Abstract
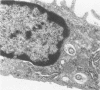
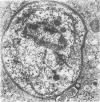
Trypanosome-activated mouse peritoneal macrophages phagocytized and digested Trypanosoma brucei in vitro and in vivo, but in the absence of specific antiserum and complement the degree of phagocytosis was minimal. Ultrastructurally, the parasites attached to the macrophage by their flagella, and ingestion proceeded flagellum first. Once ingested, T. brucei was degraded, presumably due to fusion of the parasite-containing phagosome with lysosomes. Contrariwise, normal mouse peritoneal macrophages displayed negligible ability to ingest T. brucei, even in the presence of specific antiserum and complement. During trypanosomiasis in deer mice (Peromyscus maniculatus), the development of hypergammaglobulinemia correlated with enhanced phagocytosis of T. brucei by macrophages, but only at early post-inoculation days (PID 5 to 15). Complement lysis of trypanosomes was not identified in these experiments. Between PID 20 to 30, antiserum and complement either had no phagocytosis-promoting ability or depressed the phagocytosis of T. brucei by macrophages. These results indicate that both specific antibody and complement contribute to the ingestion of T. brucei by activated macrophages, but that parasite antigenic variation effectively abrogates the phagocytic defense mechanism.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H. J., McQuillen N. K. Interaction and transformation of Leishmania donovani within in vitro cultured cells. An electron microscopical study. Am J Trop Med Hyg. 1972 Nov;21(6):873–879. doi: 10.4269/ajtmh.1972.21.873. [DOI] [PubMed] [Google Scholar]
- Alexander J. Effect of the antiphagocytic agent cytochalasin B on macrophage invasion by Leishmania mexicana promastigotes and Trypanosoma cruzi epimastigotes. J Protozool. 1975 May;22(2):237–240. doi: 10.1111/j.1550-7408.1975.tb05858.x. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boreham P. F. Kinin release and the immune reaction in human trypanosomiasis caused by Trypanosoma rhodesiense. Trans R Soc Trop Med Hyg. 1970;64(3):394–400. doi: 10.1016/0035-9203(70)90175-6. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM M. P., LUMSDEN W. H., WEBBER W. A. PRESERVATION OF VIABLE TRYPANOSOMES IN LYMPH TUBES AT LOW TEMPERATURE. Exp Parasitol. 1963 Dec;14:280–284. doi: 10.1016/0014-4894(63)90032-8. [DOI] [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Greenblatt C. L., Tyroler E. Trypanosoma lewisi: in vitro behavior of rat spleen cells. Exp Parasitol. 1971 Dec;30(3):363–374. doi: 10.1016/0014-4894(71)90100-7. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel M., Robertson E. In vitro attachment of trypanosomes to plastic. Experientia. 1976 Apr 15;32(4):464–466. doi: 10.1007/BF01920798. [DOI] [PubMed] [Google Scholar]
- Jarvinen J. A., Dalmasso A. P. Trypanosoma musculi infections in normocomplementemic, C5-deficient, and C3-depleted mice. Infect Immun. 1977 May;16(2):557–563. doi: 10.1128/iai.16.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGE D. E., LYSENKO M. G. In vitro phagocytosis of Trypanosoma lewisi by rat exudative cells. Exp Parasitol. 1960 Sep;10:39–42. doi: 10.1016/0014-4894(60)90081-3. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. C., Twohy D. W. Infection of macrophages in culture by leptomonads of Leishmania donovani. J Protozool. 1967 Nov;14(4):781–789. doi: 10.1111/j.1550-7408.1967.tb02078.x. [DOI] [PubMed] [Google Scholar]
- Moulton J. E., Coleman J. L., Thompson P. S. Pathogenesis of Trypanosoma equiperdum in deer mice (Peromyscus maniculatus). Am J Vet Res. 1974 Jul;35(7):961–976. [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. I. The role of the macrophage. Immunology. 1974 Nov;27(5):815–824. [PMC free article] [PubMed] [Google Scholar]
- NORTH R. J., MACKANESS G. B. ELECTRON MICROSCOPICAL OBSERVATIONS ON THE PERITONEAL MACROPHAGES OF NORMAL MICE AND MICE IMMUNISED WITH LISTERIA MONOCYTOGENES. I. STRUCTURE OF NORMAL MACROPHAGES AND THE EARLY CYTOPLASMIC RESPONSE TO THE PRESENCE OF INGESTED BACTERIA. Br J Exp Pathol. 1963 Dec;44:601–607. [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees J. M. Immunoglobulin changes after infection of sheep with Trypanosoma vivax. Trans R Soc Trop Med Hyg. 1969;63(1):124–125. [PubMed] [Google Scholar]
- Stevens D. R., Moulton J. E. Experimental meningoencephalitis in Trypanosoma brucei infection of deer mice (Peromyscus maniculatus). A light, immunofluorescent, and electron microscopic study. Acta Neuropathol. 1977 Jun 15;38(3):173–180. doi: 10.1007/BF00688062. [DOI] [PubMed] [Google Scholar]
- Takayanagi T., Nakatake Y., Enriquez G. L. Attachment and ingestion of Trypanosoma gambiense to the rat macrophage by specific antiserum. J Parasitol. 1974 Apr;60(2):336–339. [PubMed] [Google Scholar]
- Takayanagi T., Nakatake Y. Trypanosoma gambiense: the binding activity of antiserum to macrophages. Exp Parasitol. 1977 Jun;42(1):21–26. doi: 10.1016/0014-4894(77)90057-1. [DOI] [PubMed] [Google Scholar]
- Takayanagi T., Nakatke Y., Enriquez G. L. Tyrpanosoma gambiense: phagocytosis in vitro. Exp Parasitol. 1974 Aug;36(1):106–113. doi: 10.1016/0014-4894(74)90116-7. [DOI] [PubMed] [Google Scholar]
- Weidner E. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd. 1975 Aug 21;47(1):1–9. doi: 10.1007/BF00418060. [DOI] [PubMed] [Google Scholar]