]
- 32.Peth J, Regen F, Bajbouj M, Heuser I, Anghelescu I, Hornung OP. The influence of daytime napping versus controlled activity on the subjective well-being of patients with major depression. Psychiatry Res 2012;200:368–373. [DOI] [PubMed] [Google Scholar]
- 33.Nasrollah L, Maradey-Romero C, Jha LK, Gadam R, Quan SF, Fass R. Naps are more commonly associated with gastroesophageal reflux, compared with nocturnal sleep. Clin Gastroenterol Hepatol Epub 2014 Jun 4. [DOI] [PubMed]
- 34.Romero JP, Benito-León J, Mitchell AJ, Trincado R, Bermejo-Pareja F. Under reporting of dementia deaths on death certificates using data from a population-based study (NEDICES). J Alzheimers Dis 2014;39:741–748. [DOI] [PubMed] [Google Scholar]
- 35.Romero JP, Benito-León J, Louis ED, Bermejo-Pareja F. Under reporting of dementia deaths on death certificates: a systematic review of population-based cohort studies. J Alzheimers Dis 2014;41:213–221. [DOI] [PubMed] [Google Scholar]