The term nociception was originally defined by Sherrington1 as the neural process by which high-threshold stimuli (which cause tissue injury) are detected. This is distinct from pain, which is defined by the quality of the sensory percept (i.e., unpleasant), is not always evoked by a noxious stimulus, and can also be experienced in the absence of a noxious stimulus. In man, recessive loss of function mutations in the SCN9A gene, encoding the α subunit of Nav1.7, results in anosmia and the congenital inability to experience pain,2–4 but there have been few reports of detailed sensory testing in these patients.
Case report.
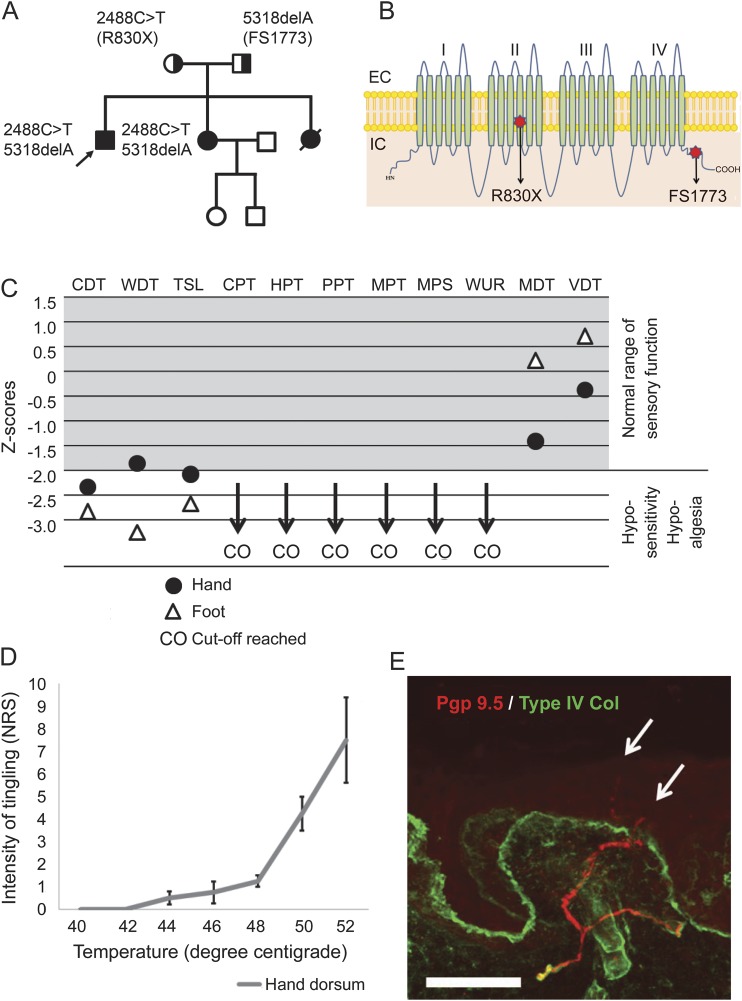
A 27-year-old man who participated in the Painful Channelopathies Study (NRES-UK reference: 12/LO/0017) had a history of self-mutilating behavior during childhood and multiple painless injuries with normal cognitive development. He had never perceived pain but reported that otherwise somatosensation was normal. In his late teens, he learned to avoid injury by attending to what he described as a tingling sensation, which was not unpleasant but that he noted only occurred in the context of threatened tissue injury. The family pedigree is shown in the figure, A. He had 2 sisters, one of whom died in childhood as a consequence of sepsis. The surviving sister also has congenital inability to experience pain.
Figure. Detailed sensory phenotyping in a patient with compound heterozygous null mutations in Nav1.7.
(A) The family pedigree shows the proband (arrow) and 2 sisters with congenital inability to experience pain. (B) Cartoon shows the structure of Nav1.7 showing the typical 4-domain structure of voltage-gated sodium channels each with 6 transmembrane segments. The mutations are as follows: a premature stop codon at arginine 830 (within domain 2) and a frameshift at glutamate 1773 within the C-terminal domain. (C) Graphical representation of sensory testing in the proband expressed as Z scores: cold detection threshold (CDT), warm detection threshold (WDT), thermal sensory limen (TSL), cold pain threshold (CPT), heat pain threshold (HPT), pressure pain threshold (PPT), mechanical pain threshold (MPT), mechanical pain sensitivity (MPS), wind-up ratio (WUR), mechanical detection threshold (MDT), and vibration detection threshold (VDT). (D) Graph represents the patient's rating of his tingling sensation on a numerical rating scale (means ± SE) in response to suprathreshold thermal stimuli delivered in a randomized manner. The threshold for this distinct sensation is clearly in the noxious range and the intensity of the sensation encodes the strength of the thermal stimulus. (E) Photomicrograph of skin section immunostained for PGP 9.5 (red) to demonstrate nerve fibers and collagen type IV (green) to show the basement membrane. Fibers are clearly seen in the dermal plexus and some are crossing into the epidermis (arrows); however, the intraepidermal nerve fiber densityis reduced to 3.98 fibers/mm. Scale bar: 50 µm.
The proband presented with short stature of 149 cm. He was anosmic. Tone and power were normal throughout and all deep tendon reflexes were preserved. He had no impairment in light touch, vibration detection, or proprioception. Pinprick was not perceived as painful although he could detect that it was pointed and a flare developed shortly after stimulation.
Genetic testing for SCN9A mutations in the index case and his sister (figure, A) revealed compound heterozygous mutations (figure, B). Nerve conduction studies demonstrated small-amplitude sural sensory nerve action potentials (table e-1 on the Neurology® Web site at Neurology.org). Quantitative sensory testing was performed according to the German Neuropathic Pain Network5 (e-Methods). He did not experience pain (i.e., there was no unpleasant sensation) in response to any stimulus applied including extremes of temperature or mechanical stimuli. His mechanical detection and vibration detection thresholds were normal. He was hyposensitive to warm and cool stimuli (figure, C). High temperatures and strong mechanical stimuli evoked a mild tingling sensation. This sensation was never unpleasant but he had learned to use it as an injury signal. We therefore performed suprathreshold thermal stimulation by giving randomized thermal stimuli and asking him to rate the intensity of this sensation (figure, D). The threshold for this stimulus was in the noxious range at 42°C and the intensity of the sensation could encode the strength of the stimulus. Skin biopsy taken from the distal leg showed that intraepidermal nerve fibers were present although at a density below the lower limit of normal (figure, E).
Discussion.
The index case reported in this kindred demonstrated insensitivity to pain, normal large-fiber sensory function, and anosmia: typical features of congenital insensitivity to pain (CIP) secondary to SCN9A mutations. Genetic testing revealed compound heterozygous mutations in SCN9A: a premature stop codon in coding exon 15 (c.2488C>T), which has previously been reported,3 and a previously unreported 1-bp deletion within coding exon 26 (c.5318delA), which induces a frameshift at position 1773 in the C-terminal domain of the channel.
The patient had identified a sensation that was not unpleasant but only occurred in the context of strong mechanical or thermal stimuli that could cause tissue injury. The sensation was mild, localized to the stimulus, and had a tingling quality to it. He used this sensation as a warning signal and had managed to reduce the frequency of injury. It is currently unclear as to the mechanism by which he can detect tissue injury in the absence of pain. Nociceptors still innervate the skin, albeit at a lower density than in age-matched controls. The proposed mechanisms through which null mutations in Nav1.7 result in inability to experience pain include impaired transduction in nociceptor terminals, action potential transmission along the axon, and release of neurotransmitter from central terminals of sensory afferents.6,7 It is possible that a small population of nociceptive afferents are able to function in the absence of Nav1.7 but provide insufficient input to drive pain sensation. This case further emphasizes that the detection of noxious stimuli and the sensation of pain are distinct and could also have practical implications if other patients with CIP can train themselves to attend to sensations that may indicate potential tissue injury but are not painful.
Supplementary Material
Footnotes
Supplemental data at Neurology.org
Author contributions: Dr. D.L. Bennett: study concept and design, analysis and interpretation, study supervision, critical revision of the manuscript for important intellectual content. Dr. S.B. McMahon: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. J.N. Wood: analysis and interpretation, critical revision of the manuscript for important intellectual content. J.D. Ramirez: study concept and design, acquisition of data, analysis and interpretation, generating first draft of manuscript. A.M. Habib: acquisition of data, analysis and interpretation. A. Themistocleous: acquisition of data, analysis and interpretation. J.J. Cox: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content.
Study funding: Supported by a strategic award by the Wellcome Trust to the London Pain Consortium (ref. no. 083259) and a Medical Research Council career development fellowship.
Disclosure: J. Ramirez is sponsored by a Francisco Jose de Caldas Scholarship from Colciencias, through LASPAU, Harvard University. He was also funded by the Wellcome Trust–funded London Pain Consortium (ref. no. 083259). A. Habib is funded by an MRC Research Career Development fellowship. J. Cox is funded by an MRC Research Career Development fellowship. A. Themistocleous is funded by the Wellcome Trust–funded London Pain Consortium (ref. no. 083259). S. McMahon is a member of the Innovative Medicines Initiative Europain (grant 115007) and has acted as a consultant for Pfizer, Astra Zeneca, Mundipharma, and Bayer. He has received grant funding support from Astra Zeneca, Pfizer, and Covergence. He is a Wellcome Trust senior investigator and a member of the Wellcome Trust–funded London Pain Consortium (ref. no. 083259). J. Wood is a member of the Innovative Medicines Initiative Europain (grant 115007). He is also funded by the Medical Research Council, the BBSRC, is a Wellcome Trust senior investigator and a member of the Wellcome Trust–funded London Pain Consortium (ref. no. 083259). D. Bennett is a member of the Innovative Medicines Initiatives (public–private partnerships between the EU and EFPIA), Europain (grant 115007), and Stembancc. He has acted as a consultant for Pfizer. David Bennett is a senior Wellcome Trust Clinical Scientist Fellow (ref. no. 095698z/11/z) and a member of the Wellcome Trust–funded London Pain Consortium (ref. no. 083259). Go to Neurology.org for full disclosures. The Article Processing Charge was paid by the Wellcome Trust.
References
- 1.Sherrington CS. Qualitative differences of spinal reflex corresponding with qualitative difference of cutaneous stimulus. J Physiol 1903;30:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox JJ, Reimann F, Nicholas AK, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006;444:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg YP, MacFarlane J, MacDonald ML, et al. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet 2007;71:311–319. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad S, Dahllund L, Eriksson AB, et al. A stop codon mutation in SCN9A causes lack of pain sensation. Hum Mol Genet 2007;16:2114–2121. [DOI] [PubMed] [Google Scholar]
- 5.Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123:231–243. [DOI] [PubMed] [Google Scholar]
- 6.Raouf R, Rugiero F, Kiesewetter H, et al. Sodium channels and mammalian sensory mechanotransduction. Mol Pain 2012;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minett MS, Nassar MA, Clark AK, et al. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat Commun 2012;3:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.