Abstract
Modulation of host cell signaling and cellular functions is key to intracellular survival of pathogenic bacteria. Intracellular growth has several advantages e.g. escape from the humoral immune response and access to a stable nutrient rich environment. Growth in such a preferred niche comes at the price of an ongoing competition between the bacteria and the host as well as other microbes that compete for the very same host resources. This requires specialization and constant evolution of dedicated systems for adhesion, invasion and accommodation. Interestingly, obligate intracellular bacteria of the order Chlamydiales have evolved an impressive degree of control over several important host cell functions. In this review we summarize how Chlamydia controls its host cell with a special focus on signal transduction and cellular modulation.
Keywords: Chlamydia, Invasion, Inclusion, Type III secretion, Tarp, Inc, Signaling, Trafficking
Introduction
Chlamydia trachomatis is an important human pathogen and the best investigated member of the order Chlamydiales[1]. Infection with C. trachomatis is among the most frequent causes of sexual transmitted diseases (STD). Infections of the upper inner eyelid eventually leading to scarring blindness (trachoma) are worldwide among the most frequently occurring ocular infections with nearly 140 million infected and 500 million at risk (source WHO). C. pneumoniae is a common agent of respiratory disease with sero-positivity as high as 30-45% in adults [2,3] and association with chronic diseases like arteriosclerosis or lung cancer [4,5].
Chlamydiae are obligate intracellular bacteria with a gram-negative atypical cell wall [6]. Growth is characterized by a distinct biphasic cycle of development. The extracellular infectious elementary bodies (EB) adhere and upon internalization into the host cell start an infectious cycle. Once internalized, EB quickly differentiate into metabolically active, dividing reticulate bodies (RB). The common perception of EB as metabolically inactive has recently been challenged by the discovery of developmental form specific metabolic requirements [7]. Adherence of an EB to the host cell is mediated by bacteria-host receptor interactions that initiate signaling via the adhesin-bound receptor and concomitantly by other bacterial effector proteins to trigger the rapid internalization of the bacteria [8-12]. Bacterial uptake leads to formation of a heavily modified pathogen containing vacuole termed inclusion [13]. Modification of the inclusion is required to prevent endolysosomal fusion and to direct acquisition of various metabolites or nutrients e.g. iron or sphingomyelin [14-17]. At the end of the infectious cycle Chlamydia is released from the host cell by lysis or a process that has been termed extrusion [18,19]. Chlamydia is able to enter a reversible persistent state through limitation of either nutrients (e.g. iron, amino acids) or application of antibiotics (e.g. penicillin) [20,21]. Persistence is characterized by formation of aberrant bodies, an incomplete developmental cycle, ongoing metabolic activity and altered gene expression [22,23]. Upon removal of the persistence inducer Chlamydia can reactivate and enter an acute developmental cycle.
Modulation of various host cell processes by Chlamydia is a prerequisite to complete the developmental cycle. Manipulation of the host cell requires specialized secretion systems e.g. the type three secretion system (TTSS) and its effector proteins and the respective genes for the TTSS can be found in all sequenced chlamydial genomes [24,25]. Other factors include e.g. the adhesins/invasins polymorphic membrane protein D (PmpD) [12,26] and outer membrane complex B (OmcB) [27]. Here, we review Chlamydia-induced signaling and the required bacterial effectors and sort both according to infection time and intracellular location. We apologize to all the authors, whose work could not be discussed in this review due to space constraints. For in depth reading we refer the interested reader to a recently published book [28].
Adhesion
Efficient adhesion to host cells is a prerequisite for invasion and intracellular life and usually requires several adhesins. Chlamydia has evolved a number of ways to attach to various host cells and infect different tissues according to serovariant and species [29,30]. Early research focused on the role of the abundant major outer membrane protein (MOMP) as an adhesin [31] (Figure 1A-B). Blocking the exposed variable MOMP domains using specific antibodies disturbed binding to the host cell [32]. Chlamydia muridarum MOMP has been described to mediate attachment to host cells as a cytoadhesin [33]. Further, MOMP from various chlamydial species is glycosylated (mainly D-mannose-rich) and this modification is critical for MOMP adhesion [34-36]. The mannose-6-phosphate / insulin-like growth factor receptor 2 (M6PR/IGFR2) has been suggested as the host receptor for MOMP, since the MOMP glycan moiety is similar to the M6PR ligand mannose-6-phosphate and blocking the M6PR prevents C. pneumoniae attachment and invasion [37].
Figure 1.
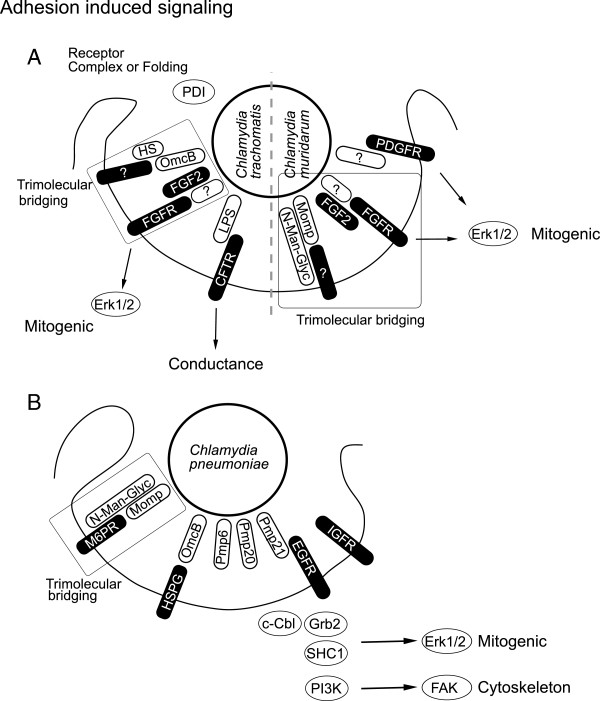
Adhesion induced signaling. A, Adhesin-receptor pairs are ill defined for the closely related pathogens C. trachomatis and C. muridarum. Several surface proteins like lipopolysaccharide (LPS), major outer membrane protein (MOMP), outer membrane complex B (OmcB) and polymorphic membrane protein (Pmp21) have been suggested as potential bacterial adhesins. A trimolecular bridge is thought to connect MOMP, OmcB and FGFR to their host or bacterial counterpart, respectively. Binding to host receptors like fibroblast growth factor receptor (FGFR) or platelet derived growth factor receptor (PDGFR) induces mitogenic signaling via extracellular-signal-regulated kinase 1/2 (Erk1/2). Receptor surface presentation and folding via protein disulfide isomerase (PDI) shows the necessity for specific host receptor binding. B, C. pneumoniae binds to its host cell in a bimolecular fashion via OmcB heparin sufate proteoglycan (HSPG) interaction. Binding between OmcB and HSPG is probably a reversible initial reversible binding step followed by irreversible specific binding. One adhesin receptor pair involved is Pmp21 – EGFR. The Pmp21 – EGFR interaction then triggers invasion of Chlamydia. Further, binding to EGFR also recruits growth factor receptor bound 2 (Grb2), Cas-Br-M (murine) ecotropic retroviral transforming sequence (c-Cbl), SHC (Src homology 2 domain containing) transforming protein 1 (SHC1) and phosphatidyl-inositol-3-kinase (PI3K) signaling, which initiates mitogenic Erk1/2 signaling as well as cytoskeletal rearrangements via focal adhesion kinase (FAK). Pmp6 and 20 have been suggested as additional adhesins on the bacterial side, while insulin growth factor receptor (IGFR) has been indicated on the host side.
Also, heparan sulfate-like glycosaminoglycan (GAG) attached to Chlamydia has been shown to bridge host and bacterium [38] (Figure 1A-B). Cleaving this GAG compound from the bacteria renders them non-adhesive, while addition of exogenous heparan sulfate restored attachment. GAG of a size similar to heparin or heparan sulfate has subsequently been found in the inclusion produced by Chlamydia[39]. Chlamydial synthesis of GAG is consistent with the observation that C. trachomatis also infects CHO cells deficient in heparin sulfate biosynthesis [40]. Outer membrane complex B (OmcB), a cysteine rich membrane protein, has been described to bind to GAG [41,42]. Further, GAG binding varies depending on the specific serovariant [27,43] and this binding has recently been attributed to a strain specific motif within the N-terminus of OmcB [44]. Variation in GAG binding has been suggested to co-determine cell type specificity [45].
Most of the studies performed so far on Chlamydia-host binding focused on bacterial adhesins and only limited data are available on the nature of host cell receptor(s). Correct surface presentation of specific host proteins has been suggested to be important using CHO cells expressing a defective protein disulfide isomerase (PDI) [46,47]. In this model PDI is most likely involved in the folding, surface presentation or receptor complex formation (Figure 1A). Attachment of C. trachomatis to host cells has been shown to require sulfation but no specific receptors were identified [48]. More recently, epidermal growth factor receptor (EGFR/ERBB) has been shown to be the host receptor for C. pneumoniae Pmp21, but not Pmp21 of C. trachomatis[12] (Figure 1A-B). Residual adhesion and invasion upon EGFR depletion indicates that other receptors are involved in adherence [12]. In case of C. trachomatis, lipopolysaccharide (LPS) has been demonstrated to be a ligand for the human cystic fibrosis transmembrane conductance regulator (CFTR) [49]. The closely related mouse pathogen C. muridarum engages the Fibroblast growth factor receptor (FGFR) for invasion. In this case, fibroblast growth factor 2 (FGF2) binds to C. muridarum and mediates invasion via FGFR [50]. The bacterial ligand for FGFR is still unknown (Figure 1A).
Adhesion accompanied signaling
Until today a systematic approach to identify host receptors for C. trachomatis and C. pneumoniae e.g. by applying RNA interference has not been undertaken. One difficulty may be receptor redundancy that prevents the straightforward identification of receptors by single knockdowns. On the bacterial side the upcoming establishment of a genetic system just recently opened the door to systematic forward genetic searches in Chlamydia. We can learn a lot about bacteria-induced signaling from the recently discovered adhesin – receptor pair Pmp21 - EGFR [12]. Pmp21 coated latex beads are endocytosed in an EGFR-dependent manner demonstrating that Pmp21 is sufficient to trigger invasion [12]. The C. trachomatis homolog PmpD has also been implicated in adhesion, however direct experimental evidence for its function as adhesin is still missing [26]. Binding of Pmp21 to EGFR activates the receptor leading to formation of a complex with the adaptor protein growth factor receptor bound-2 (Grb2) and the ubiquitin ligase Cas-Br-M (murine) ecotropic retroviral transforming sequence (c-Cbl). EGFR activation subsequently leads to extracellular-signal-regulated kinase 1/2 (Erk1/2) activation [12] (Figure 1B). C. pneumoniae invasion has been shown to be accompanied by activation of src homology containing (SHC1), Erk and phosphoinositol 3 kinase (PI3K) [51]. Apparently, SHC1, Erk and PI3K activation is initiated by EGFR activation and may together lead to FAK activation (Figure 1B). Involvement of additional adhesin – receptor pair is likely and OmcB presents a strong candidate on the bacterial side because of its heparin sulphate binding domain [27,42].
CFTR has been suggested as a potential host receptor for C. trachomatis. Binding of LPS to CFTR reduces the conductance of CFTR, however, the consequences host signaling is unclear [49] (Figure 1A). C. muridarum bound to host cells specifically recruits FGFR as well as platelet derived growth factor receptor (PDGFR), but not EGFR [50,52]. Activated FGFR and PDGFR lead to mitogenic signaling via Erk1/2, which might be similar to C. pneumoniae induced EGFR signaling. Requirements for FGF2 have also been confirmed in the human pathogenic strain C. trachomatis E indicating that activation of FGFR signaling might partially replace EGFR signaling during C. trachomatis infection [50]. Host receptors for the MOMP glycan and OmcB GAG interaction have not yet been defined. Interestingly, C. trachomatis receptor signaling and recruitment might be synergistic with signaling induced by the secreted bacterial protein Tarp [53]. Tarp interacts with several of the proteins recruited to the EGFR in a serovar- and phosphorylation-dependent manner [53,54]. Phosphorylation of Tarp in turn is mediated by multiple kinases most likely Src family kinases as well as Abl kinases [52,55,56] (Figure 2A).
Figure 2.
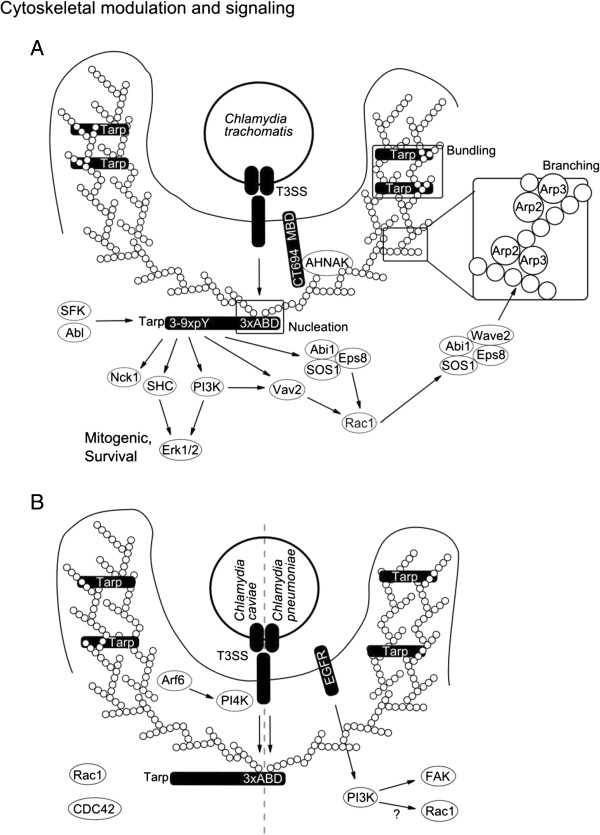
Cytoskeletal modulation and signaling. A, Invading C. trachomatis is believed to secrete a cocktail of preformed effectors into the host cell and this is supported by the two discovered factors translocated actin recruiting phosphor protein (Tarp) and CT694. Tarp initiates multiple signaling cascades, i.e. its N-terminus is phosphorylated on several tyrosine residues (pY) and the C-terminally located actin binding domains (ABD) mediate actin nucleation and bundling. Signaling via the N-terminus leads to survival signaling via extracellular-signal-regulated kinase 1/2 (Erk1/2) as well as actin branching via son of sevenless homolog 1 (SOS1) / abl-interactor 1 (Abi1) / epidermal growth factor receptor pathway substrate 8 (Eps8) / WAS protein family, member 2 (WASF2 alternative name: Wiskott-Aldrich syndrome protein family member 2 - Wave2) and actin related protein 2/3 (Arp2/3) complex. CT694 consists of a membrane binding domain (MBD) and an AHNAK nucleoprotein (AHNAK) binding region which probably links membrane to actin signaling. B, C. caviae and pneumoniae secrete Tarp which does not contain the N-terminal tyrosine phosphorylation domain. The C-terminal ABD motives are sufficient for actin bundling and nucleation and some of the functions linked to C. trachomatis Tarp might be executed via epidermal growth factor receptor (EGFR). Activation of the small GTPases ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein Rac1) (Rac1) and cell division cycle 42 (GTP binding protein, 25 kDa) (CDC42) has been shown for C. caviae, the bacterial factors mediating this are not yet found. C. caviae also activates ADP-ribosylation factor 6 (Arf6), which in turn activates phosphatidyl-inositol-4-kinase (PI4K) and this might take over the membrane-actin modulating function of CT694.
Cytoskeletal rearrangements
Initial studies on Chlamydia invasion indicated the involvement of both actin-dependent and -independent mechanisms. Invasion was suggested to take place either through phagocytosis- (actin dependent) or pinocytosis-like (actin independent) processes [57]. These observations were supported by the differential sensitivity of C. trachomatis serovariants towards the f-actin disrupting agent cytochalasin D [58]. However, more and more investigations focused on actin driven processes. One reason is that recruitment of actin to the invasion site was directly shown [59] and found to be dependent on a bacterial structural component, which was subsequently identified to be the translocated actin recruiting phosphoprotein (Tarp) [8,59] (Figure 2A). Tarp is synthesized during the late stages of infection and is most likely secreted into the host cell via the TTSS [8,60,61]. Surprisingly, Tarp tyrosine phosphorylation and actin recruitment are not coupled [62]. It turned out that Tarp is a nucleator of actin since it contains several actin-binding domains (ABD) with similarity to WH2 domain proteins. In addition, a proline rich region in Tarp may enhance actin oligomerization [63]. Tarp-mediated actin binding is conserved across species and is likely to be required for chlamydial invasion as invasion was blocked by anti-ABD sera [64]. Actin nucleation and bundling activities are separated in different ABD and the rate of actin polymerization is synergistic with the host Arp2/3 complex emphasizing the complexity of bacterially induced cytoskeletal modulation [65,66] (Figure 2A). Many pathogens require several cytoskeletal modulators for efficient invasion of their host cells. The chlamydial effector CT694 was discovered more recently and similarly to Tarp shows late expression and early secretion [9]. A search for cellular interaction partners identified the C-terminus of CT694 as a domain that interacts with host AHNAK and actin [9]. AHNAK is a localized to the apical plasma membrane where it interacts with actin to maintain the architecture of polarized cells [9]. In addition, AHNAK plays a role as a scaffold protein, thereby connecting protein kinase C alpha (PKCα) and phospholipase C gamma (PLCγ) signaling [9]. The N-terminus contains a membrane localization domain suggesting that CT694 functions in actin modulation during invasion [67] (Figure 2A).
Small GTPases are important modulators of actin dynamics and downstream signaling and many bacteria evolved ways to modulate host GTPases. C. trachomatis requires the small GTPase ras-related C3 botulinum toxin substrate 1 (Rac1) but not cell division cycle 42 (Cdc42) or ras homolog gene family member A (RhoA) for invasion [68]. Rac1 has been shown to interact with abl interactor 1 (Abi1) and WAS protein family, member 2 (WASF2; also known as Wiskott-Aldrich syndrome protein family member 2 - Wave2) in order to regulate the actin-related protein complex 2/3 (Arp2/3) and thus modulates actin recruitment and branching [69]. Activation of Rac1 might be Tarp dependent as phosphorylated Tarp interacts with the Abi1 / son of sevenless homolog 1 (SOS1) / epidermal growth factor receptor pathway substrate 8 (Eps8), vav 2 guanine nucleotide exchange factor (Vav2) and phosphoinositol 3 kinase (PI3K) upstream of Rac1 [53,54]. The requirement of GTPase for invasion differs among Chlamydia species as C. caviae needs the small GTPases Rac1 and Cdc42 but not RhoA during invasion [70] (Figure 2A-B).
Tarp from C. caviae does not possess the phosphorylation sites required for Rac activation; this suggests that another bacterial factor for the activation of Rac1 and/or Cdc42 exists. One pathway to Rac1 activation during C. pneumoniae invasion could stem from EGFR-mediated PI3K activation and it is tempting to speculate that EGFR contributes to Rac1 activation during C. pneumoniae infection in an analogous fashion as phosphorylated TARP does during C. trachomatis infection (Figure 2A-B). So far, data on the role of EGFR for C. caviae and Rho GTPases for C. pneumoniae infection are still missing, respectively. Another GTPase involved in remodeling of the actin cytoskeleton during C. caviae invasion is ADP ribosylation factor 6 (Arf6) [71]. Arf6 activates phosphatidylinositol 4-phosphate 5-kinase (PI4K) which is important for plasma membrane modulation during actin rearrangement, suggesting a similar function as has been proposed for CT694. A bacterial component activating Arf6 has not been described and awaits further investigation (Figure 2B).
Establishment of the inclusion
The exact origin of the endosomal membrane is a matter of ongoing research. Caveolin [72,73], membrane rafts [73,74] and clathrin-mediated [75,76] endosome formation have been suggested as entry route for Chlamydia. However, these findings are still a matter of discussion since these pathways of endosome formation have not been confirmed by others [77,78]. This may be partly due to the use of different chlamydial species in these reports (C. trachomatis vs. C. pneumoniae vs. C. caviae) since these species differ not only in their host receptor but also in their invasion-mediated signaling. Due to these differences, varying experimental conditions had to be used e.g. for cell culture infection (centrifuge assisted vs. static). In analogy to influenza virus entry [79] and considering actin-dependent and -independent invasion mechanisms as well as differences in adhesion and entry signaling between species, a multi-route entry is likely.
Beside the ongoing discussion on the endosomal origin in Chlamydia infection consensus exists that once the endosome is formed it quickly separates from the endosomal route and starts to acquire sphingomyelin from the exocytic route [17,80] (Figure 3). Vacuolar pH stays above 6.0 indicating that lysosomal fusion is prevented [81]. Acquisition of sphingomyelin and prevention of lysosomal degradation require bacterial components since inhibition of bacterial transcription and translation interfere with these processes [82]. Interestingly, Chlamydia may use preformed early secreted or surface presented effectors to prevent lysosomal degradation as lysosomal maturation is delayed even in the presence of bacterial translation inhibitors [83]. Only a limited number of early chlamydial effectors have yet been characterized. Tarp and CT694, two of these effectors involved in actin modulation have been discussed in the previous section. A recent report describes ChlaOTU as another early effector with deubiquitinating activity [84]. Formation of endosomes with C. caviae is accompanied by extensive ubiquitination, which is likely removed through the action of ChlaOTU. Interaction between ChlaOTU and host autophagy receptor NDP52 has been observed but appears to be dispensable for infection [84]. ChlaOTU is well conserved in C. pneumoniae but homology in C. trachomatis and C. muridarum is weak [84]. Transport of early inclusions of C. trachomatis and C. pneumoniae proceeds in a microtubule and Src family kinase dependent manner resulting in transport to the microtubule organizing center (MTOC) [85-87]. Interestingly, inclusions of the nonhuman chlamydial species C. caviae and C. muridarum are not transported to the MTOC [87]. Transport to the MTOC requires host cell vesicle transport and is dynein dependent but p50 dynamitin independent, as was shown by microinjection of antibodies against these proteins [86]. Antibodies directed against the plus end motor protein kinesin did not affect the transport while p150 (Glued) (subunit of the dynactin complex) co-localized to the endosome. The absence of p50 dynamitin which links vesicular cargo to dynein suggests that a bacterial factor within the endosomal membrane exerts this function [86]. During transport to the MTOC, the Chlamydia-containing endosome quickly deviates from the endosomal route, i.e. it is negative for endosomal fluid phase as well as lysosomal markers [88,89]. The exocytic Golgi to plasma membrane pathway is interrupted and Chlamydia-harboring endosome aquires sphingomyelin [17,80]. Interruption of Golgi derived exocytic transport might require the manipulation of small Rab GTPases, e.g. it has been shown that sphingomyelin acquisition is controlled by Rab14 around 10 hours post infection [90]. It remains to be investigated whether this process is controlled via interaction with early-secreted bacterial proteins, however, most of the investigated small Rab GTPases are recruited to the maturing inclusion [91]. Rab GTPases are selectively recruited in a species-dependent and -independent manner, probably through interaction with inclusion membrane proteins [91]. Selective recruitment of Rab GTPases regulates the interaction with various host organelles and this is supported by recruitment of several Rab interactors e.g. Bicaudal D1 (Rab 6 interactor), oculocerebrorenal syndrome of Lowe (OCRL1, interacts with multiple Rabs) and RAB11 family interacting protein 2 (Rab11FIP2, Rab11 and 14 interactor) [92-94]. Intracellular development of the inclusion is accompanied by extensive lipid acquisition from various sources. One of the major lipid sources appears to be the Golgi apparatus [17,95,96] that is fragmented during C. trachomatis infection probably to facilitate lipid transport to the inclusion [97]. Fragmentation of the Golgi and ceramide acquisition has been suggested to depend on Rab6/11 [98] and this process might be specific for C. trachomatis as it was not yet described for any other chlamydial species. The Golgi as the major lipid source is supported by preferential interception of basolaterally directed Golgi derived exocytic vesicles and recruitment of the trans-Golgi Snare syntaxin 6 (STX6) to the inclusion [99,100]. In line with this, Chlamydia intercepts retrograde intra-Golgi trafficking through recruitment of GS15 positive Conserved Oligomeric Golgi (COG) complex vesicles [101]. Additionally, optimal growth requires control of lipid trafficking from CD63-positive late endocytic multivesicular bodies, acquisition of cytoplasmic lipid droplets as well as recruitment of the high density lipoprotein (HDL) biogenesis machinery [102-104]. Recent results obtained for C. muridarum indicate that sphingomyelin acquisition might proceed in both vesicle-dependent as well as independent manner [105]. Vesicular trafficking via ADP-ribosylation factor 1 (Arf1) and Golgi-specific brefeldin A resistance factor 1 (GBF1) was found to be mainly required for inclusion membrane growth and stability but not for bacterial replication. Conversely, vesicular independent transport via the lipid carrier ceramide transfer protein (CERT) which is involved in endoplasmic reticulum (ER) to trans-Golgi transport as well as acquisition of VAMP (vesicle-associated membrane protein)-associated protein A (VAP-A), sphingomyelin synthase 1 and 2 (SMS1 and 2) to the inclusion are required for bacterial replication [105]. The situation appears to be more complex as various trafficking pathways regulate sphingolipid acquisition [99,106]. Elucidating the complexity of trafficking and lipid acquisition may require the establishment of fully polarized infection models for Chlamydia infection.
Figure 3.
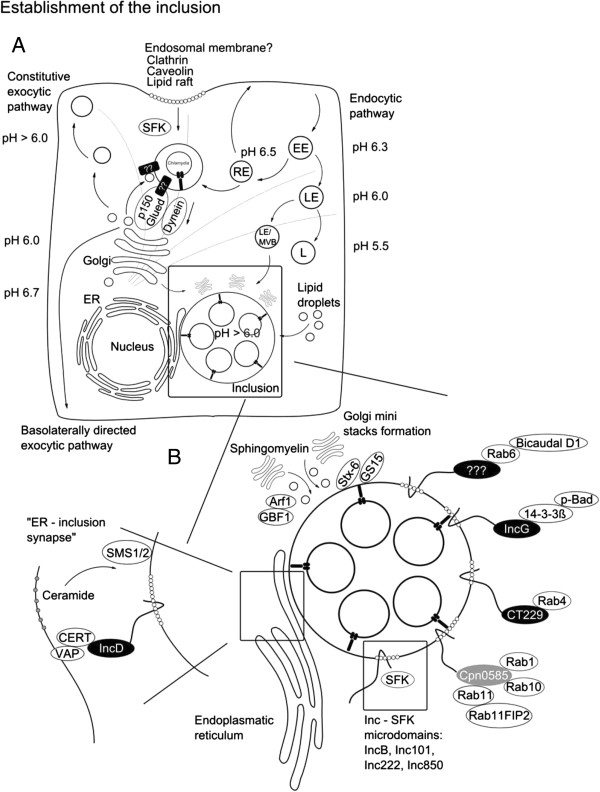
Establishment of the inclusion. A, The origin of the endosomal membrane of Chlamydia is not yet solved. Entry via multiple routes was suggested e.g. clathrin, caveolin or lipid raft dependent. After invasion early inclusions deviate from the endosomal route and acquires sphingolipids from the basolaterally directed exocytic route. Bacterial factors directing the exocytic trafficking towards the inclusion are not yet known. Similar to the Golgi (the most important inclusion lipid source) the inclusion pH stays above 6.0. Other lipid sources are via CD63+ late endosomes/ multivesicular body (LE/MVB), lipid droplets and through recruitment of the high density lipoprotein (HDL) synthesis machinery. EE (Early endosome), LE (Late endosome), RE (Recycling endosome) and L (Lysosomes). Transport of the inclusion to the microtubule organizing center (MTOC) requires Src family kinases (SFK), dynein, the dynactin subunit p150 Glued and an unidentified bacterial factor. Positioning and growth of the inclusion is accompanied by Golgi ministack formation in C. trachomatis. B, Interaction with various cellular organelles is mediated via a wide set of inclusion membrane proteins. IncG, CT229 and Cpn0585 are among the best investigated Inc proteins shown to interact with host proteins. Apoptosis is controlled via IncG/14-3-3 beta/ BCL2-associated agonist of cell death (p-Bad) interaction. Organelle identity is probably mediated via CT229 which interacts with Rab4 and Cpn0585 (interacts with Rab1, 10 and 11). Moreover, Incs segregate into micro domains and this is often associated with SFK co-localization. ER – inclusion synapses have been suggested to be an additional routes of lipid uptake. This is mediated via IncD/ collagen, type IV, alpha 3 (Goodpasture antigen) binding protein (CERT) / VAMP (vesicle-associated membrane protein)-associated protein A, 33 kDa (VAP-A) interaction. CERT is a lipid carrier suggested to transfer ceramide into the inclusion membrane where ceramide is converted to sphingomyelin via sphingomyelin synthase 1/2 (SMS1/2).
Contact area - proteins in the inclusion membrane
Upon completion of invasion chlamydial proteins heavily modify the endosomal membrane. Bacterial proteins present in the membrane of the inclusion and directed towards the cytoplasmic face of the inclusion membrane are likely to mediate early (positioning), mid (organelle fusion, survival control) and late (survival control, egress) effects and thereby critically regulate replication. More than 50 chlamydial proteins were detected in the inclusion membrane by immunofluorescence microscopy using specific antibodies [107]. A characteristic feature of the inclusion membrane (Inc) proteins [15] is a large hydrophobic bi-lobed transmembrane region which is useful for the in silico prediction of Inc proteins throughout the order of Chlamydiales[108-113]. Inc proteins share little sequence identity with each other, are unique for the order Chlamydiales and represent between 7-10% of the respective species proteomes [113]. Secretion of Inc proteins has been suggested to be TTSS dependent and this has been confirmed in a heterologous Shigella and Yersinia systems as well as by using chemical inhibitors of TTSS [114,115].
IncA is so far the best characterized Inc protein and has been shown to mediate inclusion fusogenicity through interaction of its soluble N-ethylmaleimide-sensitive-factor attachment receptor (SNARE) like cytoplasmic coiled-coil domains forming tetramer bundles [116-119]. Propagation of C. trachomatis was dramatically reduced in the presence of TTSS inhibitors and treatment of infected cells with TTSS inhibitors prevented translocation of IncA as well as inclusion fusion [120]. IncA homotypic interaction might be the exception rather than the rule among Inc proteins and more recent data suggest additional interactions with host SNARE proteins [119]. Host proteins have been identified as interaction partners for many of the investigated Inc proteins that could explain how Chlamydia modulates host cell physiology. Interaction of IncG and host 14-3-3 beta was the first described example, which was later linked to the recruitment of phosphorylated host Bcl-2-associated agonist of cell death (Bad) and indicated to be one part of chlamydial interference with apoptosis signaling [119,121] (Figure 3). Recently, Inc proteins were identified as regulators of species-specific Rab GTPase inclusion recruitment [91]. CT229 was found to interact with Rab4, while Cpn0585 interacts with Rab1, 10 and 11 [122,123]. Thus, recruitment of Rab GTPases via Inc proteins could explain inclusion-mediated regulation and control of vesicular trafficking inside the eukaryotic host cell. A subset of Inc proteins, i.e. IncB, Inc101, Inc222 and Inc850 have been shown to associate with active Src family kinases (SFK) in micro-domains and this regulates interaction with the microtubule network and maybe even SFK-mediated sphingolipid acquisition [106,124]. IncD interaction with CERT represents another example of how Chlamydia exerts control over sphingolipid acquisition and suggests a bridging function at inclusion ER junction sites between IncD, CERT and VAPs [125,126].
Finally, exit mechanisms might also be governed through Inc interactions as shown for the interaction between CT228 and Myosin phosphatase-targeting subunit 1 (MYPT1) [127]. Chlamydial host cell exit takes place either through a series of cysteine protease mediated proteolytic steps or extrusion, which describes an actin, N-Wasp, Myosin-II and Rho GTPase-dependent exit mechanism [18]. Both, the active as well as inactive forms of MYPT1 were recruited to the inclusion membrane. Phosphorylated inactive MYPT1 co-localized in SFK micro domains with myosin light chain 2 (MLC2), myosin light chain kinase (MLCK), myosin IIA and B. Inactivation of either MLC2, MLCK, myosin IIA or B reduced chlamydial extrusion; thus, the suggested role of CT228 mediated MYPT1 regulation is a shift of exit mechanism in response to certain environmental stimuli [127]. These examples suggest that understanding the function of chlamydial Inc and host protein complexes will be key for a deeper understanding on the mechanism how Chlamydia modulates the host cell. This assumption asks for a systematic investigation of Inc proteins and inclusion membrane content.
Future directions
Due to the unique intracellular lifestyle in a membrane-bound vacuolar environment, Chlamydia spp. have to exploit various routes of invasion and mechanisms to maintain their niche. Here, we have summarized how Chlamydia modulates cellular signaling and membrane trafficking. It is apparent that significant effort is required to fully understand how Chlamydia occupies its niche. Some of the open tasks are e.g. identification of the adhesin host receptor repertoire, clarification of the first steps of invasion, species specificity, infection of polarized epithelial cells and transfer into in vivo models. Further, although the number of proteins interacting with the bacterial factors is constantly growing, functional analysis of these interactions is still in its infancy and awaiting the full use of the newly developed chlamydial genetics. Applying the power of forward genetic approaches will help to identify bacterial effectors that orchestrate the complex chlamydial adaptation in its unique niche inside the host cell.
Abbreviations
EB: Elementary bodies; RB: Reticulate bodies.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AM and TR: drafted and revised the manuscript. Both authors read and approved the final manuscript.
Contributor Information
Adrian Mehlitz, Email: adrian.mehlitz@biozentrum.uni-wuerzburg.de.
Thomas Rudel, Email: thomas.rudel@biozentrum.uni-wuerzburg.de.
Acknowledgments
We thank Andreas Demuth for comments and corrections. This work was supported through the DFG priority program SPP1580 to A.M. and T.R. This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing.
References
- Everett KD, Bush RM, Andersen AA. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. 1999;49 Pt 2:415–440. doi: 10.1099/00207713-49-2-415. [DOI] [PubMed] [Google Scholar]
- Thom DH, Grayston JT, Wang SP, Kuo CC, Altman J. Chlamydia pneumoniae strain TWAR, Mycoplasma pneumoniae, and viral infections in acute respiratory disease in a university student health clinic population. Am J Epidemiol. 1990;132:248–256. doi: 10.1093/oxfordjournals.aje.a115654. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Kuo CC, Wang SP, Grayston JT. Serological response to Chlamydia pneumoniae infection. J Clin Microbiol. 1990;28:1261–1264. doi: 10.1128/jcm.28.6.1261-1264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman AJ, Jackson LA, Vaughan TL. Chlamydia pneumoniae and lung cancer: epidemiologic evidence. Cancer Epidemiol Biomarkers Prev. 2005;14:773–778. doi: 10.1158/1055-9965.EPI-04-0599. [DOI] [PubMed] [Google Scholar]
- Mahony JB, Coombes BK. Chlamydia pneumoniae and atherosclerosis: does the evidence support a causal or contributory role? FEMS Microbiol Lett. 2001;197:1–9. doi: 10.1111/j.1574-6968.2001.tb10574.x. [DOI] [PubMed] [Google Scholar]
- Henrichfreise B, Schiefer A, Schneider T, Nzukou E, Poellinger C, Hoffmann TJ, Johnston KL, Moelleken K, Wiedemann I, Pfarr K. et al. Functional conservation of the lipid II biosynthesis pathway in the cell wall-less bacteria Chlamydia and Wolbachia: why is lipid II needed? Mol Microbiol. 2009;73:913–923. doi: 10.1111/j.1365-2958.2009.06815.x. [DOI] [PubMed] [Google Scholar]
- Omsland A, Sager J, Nair V, Sturdevant DE, Hackstadt T. Developmental stage-specific metabolic and transcriptional activity of Chlamydia trachomatis in an axenic medium. Proc Natl Acad Sci U S A. 2012;109:19781–19785. doi: 10.1073/pnas.1212831109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, Carabeo RA, Hackstadt T. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci U S A. 2004;101:10166–10171. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hower S, Wolf K, Fields KA. Evidence that CT694 is a novel Chlamydia trachomatis T3S substrate capable of functioning during invasion or early cycle development. Mol Microbiol. 2009;72:1423–1437. doi: 10.1111/j.1365-2958.2009.06732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham AP, Jaafar ZA, Kemege KE, Middaugh CR, Hefty PS. Biophysical characterization of Chlamydia trachomatis CT584 supports its potential role as a type III secretion needle tip protein. Biochemistry. 2009;48:10353–10361. doi: 10.1021/bi901200y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CB, Bulir DC, Emdin CA, Pirie RM, Porfilio EA, Slootstra JW, Mahony JB. Chlamydia pneumoniae CdsL regulates CdsN ATPase activity, and disruption with a peptide mimetic prevents bacterial invasion. Front Microbiol. 2011;2:21. doi: 10.3389/fmicb.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleken K, Becker E, Hegemann JH. The Chlamydia pneumoniae invasin protein Pmp21 recruits the EGF receptor for host cell entry. PLoS Pathog. 2013;9:e1003325. doi: 10.1371/journal.ppat.1003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scidmore-Carlson MA, Shaw EI, Dooley CA, Fischer ER, Hackstadt T. Identification and characterization of a Chlamydia trachomatis early operon encoding four novel inclusion membrane proteins. Mol Microbiol. 1999;33:753–765. doi: 10.1046/j.1365-2958.1999.01523.x. [DOI] [PubMed] [Google Scholar]
- Al-Younes HM, Rudel T, Brinkmann V, Szczepek AJ, Meyer TF. Low iron availability modulates the course of Chlamydia pneumoniae infection. Cell Microbiol. 2001;3:427–437. doi: 10.1046/j.1462-5822.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- Rockey DD, Heinzen RA, Hackstadt T. Cloning and characterization of a Chlamydia psittaci gene coding for a protein localized in the inclusion membrane of infected cells. Mol Microbiol. 1995;15:617–626. doi: 10.1111/j.1365-2958.1995.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Ojcius DM, Hellio R, Dautry-Varsat A. Distribution of endosomal, lysosomal, and major histocompatability complex markers in a monocytic cell line infected with Chlamydia psittaci. Infect Immun. 1997;65:2437–2442. doi: 10.1128/iai.65.6.2437-2442.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T, Rockey DD, Heinzen RA, Scidmore MA. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 1996;15:964–977. [PMC free article] [PubMed] [Google Scholar]
- Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci U S A. 2007;104:11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin E, Kirker K, Zuck M, James G, Hybiske K. Actin recruitment to the Chlamydia inclusion is spatiotemporally regulated by a mechanism that requires host and bacterial factors. PLoS One. 2012;7:e46949. doi: 10.1371/journal.pone.0046949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick PB. Chlamydia trachomatis persistence in vitro: an overview. J Infect Dis. 2010;201(Suppl 2):S88–S95. doi: 10.1086/652394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan RJ, Mathews SA, Mukhopadhyay S, Summersgill JT, Timms P. Chlamydial persistence: beyond the biphasic paradigm. Infect Immun. 2004;72:1843–1855. doi: 10.1128/IAI.72.4.1843-1855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belland RJ, Zhong G, Crane DD, Hogan D, Sturdevant D, Sharma J, Beatty WL, Caldwell HD. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci U S A. 2003;100:8478–8483. doi: 10.1073/pnas.1331135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer AP, Mehlitz A, Mollenkopf HJ, Meyer TF. Gene expression profiles of Chlamydophila pneumoniae during the developmental cycle and iron depletion-mediated persistence. PLoS Pathog. 2007;3:e83. doi: 10.1371/journal.ppat.0030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Wilson DP, Myers G, Timms P, Bavoil PM. Type III secretion a la Chlamydia. Trends Microbiol. 2007;15:241–251. doi: 10.1016/j.tim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Beeckman DS, Vanrompay DC. Bacterial secretion systems with an emphasis on the chlamydial Type III secretion system. Curr Issues Mol Biol. 2010;12:17–41. [PubMed] [Google Scholar]
- Wehrl W, Brinkmann V, Jungblut PR, Meyer TF, Szczepek AJ. From the inside out–processing of the Chlamydial autotransporter PmpD and its role in bacterial adhesion and activation of human host cells. Mol Microbiol. 2004;51:319–334. doi: 10.1046/j.1365-2958.2003.03838.x. [DOI] [PubMed] [Google Scholar]
- Moelleken K, Hegemann JH. The Chlamydia outer membrane protein OmcB is required for adhesion and exhibits biovar-specific differences in glycosaminoglycan binding. Mol Microbiol. 2008;67:403–419. doi: 10.1111/j.1365-2958.2007.06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Bavoil PM. Intracellular Pathogens I: Chlamydiales. Washington, D.C.: ASM Press; 2012. [Google Scholar]
- Campbell LA, Kuo CC. Interactions of chlamydia with the host cells that mediate attachment and uptake. Chlamydia genomics and pathogenesis. Horizon Bioscience. 2006;1:505–522. [Google Scholar]
- Moelleken K, Hegemann JH. In: Intracellular Pathogens I: Chlamydiales. Tan M, Bavoil PM, editor. Washington, D.C.: ASM Press; 2012. Chlamydial adhesion and adhesins. [Google Scholar]
- Su H, Watkins NG, Zhang YX, Caldwell HD. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Caldwell HD. In vitro neutralization of Chlamydia trachomatis by monovalent Fab antibody specific to the major outer membrane protein. Infect Immun. 1991;59:2843–2845. doi: 10.1128/iai.59.8.2843-2845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Raymond L, Rockey DD, Fischer E, Hackstadt T, Caldwell HD. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc Natl Acad Sci U S A. 1996;93:11143–11148. doi: 10.1073/pnas.93.20.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson AF, Kuo CC. Evidence that the major outer membrane protein of Chlamydia trachomatis is glycosylated. Infect Immun. 1991;59:2120–2125. doi: 10.1128/iai.59.6.2120-2125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson AF, Kuo CC. Binding of the glycan of the major outer membrane protein of Chlamydia trachomatis to HeLa cells. Infect Immun. 1994;62:24–28. doi: 10.1128/iai.62.1.24-28.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C, Takahashi N, Swanson AF, Ozeki Y, Hakomori S. An N-linked high-mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J Clin Invest. 1996;98:2813–2818. doi: 10.1172/JCI119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puolakkainen M, Kuo CC, Campbell LA. Chlamydia pneumoniae uses the mannose 6-phosphate/insulin-like growth factor 2 receptor for infection of endothelial cells. Infect Immun. 2005;73:4620–4625. doi: 10.1128/IAI.73.8.4620-4625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JP, Stephens RS. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-O. [DOI] [PubMed] [Google Scholar]
- Rasmussen-Lathrop SJ, Koshiyama K, Phillips N, Stephens RS. Chlamydia-dependent biosynthesis of a heparan sulphate-like compound in eukaryotic cells. Cell Microbiol. 2000;2:137–144. doi: 10.1046/j.1462-5822.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Poteralski JM, Olinger L. Interaction of Chlamydia trachomatis with mammalian cells is independent of host cell surface heparan sulfate glycosaminoglycans. Infect Immun. 2006;74:1795–1799. doi: 10.1128/IAI.74.3.1795-1799.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Koshiyama K, Lewis E, Kubo A. Heparin-binding outer membrane protein of chlamydiae. Mol Microbiol. 2001;40:691–699. doi: 10.1046/j.1365-2958.2001.02418.x. [DOI] [PubMed] [Google Scholar]
- Fadel S, Eley A. Chlamydia trachomatis OmcB protein is a surface-exposed glycosaminoglycan-dependent adhesin. J Med Microbiol. 2007;56:15–22. doi: 10.1099/jmm.0.46801-0. [DOI] [PubMed] [Google Scholar]
- Fadel S, Eley A. Differential glycosaminoglycan binding of Chlamydia trachomatis OmcB protein from serovars E and LGV. J Med Microbiol. 2008;57:1058–1061. doi: 10.1099/jmm.0.2008/001305-0. [DOI] [PubMed] [Google Scholar]
- Fechtner T, Stallmann S, Moelleken K, Meyer KL, Hegemann JH. Characterization of the interaction between the chlamydial adhesin OmcB and the human host cell. J Bacteriol. 2013;195(23):5323–5333. doi: 10.1128/JB.00780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick EJ, Travelstead A, Cooper MD. Comparative studies of glycosaminoglycan involvement in Chlamydia pneumoniae and C. trachomatis invasion of host cells. J Infect Dis. 2003;187:1291–1300. doi: 10.1086/374056. [DOI] [PubMed] [Google Scholar]
- Fudyk T, Olinger L, Stephens RS. Selection of mutant cell lines resistant to infection by Chlamydia spp [corrected] Infect Immun. 2002;70:6444–6447. doi: 10.1128/IAI.70.11.6444-6447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant CG, Stephens RS. Chlamydia attachment to mammalian cells requires protein disulfide isomerase. Cell Microbiol. 2007;9:222–232. doi: 10.1111/j.1462-5822.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- Rosmarin DM, Carette JE, Olive AJ, Starnbach MN, Brummelkamp TR, Ploegh HL. Attachment of Chlamydia trachomatis L2 to host cells requires sulfation. Proc Natl Acad Sci U S A. 2012;109:10059–10064. doi: 10.1073/pnas.1120244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajonuma LC, Fok KL, Ho LS, Chan PK, Chow PH, Tsang LL, Wong CH, Chen J, Li S, Rowlands DK. et al. CFTR is required for cellular entry and internalization of Chlamydia trachomatis. Cell Biol Int. 2010;34:593–600. doi: 10.1042/CBI20090227. [DOI] [PubMed] [Google Scholar]
- Kim JH, Jiang S, Elwell CA, Engel JN. Chlamydia trachomatis co-opts the FGF2 signaling pathway to enhance infection. PLoS Pathog. 2011;7:e1002285. doi: 10.1371/journal.ppat.1002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes BK, Mahony JB. Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of HEp2 cells. Cell Microbiol. 2002;4:447–460. doi: 10.1046/j.1462-5822.2002.00203.x. [DOI] [PubMed] [Google Scholar]
- Elwell CA, Ceesay A, Kim JH, Kalman D, Engel JN. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog. 2008;4:e1000021. doi: 10.1371/journal.ppat.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlitz A, Banhart S, Maurer AP, Kaushansky A, Gordus AG, Zielecki J, Macbeath G, Meyer TF. Tarp regulates early Chlamydia-induced host cell survival through interactions with the human adaptor protein SHC1. J Cell Biol. 2010;190:143–157. doi: 10.1083/jcb.200909095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BJ, Mutchler C, Al Khodor S, Grieshaber SS, Carabeo RA. Chlamydial entry involves TARP binding of guanine nucleotide exchange factors. PLoS Pathog. 2008;4:e1000014. doi: 10.1371/journal.ppat.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett TJ, Dooley CA, Mead DJ, Hackstadt T. Chlamydia trachomatis tarp is phosphorylated by src family tyrosine kinases. Biochem Biophys Res Commun. 2008;371:339–344. doi: 10.1016/j.bbrc.2008.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlitz A, Banhart S, Hess S, Selbach M, Meyer TF. Complex kinase requirements for Chlamydia trachomatis Tarp phosphorylation. FEMS Microbiol Lett. 2008;289:233–240. doi: 10.1111/j.1574-6968.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- Reynolds DJ, Pearce JH. Characterization of the cytochalasin D-resistant (pinocytic) mechanisms of endocytosis utilized by chlamydiae. Infect Immun. 1990;58:3208–3216. doi: 10.1128/iai.58.10.3208-3216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm N, Wyrick PB. Cytoskeletal requirements in Chlamydia trachomatis infection of host cells. Infect Immun. 1995;63:324–332. doi: 10.1128/iai.63.1.324-332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo RA, Grieshaber SS, Fischer E, Hackstadt T. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect Immun. 2002;70:3793–3803. doi: 10.1128/IAI.70.7.3793-3803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkworth AJ, Malcolm DS, Pedrosa AT, Roguska K, Shahbazian S, Graham JE, Hayward RD, Carabeo RA. Chlamydia trachomatis Slc1 is a type III secretion chaperone that enhances the translocation of its invasion effector substrate TARP. Mol Microbiol. 2011;82:131–144. doi: 10.1111/j.1365-2958.2011.07802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison WP, Hackstadt T. Induction of type III secretion by cell-free Chlamydia trachomatis elementary bodies. Microb Pathog. 2008;45:435–440. doi: 10.1016/j.micpath.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton DR, Dooley CA, Grieshaber SS, Carabeo RA, Fields KA, Hackstadt T. Tyrosine phosphorylation of the chlamydial effector protein Tarp is species specific and not required for recruitment of actin. Infect Immun. 2005;73:3860–3868. doi: 10.1128/IAI.73.7.3860-3868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett TJ, Fischer ER, Mead DJ, Hackstadt T. Chlamydial TARP is a bacterial nucleator of actin. Proc Natl Acad Sci U S A. 2006;103:15599–15604. doi: 10.1073/pnas.0603044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett TJ, Miller NJ, Dooley CA, Hackstadt T. The conserved Tarp actin binding domain is important for chlamydial invasion. PLoS Pathog. 2010;6:e1000997. doi: 10.1371/journal.ppat.1000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiwani S, Alvarado S, Ohr RJ, Romero A, Nguyen B, Jewett TJ. Chlamydia trachomatis Tarp harbors distinct G and F actin binding domains that bundle actin filaments. J Bacteriol. 2013;195:708–716. doi: 10.1128/JB.01768-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiwani S, Ohr RJ, Fischer ER, Hackstadt T, Alvarado S, Romero A, Jewett TJ. Chlamydia trachomatis Tarp cooperates with the Arp2/3 complex to increase the rate of actin polymerization. Biochem Biophys Res Commun. 2012;420:816–821. doi: 10.1016/j.bbrc.2012.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock HD, Hower S, Fields KA. Domain analyses reveal that Chlamydia trachomatis CT694 protein belongs to the membrane-localized family of type III effector proteins. J Biol Chem. 2012;287:28078–28086. doi: 10.1074/jbc.M112.386904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo RA, Grieshaber SS, Hasenkrug A, Dooley C, Hackstadt T. Requirement for the Rac GTPase in Chlamydia trachomatis invasion of non-phagocytic cells. Traffic. 2004;5:418–425. doi: 10.1111/j.1398-9219.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- Carabeo RA, Dooley CA, Grieshaber SS, Hackstadt T. Rac interacts with Abi-1 and WAVE2 to promote an Arp2/3-dependent actin recruitment during chlamydial invasion. Cell Microbiol. 2007;9:2278–2288. doi: 10.1111/j.1462-5822.2007.00958.x. [DOI] [PubMed] [Google Scholar]
- Subtil A, Wyplosz B, Balana ME, Dautry-Varsat A. Analysis of Chlamydia caviae entry sites and involvement of Cdc42 and Rac activity. J Cell Sci. 2004;117:3923–3933. doi: 10.1242/jcs.01247. [DOI] [PubMed] [Google Scholar]
- Balana ME, Niedergang F, Subtil A, Alcover A, Chavrier P, Dautry-Varsat A. ARF6 GTPase controls bacterial invasion by actin remodelling. J Cell Sci. 2005;118:2201–2210. doi: 10.1242/jcs.02351. [DOI] [PubMed] [Google Scholar]
- Norkin LC, Wolfrom SA, Stuart ES. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp Cell Res. 2001;266:229–238. doi: 10.1006/excr.2001.5202. [DOI] [PubMed] [Google Scholar]
- Stuart ES, Webley WC, Norkin LC. Lipid rafts, caveolae, caveolin-1, and entry by Chlamydiae into host cells. Exp Cell Res. 2003;287:67–78. doi: 10.1016/S0014-4827(03)00059-4. [DOI] [PubMed] [Google Scholar]
- Jutras I, Abrami L, Dautry-Varsat A. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect Immun. 2003;71:260–266. doi: 10.1128/IAI.71.1.260-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybiske K, Stephens RS. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect Immun. 2007;75:3925–3934. doi: 10.1128/IAI.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M, Kihlstrom E. Mobilization of F-actin and clathrin during redistribution of Chlamydia trachomatis to an intracellular site in eucaryotic cells. Infect Immun. 1991;59:4465–4472. doi: 10.1128/iai.59.12.4465-4472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleti H, Benmerah A, Ojcius DM, Cerf-Bensussan N, Dautry-Varsat A. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth. J Cell Sci. 1999;112(Pt 10):1487–1496. doi: 10.1242/jcs.112.10.1487. [DOI] [PubMed] [Google Scholar]
- Gabel BR, Elwell C, van Ijzendoorn SC, Engel JN. Lipid raft-mediated entry is not required for Chlamydia trachomatis infection of cultured epithelial cells. Infect Immun. 2004;72:7367–7373. doi: 10.1128/IAI.72.12.7367-7373.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. Endocytosis of influenza viruses. Microbes Infect. 2004;6:929–936. doi: 10.1016/j.micinf.2004.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Hackstadt T. Sphingomyelin trafficking in Chlamydia pneumoniae-infected cells. Cell Microbiol. 2001;3:145–152. doi: 10.1046/j.1462-5822.2001.00098.x. [DOI] [PubMed] [Google Scholar]
- Schramm N, Bagnell CR, Wyrick PB. Vesicles containing Chlamydia trachomatis serovar L2 remain above pH 6 within HEC-1B cells. Infect Immun. 1996;64:1208–1214. doi: 10.1128/iai.64.4.1208-1214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scidmore MA, Rockey DD, Fischer ER, Heinzen RA, Hackstadt T. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect Immun. 1996;64:5366–5372. doi: 10.1128/iai.64.12.5366-5372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scidmore MA, Fischer ER, Hackstadt T. Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect Immun. 2003;71:973–984. doi: 10.1128/IAI.71.2.973-984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado AR, Essid M, Perrinet S, Balana ME, Yoder N, Dehoux P, Subtil A. The chlamydial OTU domain-containing protein ChlaOTU is an early type III secretion effector targeting ubiquitin and NDP52. Cell Microbiol. 2013;15(12):2064–2079. doi: 10.1111/cmi.12171. [DOI] [PubMed] [Google Scholar]
- Clausen JD, Christiansen G, Holst HU, Birkelund S. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol Microbiol. 1997;25:441–449. doi: 10.1046/j.1365-2958.1997.4591832.x. [DOI] [PubMed] [Google Scholar]
- Grieshaber SS, Grieshaber NA, Hackstadt T. Chlamydia trachomatis uses host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process. J Cell Sci. 2003;116:3793–3802. doi: 10.1242/jcs.00695. [DOI] [PubMed] [Google Scholar]
- Mital J, Hackstadt T. Diverse requirements for SRC-family tyrosine kinases distinguish chlamydial species. MBio. 2011. doi: 10.1128/mBio.00031-11. [DOI] [PMC free article] [PubMed]
- Heinzen RA, Scidmore MA, Rockey DD, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Younes HM, Rudel T, Meyer TF. Characterization and intracellular trafficking pattern of vacuoles containing Chlamydia pneumoniae in human epithelial cells. Cell Microbiol. 1999;1:237–247. doi: 10.1046/j.1462-5822.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- Capmany A, Damiani MT. Chlamydia trachomatis intercepts Golgi-derived sphingolipids through a Rab14-mediated transport required for bacterial development and replication. PloS one. 2010;5:e14084. doi: 10.1371/journal.pone.0014084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzomp KA, Scholtes LD, Briggs BJ, Whittaker GR, Scidmore MA. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect Immun. 2003;71:5855–5870. doi: 10.1128/IAI.71.10.5855-5870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead AM, Jung JY, Smirnov A, Kaufer S, Scidmore MA. Multiple host proteins that function in phosphatidylinositol-4-phosphate metabolism are recruited to the chlamydial inclusion. Infect Immun. 2010;78:1990–2007. doi: 10.1128/IAI.01340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead AR, Rzomp KA, Scidmore MA. The Rab6 effector Bicaudal D1 associates with Chlamydia trachomatis inclusions in a biovar-specific manner. Infect Immun. 2007;75:781–791. doi: 10.1128/IAI.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva N, Capmany A, Damiani MT. Rab11-family of interacting protein 2 associates with chlamydial inclusions through its Rab-binding domain and promotes bacterial multiplication. Cell Microbiol. 2013;15:114–129. doi: 10.1111/cmi.12035. [DOI] [PubMed] [Google Scholar]
- Carabeo RA, Mead DJ, Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci U S A. 2003;100:6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T, Scidmore MA, Rockey DD. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci U S A. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer D, Lipinski AR, Machuy N, Karlas A, Wehrens A, Siedler F, Brinkmann V, Meyer TF. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- Rejman Lipinski A, Heymann J, Meissner C, Karlas A, Brinkmann V, Meyer TF, Heuer D. Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent Golgi fragmentation. PLoS Pathog. 2009;5:e1000615. doi: 10.1371/journal.ppat.1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ER, Fischer ER, Mead DJ, Hackstadt T. The chlamydial inclusion preferentially intercepts basolaterally directed sphingomyelin-containing exocytic vacuoles. Traffic. 2008;9:2130–2140. doi: 10.1111/j.1600-0854.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ER, Mead DJ, Dooley CA, Sager J, Hackstadt T. The trans-Golgi SNARE syntaxin 6 is recruited to the chlamydial inclusion membrane. Microbiology. 2011;157:830–838. doi: 10.1099/mic.0.045856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrovskaya ID, Szwedo JW, Goodwin A, Lupashina TV, Nagarajan UM, Lupashin VV. Chlamydia trachomatis hijacks intra-Golgi COG complex-dependent vesicle trafficking pathway. Cell Microbiol. 2012;14:656–668. doi: 10.1111/j.1462-5822.2012.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WL. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J Cell Sci. 2006;119:350–359. doi: 10.1242/jcs.02733. [DOI] [PubMed] [Google Scholar]
- Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A. 2008;105:9379–9384. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JV, Naher N, Abdelrahman YM, Belland RJ. Host HDL biogenesis machinery is recruited to the inclusion of Chlamydia trachomatis-infected cells and regulates chlamydial growth. Cell Microbiol. 2012;14:1497–1512. doi: 10.1111/j.1462-5822.2012.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell CA, Jiang S, Kim JH, Lee A, Wittmann T, Hanada K, Melancon P, Engel JN. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog. 2011;7:e1002198. doi: 10.1371/journal.ppat.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital J, Hackstadt T. Role for the SRC family kinase Fyn in sphingolipid acquisition by chlamydiae. Infect Immun. 2011;79:4559–4568. doi: 10.1128/IAI.05692-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chen C, Chen D, Wu Y, Zhong Y, Zhong G. Characterization of fifty putative inclusion membrane proteins encoded in the Chlamydia trachomatis genome. Infect Immun. 2008;76:2746–2757. doi: 10.1128/IAI.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannantine JP, Stamm WE, Suchland RJ, Rockey DD. Chlamydia trachomatis IncA is localized to the inclusion membrane and is recognized by antisera from infected humans and primates. Infect Immun. 1998;66:6017–6021. doi: 10.1128/iai.66.12.6017-6021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannantine JP, Griffiths RS, Viratyosin W, Brown WJ, Rockey DD. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell Microbiol. 2000;2:35–47. doi: 10.1046/j.1462-5822.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- Toh H, Miura K, Shirai M, Hattori M. In silico inference of inclusion membrane protein family in obligate intracellular parasites chlamydiae. DNA Res. 2003;10:9–17. doi: 10.1093/dnares/10.1.9. [DOI] [PubMed] [Google Scholar]
- Heinz E, Rockey DD, Montanaro J, Aistleitner K, Wagner M, Horn M. Inclusion membrane proteins of Protochlamydia amoebophila UWE25 reveal a conserved mechanism for host cell interaction among the Chlamydiae. J Bacteriol. 2010;192:5093–5102. doi: 10.1128/JB.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter EI, Martens C, Hackstadt T. Evolution and conservation of predicted inclusion membrane proteins in chlamydiae. Comp Funct Genomics. 2012;2012:362104. doi: 10.1155/2012/362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehoux P, Flores R, Dauga C, Zhong G, Subtil A. Multi-genome identification and characterization of chlamydiae-specific type III secretion substrates: the Inc proteins. BMC Genomics. 2011;12:109. doi: 10.1186/1471-2164-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Parsot C, Dautry-Varsat A. Secretion of predicted Inc proteins of Chlamydia pneumoniae by a heterologous type III machinery. Mol Microbiol. 2001;39:792–800. doi: 10.1046/j.1365-2958.2001.02272.x. [DOI] [PubMed] [Google Scholar]
- Fields KA, Mead DJ, Dooley CA, Hackstadt T. Chlamydia trachomatis type III secretion: evidence for a functional apparatus during early-cycle development. Mol Microbiol. 2003;48:671–683. doi: 10.1046/j.1365-2958.2003.03462.x. [DOI] [PubMed] [Google Scholar]
- Suchland RJ, Rockey DD, Bannantine JP, Stamm WE. Isolates of Chlamydia trachomatis that occupy nonfusogenic inclusions lack IncA, a protein localized to the inclusion membrane. Infect Immun. 2000;68:360–367. doi: 10.1128/IAI.68.1.360-367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzhanov D, Barnes J, Hruby DE, Rockey DD. Chlamydial development is blocked in host cells transfected with Chlamydophila caviae incA. BMC Microbiol. 2004;4:24. doi: 10.1186/1471-2180-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Nilges M, Dautry-Varsat A, Subtil A. Conservation of the biochemical properties of IncA from Chlamydia trachomatis and Chlamydia caviae: oligomerization of IncA mediates interaction between facing membranes. J Biol Chem. 2004;279:46896–46906. doi: 10.1074/jbc.M407227200. [DOI] [PubMed] [Google Scholar]
- Delevoye C, Nilges M, Dehoux P, Paumet F, Perrinet S, Dautry-Varsat A, Subtil A. SNARE protein mimicry by an intracellular bacterium. PLoS Pathog. 2008;4:e1000022. doi: 10.1371/journal.ppat.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschiol S, Bailey L, Gylfe A, Sundin C, Hultenby K, Bergstrom S, Elofsson M, Wolf-Watz H, Normark S, Henriques-Normark B. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc Natl Acad Sci U S A. 2006;103:14566–14571. doi: 10.1073/pnas.0606412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke P, Welter-Stahl L, Ying S, Hansen J, Hacker G, Darville T, Ojcius DM. Recruitment of BAD by the Chlamydia trachomatis vacuole correlates with host-cell survival. PLoS Pathog. 2006;2:e45. doi: 10.1371/journal.ppat.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes C, Rzomp KA, Tvinnereim A, Scidmore MA, Wizel B. Chlamydia pneumoniae inclusion membrane protein Cpn0585 interacts with multiple Rab GTPases. Infect Immun. 2007;75:5586–5596. doi: 10.1128/IAI.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzomp KA, Moorhead AR, Scidmore MA. The GTPase Rab4 interacts with Chlamydia trachomatis inclusion membrane protein CT229. Infect Immun. 2006;74:5362–5373. doi: 10.1128/IAI.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital J, Miller NJ, Fischer ER, Hackstadt T. Specific chlamydial inclusion membrane proteins associate with active Src family kinases in microdomains that interact with the host microtubule network. Cell Microbiol. 2010;12:1235–1249. doi: 10.1111/j.1462-5822.2010.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derre I, Swiss R, Agaisse H. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog. 2011;7:e1002092. doi: 10.1371/journal.ppat.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoux M, Clare DK, Saibil HR, Hayward RD. Chlamydiae assemble a pathogen synapse to hijack the host endoplasmic reticulum. Traffic. 2012;13:1612–1627. doi: 10.1111/tra.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter EI, Barger AC, Nair V, Hackstadt T. Chlamydia trachomatis Inclusion Membrane Protein CT228 Recruits Elements of the Myosin Phosphatase Pathway to Regulate Release Mechanisms. Cell Rep. 2013;3:1921–1931. doi: 10.1016/j.celrep.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


