Abstract
The Mediterranean fruit fly (Medfly) is one of the world's most economically damaging pests. It displays highly seasonal population dynamics, and the environmental conditions suitable for its abundance are not constant throughout the year in most places. An extensive literature search was performed to obtain the most comprehensive data on the historical and contemporary spatio-temporal occurrence of the pest globally. The database constructed contained 2328 unique geo-located entries on Medfly detection sites from 43 countries and nearly 500 unique localities, as well as information on hosts, life stages and capture method. Of these, 125 localities had information on the month when Medfly was recorded and these data were complemented by additional material found in comprehensive databases available online. Records from 1980 until present were used for medfly environmental niche modeling. Maximum Entropy Algorithm (MaxEnt) and a set of seasonally varying environmental covariates were used to predict the fundamental niche of the Medfly on a global scale. Three seasonal maps were also produced: January-April, May-August and September-December. Models performed significantly better than random achieving high accuracy scores, indicating a good discrimination of suitable versus unsuitable areas for the presence of the species.
Introduction
Many invasive alien species are directly associated with biodiversity loss, ecosystem service changes, and negative impacts on human health, agriculture, forestry and fisheries. In Europe alone, these losses and impacts are estimated to cost at least EUR 12 billion per year [1]. The Mediterranean fruit fly, Ceratitis capitata (Wiedemann), commonly referred to as Medfly, is considered one of the world's most destructive pests [2]. It is a highly polyphagus species, able to feed on over 300 hosts and known to be capable of adapting to a wide range of climates [2]–[4]. It causes significant damage to fruits and vegetables, and its economic impacts are substantial.
Medfly originated from sub-Saharan Africa and in early 19th century was identified in southern parts of Europe, from where it subsequently spread to other parts of the globe [5]. It is currently present in Mediterranean Europe and the Middle East, in most parts of Africa including Indian Ocean islands, South and Central America, western Australia and the Pacific region. It is a quarantine pest and countries with established Medfly populations have significant trade barriers imposed to their exports. The pest has been established for about a century in Hawaii and despite persistent and costly eradication efforts, is repeatedly detected into Florida and California [6], [7]. It is estimated that the cost of each of its previous incursions into the US (eradication and industry loss) ranged from US$300,000 to US$200 m [8]. Medfly outbreaks in California during the past 25 years have cost taxpayers nearly $500 million, while the Medfly outbreak in Florida's Tampa Bay region in 1997 resulted in $25 million spent on eradication [9], which is significantly less than the cost of potential establishment. It has been estimated that the cost of controlling established Medfly in the State of California alone could range between $493 million to $875 million, and imposition of trade embargo from Asian countries would result in additional revenue losses of $564 million and cost more than 14,000 jobs [10]. The eastern Mediterranean region also experienced substantial losses linked to fruit fly infestations estimated at US$192 m [11].
Comprehensive global information on Medfly occurrence, both in spatial and temporal terms, is crucial for understanding not only the current and historical extent of its occurrence, but also the conditions where it is able to survive and areas susceptible to potential invasion and establishment. For similar reasons, it is also essential to track historical spread routes and the history of invasion. Occurrence records with temporal reference are important for understanding the drivers of Medfly seasonal population dynamics, which can be valuable for guiding eradication and control strategies. Commonwealth Agricultural Bureaux International (CABI), European Plant Protection Organization (EPPO) and International Atomic Energy Agency (IAEA) are examples of institutions maintaining records on where Medfly is established [12]–[15]. These sources define Medfly presence status at the country scale and less frequently, on the provincial scale. Their quality, according to the most current spatial and temporal information, varies and there are no widely-available expert opinion maps defining the environmental range of known Medfly occurrence.
Recent years have seen a handful of studies aiming to define the potential distribution of Medfly. In a study by De Meyer et al. [16], a genetic algorithm for rule-set prediction (GARP) and principal component analysis (PCA) were used to estimate the potential geographical range of Medfly using native range distributional data derived from a database maintained by the Royal Museum for Central Africa. This data was complemented by non-native range information gathered from the literature and electronic resources. Outputs showed areas of high and low suitability for Medfly presence globally without providing information on what constituted the threshold for such categories, or any seasonal changes. CLIMEX (http://www.csiro.au/solutions/ps1h3) was used in a different study to assess the seasonal and year-to-year variation in climatic suitability for Medfly worldwide with emphasis on Argentina and Australia [17]. No occurrence data were used for the modeling, but rather parameters of its population dynamics were used, specifically a CLIMEX growth index derived from a study of Medfly populations in Thessaloniki [18]. Gutierrez and Ponti [19] also assessed the invasive potential of Medfly in California and Arizona using GRASS-GIS, based on age-structured dynamics of Medfly life stages and temperature variability in the region. MaxEnt outperformed GARP in a study [20] which aimed to assess the potential distribution of three fruit fly species including Medfly in China. A set of environmental variables describing temperature and precipitation, as well as worldwide occurrence records were used in the modelling.
It is well documented in regional studies from several areas of the world that Medfly has a highly seasonal pattern to its population dynamics [3], [21]–[29]. However, spatiotemporal datasets to quantify these patterns on a global scale have yet to be assembled, while previous global mapping of the suitability for Medfly presence has not accounted for seasonal shifts. Here we present the results of a study that has focused on constructing the most comprehensive database on confirmed Medfly occurrence records, the timing of these records and their locations. Moreover, information on hosts, life stages and capture method were also recorded. Finally, seasonally-varying gridded environmental variables including temperature, precipitation, elevation and normalized vegetation index (NDVI) were linked to these records in a niche modeling framework to produce predictions of the annual and seasonal distributions of suitability for Medfly presence on a global scale, with an aim of identifying regions that can be potential risk areas for Medfly invasions depending on the season.
Materials and Methods
Occurrence data
Confirmed detection location data for C. capitata were searched for in online open-access museum collections data, published articles, reports and conference proceedings. The literature search resulted in 158 publications and reports containing potential data to be reviewed [2]–[5], [7], . Of these publications, 101 contained information about Medfly detection that could be geolocated, and 64 contained information about the month when Medfly was observed. A database was constructed to store historical data pertaining to Medfly detection locations. For each entry, information about the author, year and type of publication, country, two administrative levels and locality, georeferenced location and source of coordinates, the quality of information about the location, year and month of the occurrence record, sampling technique, developmental stage of Medfly and host plant were recorded. This data record protocol was built upon that pioneered for recent studies of malaria vectors worldwide [162]–[165]. Locations of Medfly observations were georeferenced, either based on coordinates included in the source material or dependent on the name of the location found in the source, and the geolocation source, method and accurracy is identified in the database. Additional supporting sources of information on the Medfly occurrence locations were obtained from the Global Biodiversity Information Facility (GBIF http://data.gbif.org), Belgian Biodiversity Platform (BeBIF www.biodiversity.be) and the Royal Museum of Central Africa (http://projects.bebif.be/enbi/fruitfly) [16].
Covariates used in modeling
A suite of environmental variables was constructed in preparation for use in niche modeling in consideration of the pest's environmental limiting factors (Table 1 and 2). Medfly has four stages of development: (1) female adults deposit eggs under the skin of susceptible fruits or vegetables, where (2) eggs hatch to produce larvae. (3) Larvae feed on the pulp of the host before complete the larval development and abandon the fruit to the soil, where (4) larvae pupate and after methamorphosis adults emerge from the soil. The length of time required to complete the Medfly lifecycle in tropical summer weather conditions is in the range of 21 to 30 days [166]. In cool climates it can take well over 200 days [18], [134]. Its presence is also influenced by the availability of hosts. In tropical areas, due to overlapping host phenology, Medfly populations are able to persist year-round or during the majority of the year, whereas in temperate regions, the host-present period is considerably shorter. Medfly, as with other insects, is known to be sensitive to climate, and apart of host availability, one of the main limitations to its development is low temperature that may hinder its ability to overwinter [18], [29], plus high precipitation which may have an adverse impact on the pupae development in the soil [3], [24]. Thermal requirements of insect species are often derived in laboratory conditions and vary with developmental stage, environmental conditions and their geographic origin [167], [168]. The optimum temperature threshold for Medfly development is estimated to be between 21°C and 26.7°C, and below 10°C and above 35°C the development stops [140], [169], [170]. The ability to overwinter at high altitudes on the fringes of suitable areas is very limited and dependent on the availability of hosts favoring slow growth, inside which pest could survive [21], [82]. Given these factors, we obtained land surface temperature (LST) and normalized difference vegetation index (NDVI) images from the Advanced Very High Resolution Radiometer (AVHRR) satellite sensor. The products are available at 8×8 km spatial resolution for over a 20-year time series and downloadable via the Goddard Flight Center's Web Site (http://daac.gsfc.nasa.gov/). Digital elevation model(DEM) data were obtained from the Shuttle Radar Topography Mission (SRTM) [http://www2.jpl.nasa.gov/srtm/]. Finally, annual and quarterly average, minimum and maximum precipitation data were derived from the Worldclim database (http://www.worldclim.org/). The data represent interpolated rainfall measures derived from the world-wide network of weather stations for the time period of 1950–2000 [171].
Table 1. Data sets used in the development of ecological niche models, including source and spatial resolution.
| Variables in the annual model | Source | Spatial resolution |
| Minimum Land Surface Temperatures (LST) | AVHRR | 5 km |
| Maximum LST | AVHRR | 5 km |
| Elevation | SRTM | 5 km |
| Minimum precipitation | WorldClim | 5 km |
| Maximum precipitation | WorldClim | 5 km |
| Mean Normalized Difference Vegetation Index (NDVI) | AVHRR | 5 km |
Table 2. The list of the data sets used in the development of seasonal ecological niche models including source and the spatial resolution. Seasonal model 1: Jan–Apr, 2; May–Aug and 3: Sep–Dec.
| Variables in the seasonal model | Source | Spatial resolution | Seasonal model |
| Average LST in the season | AVHRR | 5 km | 2, 3 |
| Average LST of the coldest month in the season | AVHRR | 5 km | 1, 3 |
| Average LST of the warmest month in the season | AVHRR | 5 km | 1 |
| Elevation | SRTM | 5 km | 1, 2, 3 |
| Precipitation total of the wettest month | WorldClim | 5 km | 1, 2, 3 |
| Precipitation total of the driest month | WorldClim | 5 km | 1, 2, 3 |
| Average quarterly NDVI in the season | AVHRR | 5 km | 1, 2, 3 |
All of the gridded datasets underwent a number of processing steps prior to being used in the modelling [162], [165], [172]. The size, location and extent have been matched for every layer. For datasets where the remotely sensed information was multi-temporal, Fourier analysis was used to ordinate the data by decomposing the temporal signal into an additive series of harmonics of different seasonal frequencies [162]–[165], [172]. All of the gridded datasets were resampled to produce matching extents and a grid cell size of 5 km×5 km. The layers used in the modelling here do not include locations above 59°N, as they were not available for all datasets.
All of the data layers were tested for pairwise Pearson correlation prior to building and running the model. Although MaxEnt is known to be a stable model in the face of correlated variables [173], those with the high correlation coefficients (r> = 0.85) were excluded from the analysis. These were average precipitation and LST in the general model, and average precipitation in the seasonal model. Average LST was removed from the first season, average LST of the coldest month was removed from the second season and the average LST of the warmest month remained as a covariate only in the first season model.
Choice of seasons
The year was divided into three Medfly-relevant seasons for seasonal mapping to strike a balance between ensuring that the seasonal variation in Medfly occurrence was captured, and having sufficient datapoints to produce reliable maps for each time period. Dividing year according to calendar seasons did not correspond well with the activity of Medflies, especially in the northern hemisphere, where the seasonal differences tend to be most pronounced due to a larger proportion of land located in higher latitudes. On the northern limits of the Medfly distribution, the pest tends to be inactive between January and April. It is unable to overwinter in egg or adult stage, but there is evidence of an ability to survive low temperatures in fruit hosts as a larvae and to a significantly lesser degree, in the pupal stage [18]. The onset of pest activity starts anywhere between May and August, depending on location and condition in a particular year. The peak of Medfly activity is observed in the fall – usually between September and November, and a sharp decline is observed between November and December. This led us to divide the year into three seasons (January–April, May–August and September–December), which is both significant from the phenological point of view for Medfly, and at the same time preserves some common environmental characteristics of the seasons.
Maximum entropy modelling
The Maximum Entropy Modelling tool (MaxEnt version 3.3.3 k) was used to map the potential distribution of Medfly. The model estimates species' potential distributions by finding the maximum entropy distribution, in other words, distribution closest to uniform [174], [175]. In the model, the environmental values found at the detection localities impose certain constraints on the output distribution. The constraints are expressed as simple functions of the environmental variables called features, and each feature in the model should have a mean close to the empirical average. The model looks for a set of probability distributions that satisfy the constraints and chooses the most unconstrained one [175]. In the logistic output of the model, every grid square has an assigned value between 0 and 100, which represents the relative suitability of species occurrence.
There were many reasons that dictated the choice of this model. Most importantly, in a review of 16 species modeling methods, MaxEnt was among the best performing methods when evaluated using the area under the curve (AUC) and correlation statistics [174]. Secondly, the method holds a strict mathematical definition and can accommodate diverse types of predictor variables - both categorical and continuous. Moreover, it does not require absence data, can handle a relatively low sample size and gives a simple to interpret continuous output. Finally, the method is well documented and available for free download (http://www.cs.princeton.edu/~schapire/maxent).
The default MaxEnt model parameters have been calibrated on a wide range of data (a convergence threshold of 10−5, a maximum iteration value of 500 and the maximum number of background points as 10000). These settings are recommended to achieve good model performance for species at ecological equilibrium [176]. Because Medfly is an invasive organism, we modified these settings to better predict the nature of the potential niche of an invader. The convergence threshold was left at the default of 10−5 and the number of iterations was increased to 5000 to allow the model adequate time for convergence and avoid under- or over-prediction of the relationships. We explored the choice of features and increased model regularization. MaxEnt allows various feature types to be used by default (if there are enough sample points on species presence available), which results in complex functions. Using less or only one feature is recommended for simpler models and we chose hinge features which allow the model to fit nonlinear functions of varying complexity, but without the sudden steps of threshold features [173]. Increased regularization parameters increase the degree of level smoothing, however the AUC score of our model was consequently declining with increased regularization. Therefore, we left the regularization setting at the default of 1. The output maps illustrate the mean results of the replicated runs. There are no precise scientific guidelines that dictate the choice of settings, so our choice was based on a visual assessment of their influence on the partial dependence plots, AUC scores and the prediction maps.
The logistic habitat-suitability output values were used for the model output which is simple to interpret (probability range of occurrence between 0 and 1). The model estimates the relative influence of each variable used in the prediction. It is scaled so the sum of the relative influence of each variable adds to 100, with higher numbers indicating stronger contribution on the outcome [177]. In the case where multiple Medfly occurrence points in our constructed database were registered at a single location, only one record was used in the MaxEnt modelling. Only points of occurrence and sources from 1980 onwards were taken into consideration to build the models.
By default, MaxEnt selects its own background samples from the entire study region, which implies that this entire space is available to species and surveillance [178]. Another option is to include a mask that will allow MaxEnt to choose a background sample only from pre-selected areas. In the case of Medfly these could be areas that are accessible for Medfly – where the species currently is present and no eradication efforts are currently ongoing, and no quarantine measures against Medfly are in place. It could also include areas that were accessible to Medfly over decades. Finally, the mask could help represent the sampling bias of Medfly occurrence records, however this was not feasible here as the data comes from various sources over long temporal scales. Since the goal of this study was to represent Medfly fundamental and not realized niche, and also how environmental variables favorable for its presence are changing according to the season, plus we only have political boundaries of Medfly current presence and not expert drawn distribution maps, we did not use a mask for the general potential niche model, but we applied a mask for the three seasonal models. The mask was obtained by using a probablility threshold of Medfly occurrence equal to or above 0.1 from the general model. It helped to avoid overprediction of the model and filter out areas that can only be suitable for Medfly occurrence during a few months of the year and therefore cannot sustain populations over climatically unfavorable months.
Model testing
The accuracy of the distribution models was evaluated by partitioning the data within MaxEnt into training (75%) and testing (25%) subsets and performing validation statistical analyses on each of the partitions. Each of the settings were run on 30 replicates using a subsample run type with a random seed, so that Maxent could average the results from all of the models created. Firstly, the area under the Receiver Operating Characteristic (ROC) was used to measure model performance. The plot of the ROC curve is illustrative of the ratio of correctly classified positives to the total number of positive cases (sensitivity) versus the false positive rate (specificity) at all thresholds of presence-absence classification. In this case, we do not have actual absence data in the study. Therefore, tests show whether the model classifies presence more accurately than a random prediction. The ROC plot for a model whose predictive ability is the equivalent of random assignment will lie near the diagonal, where the true positive rate equals the false positive rate for all thresholds. AUC is therefore a good measure of the overall model performance and has a possible range of 0–1, where 0 indicates that prediction is equal to a random assignment while an AUC score of 1 indicates a perfect presence-absence prediction. Secondly, a threshold-dependent binomial test of omission was performed. If in a specific cell we observe a value of 0.10 or above, that cell is classified as suitable for Medfly. This approach transforms the prediction output from continuous into binary. The number of Medfly suitable cells was compared to the number of cells known to have had Medfly presence. A one tailed binomial test was used to find out whether the model outperformed a random model predicting Medfly to be present in the same number of cells. MaxEnt provides test statistics for binomial tests for 10 different threshold values. The extrinsic omission rate represented the fraction of the test localities that were assigned into pixels which are not predicted as suitable for Medfly. Low omission rate is highly advisable for a good model [179].
Results
Occurrence data
The search for data on Medfly historical occurrence resulted in records from 43 countries and nearly 500 unique localities (Dataset S1). The oldest records come from 1898 and the most recent from 2011. 171 locations contained information about the year of Medfly occurrence, and 125 about a specific month where Medfly was observed. The majority of the records identified Medfly occurrence at the adult stage of development, with some in the pupae and larvae stage. Some of the most common hosts included apricot, guava, peach, various types of citrus (mainly varieties of orange and mandarine), apple, fig, peach, loquat and coffee. The dominant method of recording occurrence data was through food or Trimedlure baited traps (McPhail and Jackson, but also Nadel, Maxitrap, Steiner and Lynfield) [180].
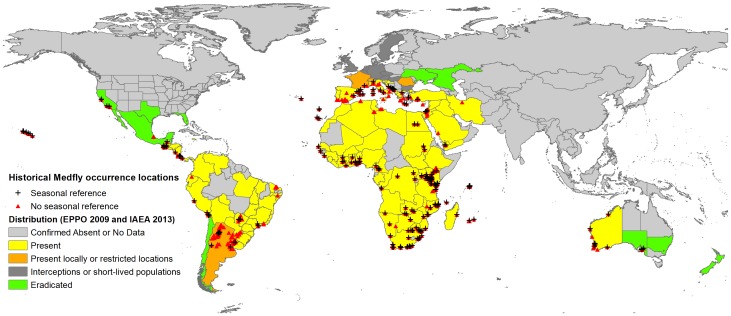
For the purpose of niche modelling, points with uncertain locations, duplicate points and those collected before 1980 were removed for the analysis. Additionally, the data were supplemented with occurrence points derived from the GBIF, BeBIF and MCA datasets, which further increased the total number of sample points available for niche modelling. In the annual model (produced using all geolocated points available), 463 unique occurrence points were used. For the seasonal models, 139 points were used for the January through April model, 158 for May through August and 157 for September through December. Figure 1 shows the locations of Medfly occurrence worldwide obtained from all of the data sources, starting from 1980 onwards. The points where information about Medfly occurrence after 1980 were available are marked in red (463 unique locations), and those marked with crosses are locations that had the month of record information on file (270 unique locations).
Figure 1. Occurrence data for Ceratitis capitata used in the study.
Data with information about the month of occurrence is marked with red triangles. Countries/regions where Medfly is present are coloured with yellow and where it is eradicated are marked with green [12], [13].
Environmental niche modelling
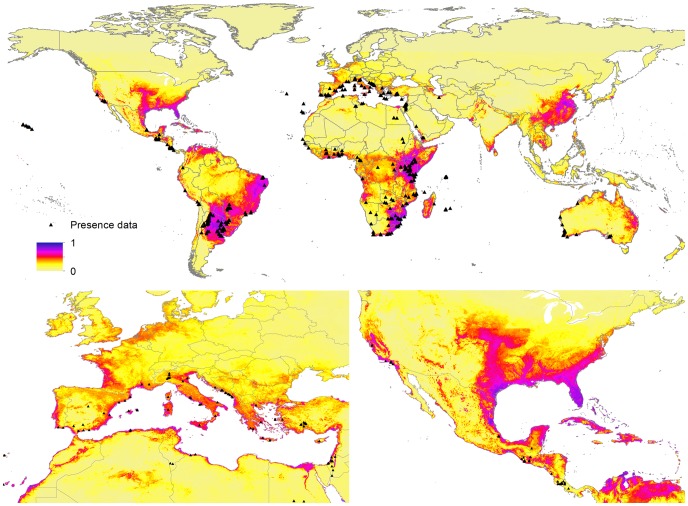
The annual Medfly niche suitability model, produced using all geolocated occurrence records since 1980, is presented in Figure 2. The largest suitable areas for Medfly presence are located in South America, east and south Africa and eastern Asia. Other suitable areas appear across a variety of climate zones, including warm temperate and semi-tropical and tropical, mostly in coastal areas. This incudes the Mediterranean basin, Gulf of Mexico, western coast of South America and coastal areas of India and Australia. The model prediction performs significantly better than random with a binomial test result of p<3.9−40. The AUC score for the training and testing datasets is 0.882 and 0.878 respectively, representing strong predictive performance, given the fractional predicted area of 0.307 (Table 3).
Figure 2. Global environmental suitability for C. capitata occurrence as predicted by MaxEnt model.
Black triangles represent presence points used in the modeling. Blue, purple and red colors show high confidence in predicted suitability, while yellow represents low confidence and predicted absence.
Table 3. Models were calibrated using training and test data (75% and 25% randomly selected occurrence points respectively).
| Model | General | Jan–Apr | May–Aug | Sep–Dec |
| No. of points | 463 | 139 | 158 | 157 |
| Mean training AUC | 0.882 | 0.891 | 0.866 | 0.881 |
| Mean test AUC | 0.878 | 0.855 | 0.848 | 0.853 |
| Test AUC standard deviation | 0.012 | 0.025 | 0.025 | 0.022 |
| Mean fractional predicted area | 0.307 | 0.376 | 0.335 | 0.336 |
| Training omission rate | 0.097 | 0.098 | 0.098 | 0.098 |
| Test omission rate | 0.118 | 0.165 | 0.130 | 0.161 |
| p value | 3.948−40 | 2.495−9 | 3.795−10 | 1.154−8 |
Area under the curve (AUC) was calculated as an average of 30 model replicate runs using subsample run type. Mean AUC and omission rate values were calculated both for test and training data. The mean omission rates are calculated at an arbitrarily chosen cumulative threshold of 10. All model omission results performed significantly better than random (p<0.0001).
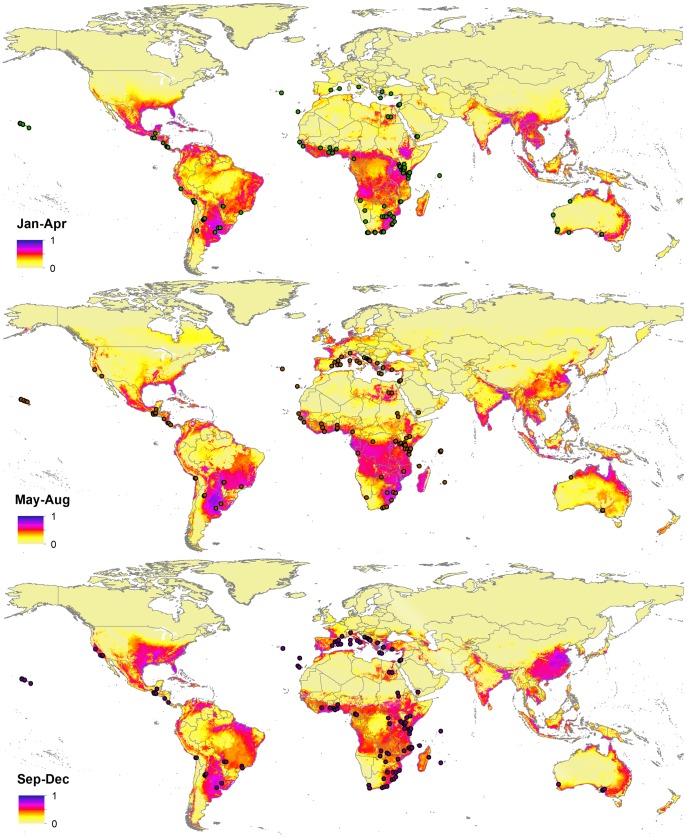
The January-April model has the highest fractional predicted area (0.376 for Jan–Apr, 0.335 for May–Aug, and 0.336 for Sept–Dec) (Figure 3). In that season, the least amount of land area in the Mediterranean basin is shown as suitable for Medfly. The area surrounding the Gulf of Mexico and Caribbean Basin, as well as the Pampa in Argentina and eastern Brazil are predicted to be highly suitable. In Africa, the highest suitability is observed in the Sahel belt, some parts of Abisynia and the southern part of the continent, including Madagascar. High suitability is also apparent in southeast Asia, where Medfly is not yet known to be established. The May–Aug season largely corresponds with summer in the northern hemisphere. Consequently, the largest proportions of areas in Europe, North America and Asia appear as suitable, compared to the other seasons. The expansion in predicted suitable range is also apparent in central Africa and northern Australia. In the Sept–Dec season, the suitability is largely contained in the Mediterranean basin in Europe, Southeast United States, but is expanded in the southern hemisphere. The AUC scores for the training data for all the seasons consistently exceed 0.86, while for the test data it remains above 0.84 (Figure S1, Table 3). As in the case of annual suitability model, the seasonal predictions return extremely low p values, indicating that the models perform significantly better than random (Table 3).
Figure 3. 3-panel seasonal maps showing the environmental suitability for C. capitata occurrence annually according to the MaxEnt model.
Dots represent the seasonal presence points used in the seasonal potential niche modelling. Blue, purple and red colors show high confidence in predicted suitability, while yellow represents low confidence and predicted absence.
Relative importance of predictor variables
Table 4 represents the relative influence of the variables on the model. The most significant environmental contributor appears to be temperature (minimum for the general model and Jan-Apr season and average for the Sep–Dec season). In May–Aug maximum precipitation appears as the most important predictor. These are followed by NDVI, DEM and minimum precipitation.
Table 4. Relative influence of the contribution of the variables to the model [%].
| Variable | General | Jan–Apr | May–Aug | Sep–Dec |
| Land Surface Temp (LST) minimum | 63.7 | 32.5 | - | 3.4 |
| LST maximum | 23.3 | 5.8 | - | - |
| Elevation (DEM) | 7.4 | 7.9 | 10.2 | 6.1 |
| Precipitation maximum | 2.8 | 5.9 | 24.7 | 14.5 |
| Normalized Difference Vegetation Index (NDVI) | 2.0 | 8.4 | 18.1 | 8.8 |
| Precipitation minimum | 0.8 | 9.1 | 3.5 | 1.1 |
| LST average | - | - | 4.2 | 17.4 |
| General Model suitability index >0.1 mask | - | 30.4 | 39.3 | 48.6 |
Discussion
The distribution model outputs represent the first global assessment of the seasonally changing potential distribution of Medfly, illustrating the significant shifts in environmental suitability that occurs throughout a typical year. Previous mapping has rarely addressed these seasonal variations, and the output maps provide a basis for global assessments of shifting invasion risks. Some areas identified in this study as highly suitable do not have Medfly populations established at present and this may be a result of either lack of introduction, eradication efforts or presence of another dominant species, such as in the case of eastern Australia, where the Queensland fruit fly has displaced Medfly [66]. The seasonal prediction maps reflect changes in the environmental suitability for Medfly, and it should be noted that while the insect may be able survive in the regions shown to be suitable for one or two of the three seasons mapped, it may likely not be able to become established, due to unsuitable conditions for the remainder of the year. In the locations close to the northern or southern boundary of Medfly distribution, the insect may be able to survive for one or several unusually warm seasons, and not be able to establish a stable population long-term.
The seasonal environmental niche mapping can be an important strategic tool for tailoring control and surveillance activities. With sufficient amounts of spatio-temporal data, the times of year when the pest is at its lowest activity stage can be identified and combined with information about commodity and passenger movements. It can facilitate prioritization and optimization of border surveillance efforts that are operating under limited resources and staffing. It can also be used to target interventions, enable or deny seasonal trade and be incorporated into risk assessments for commodity importation. This methodology, in principle, has the potential to be applied to any invasive insect species, or any organism subject to seasonal population dynamics and density. Combined with information about changes in seasonal movement of commodities at risk or passenger luggage, it could be adopted widely, both in the scientific and policy-making communities.
Our global annual suitability model presented here can be compared with previous published studies on Medfly range. One of the most recent ones was performed using two approaches: a genetic algorithm for rule-set prediction (GARP) and principal component analysis (PCA) [16]. A comprehensive native and non-native dataset on Medfly occurrence was compiled and used in modeling, together with eight environmental covariates consisting of temperature and precipitation parameters. In the model output areas of “high” and “low” suitability indicated by various shades of gray were presented, but thresholds for the division between them were not specified. The GARP model was judged to perform better by the authors; therefore we compare our results to the GARP output. It is noticeable that the MaxEnt annual model presented here tends to be more conservative and return a narrower range of Medfly suitability. It is particularly apparent in Africa and Australia. The models show less agreement in terms of suitability in Americas and good agreement on the suitability in Europe and Asia. Another previous study used CLIMEX to predict Medfly's suitable niche [17]. This model inferred the climatic conditions it can tolerate, based on the CLIMEX Growth Index. Additional modeling was then performed incorporating the effect of irrigation on Medfly abundance. Suitability was illustrated by 3 different suitability indices represented by various sized dots. In this case, the MaxEnt annual model presented here shows substantially closer agreement in terms of the most suitable areas in both the Americas and Europe, while it shows a more constrained suitability range in Africa, Australia and Asia. The study by Gutierrez and Ponti [19] represents a mechanistic approach to Medfly suitability range prediction. They developed a fine scale temperature driven and physiologically-based demographic model for Medfly in order to predict potential distribution in California, Arizona and Italy under the most recent climatic conditions for several individual years and under hypothetical climate warming. Results suggested that the climate in Arizona is outside of the climatic envelope for Medfly, whereas most of the Central Valley of California has marginal suitability, except in south coastal California. They conclude that continuous inter-annual suitability for Medfly could occur only in south coastal regions of the state. Our general Medfly suitability prediction defines a larger portion of California as being suitable – including coastal areas and the Central Valley, while the seasonal model outlined here defines the most suitable conditions as being in the third trimester. During the first trimester high altitude areas are defined as unsuitable, most likely due to low temperature and the second trimester defines most of California as moderately suitable. Stable suitability for Medfly is predicted in the southern part of the Italian peninsula, along the coast near Rome and on the plain of the Po River. Our model shows good agreement with the Gutierrez and Ponti [19] predictions here. Another MaxEnt prediction for Medfly was carried in the past, with default software settings with the exception of increased numbers of iterations. A smaller number of occurrence points was used and a wide range of climate predictors related to temperature and precipitation, without excluding those with high pairwise correlation values, was applied [20]. The model showed the highest suitability for Medfly in the southern part of China, whereas our model depicted another territory of potential invasion in the eastern part of the country.
The database constructed here includes a new level of detail regarding Medfly occurrence, including the sampling method, host type and relative abundance of Medfly in different months. In terms of data coverage, Medfly occurrence is relatively well documented in Mediterranean Europe, but most data from the Middle East comes from Israel, with little information available from other countries in the region. While there do exist comprehensive data on the native range of Medfly across most of Africa, we found few records from the northern part of the continent. Data collection for Central and South America resulted in generally sparse coverage, with Argentina being an exception, where many comprehensive studies were performed and a large amount of occurrence data are available. Only around 25% of records gathered included information about the month of Medfly occurrence. We have received information on Medfly detection locations for Hawaii and California, and no geo-referenced locations for Florida. Given the environmental sensitivity of the species and the resultant significant seasonality in distributions and abundance, future studies should ideally prioritize the collection and assembly of such valuable temporal information.
The seasonal suitability maps presented in this paper show considerably lower suitability for Medfly activity in the northern fringes of its distribution in the first 4 months of the year, which is in agreement with both gathered occurrence data, and previous studies on its seasonal dynamics in the region [3], [18]. During these months Medfly tends to overwinter as larvae in host fruits or at certain extension in a pupal stage in the ground. The adult activity is not present or is very limited. Most of the Mediterranean basin appears as unsuitable and significantly lower suitability is observed in California, where there is discussion about the pest's ability to overwinter. In May–Aug period, the suitability for Medfly extends well above its northern distribution limit in Europe, and a significantly higher suitability is observed in the Mediterranean basin. The highest suitability is observed in the last season, which corresponds well to previous studies on pest seasonal dynamics in the northern hemisphere. Lesser variability in the suitability for Medfly development is observed on the southern fringes on its distribution – especially in South America. More pronounced variability is observed in Australia, mostly in northern parts of the country, which lie outside of the current pest distribution. Few studies on the seasonal activity of Medfly outside of Europe exist and therefore it is hard to evaluate the results with the detection data. More pronounced variability in suitability is observed in near-equator or tropical locations, which might be a result of variability in precipitation and its effect on pupal development, and also host availability and phenology.
Significant uncertainties in the outputs presented here remain. The models have been built on the most comprehensive dataset of Medfly occurrence points yet assembled, but this still has a limited amount of data in many parts of the world. Moreover, the data are often lacking consistency in sampling methodology and possibly subject to errors in spatial and temporal referencing that are difficult to track. Further, while a detailed set of global seasonal environmental covariate datasets has been assembled and utilized in the modeling here, some additional factors could be taken into account in future studies, including humidity. Additionally, there are many factors that influence Medfly presence and abundance for which global spatial data do not exist – these include, for example, the distribution of competitor species, the distribution of host plants, control method coverage and produce movement patterns (natural and by fruit trade). Spatial data on these would likely improve modeling output fidelity and tackle some of the unexplained variance seen. Finer scale regional approaches might shed more light on local pest dynamics. Despite these caveats, the output maps represent the first attempt to model the global seasonal environmental suitability of one of the World's most economically damaging pest species.
Due to continuous efforts towards its elimination in many countries and trade and custom regulations that aim at reducing the risk of its importation, the presence of Medfly is not necessarily continuous across a region, but fragmented. To get a better picture of the seasonal aspects of Medfly activity, the outputs presented here need to be matched and adjusted to known Medfly suitability areas, where the pest could overwinter and become established. Future work will aim to tackle this and link the seasonal distribution maps presented here with seasonally changing commodity movement and human travel data to work towards building predictive models of Medfly importation risk and how it likely changes seasonally. The analyses presented here have shown how the suitability for Medfly changes throughout a typical year, but the riskiest movements of people and commodities for Medfly importation to suitable areas also change seasonally, and assessing reliably the risk of both Medfly importation and establishment should account for both. Such approaches can likely aid surveillance planning in prioritizing limited resources.
Conclusions
Very few studies exist on the seasonal modeling of species distributions, and these studies are usually performed on local or regional scales. For species that are highly sensitive to environmental conditions that display strong seasonal patterns in distributions and abundances, seasonal modeling of environmental suitability can be crucial in terms of understanding and predicting when and where a pest is most likely to be at the peak of its population activity, potentially informing targeted surveillance at borders. Continued effort in gathering information about Medfly detection locations, not only in terms of spatial occurrence, but recording activity on seasonal scales, can serve as a tool to understand the spatio-temporal population dynamics of the species. Increasing numbers of tools are available to model species potential distributions, and with finer resolutions of global spatial environmental datasets as well as ever increasing computing power to handle such large datasets more accurate prediction of species potential distributions can likely be performed. Even the most robust methods however, are limited in their performance where occurrence data is incomplete or scarce. Continued efforts to document Medfly and other pest species occurrence and make such records available are therefore essential for improvement of our understanding and prediction of their distributions.
Supporting Information
ROC curves. Red lines represent mean AUC and mean +/− one standard deviation (blue field). The lines show the “fit” of the model to the training and test data. Black line represents random prediction. The red line can be considered to show the real test of the models predictive power [175].
(DOC)
Medfly occurrence dataset.
(XLSX)
Acknowledgments
We thank our collaborators: Dr. Roger Magarey (North Carolina State University), Dr. Manuel Colunga-Garcia (Michigan State University), Dr. Daniel Borchert (USDA-APHIS-PPQ-CPHST), Dr. Norman Leppla, Dr. Peter Waylen, Dr. Michael Binford (University of Florida) and Paul Hornby (USDA-APHIS-PPQ). Also, we thank Dr. Peter Gething (University of Oxford) for help in assembly of the environmental grids used in the study.
Funding Statement
The study was financed by the USDA-APHIS-PPQ-CPHST Cooperative Agreement No. 12-8130-0158-CA titled “Construction of Multi-Disciplinary Tools to Assess Seasonal Risk of Mediterranean Fruit Fly Ceratitis Capitata (Medfly) importation to Florida and California.” AJT is supported by funding from NIH/NIAID (U19AI089674), the Bill & Melinda Gates Foundation (OPP110642749446, 1032350), and the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Riccardo Scalera, Genovesi P, Franz Essl, Rabitsch W (2012) The impacts of invasive alien species in Europe. Technical Report. Luxembourg: European Environmental Agency. Available: http://www.eea.europa.eu/publications/impacts-of-invasive-alien-species.
- 2. Liquido NJ, Cunningham RT, Nakagawa S (1990) Host Plants of Mediterranean Fruit Fly (Diptera: Tephritidae) on the Island of Hawaii (1949–1985 Survey). J Econ Entomol 83: 1863–1878. [Google Scholar]
- 3. Papadopoulos NT, Katsoyannos BI, Carey JR, Kouloussis NA (2001) Seasonal and Annual Occurrence of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Northern Greece. Ann Entomol Soc Am 94: 41–50 10.1603/0013-8746(2001)0940041:SAAOOT2.0.CO2 [DOI] [PubMed] [Google Scholar]
- 4. Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Hendrichs J, Carey JR, et al. (2001) Early Detection and Population Monitoring of Ceratitis capitata (Diptera: Tephritidae) in a Mixed-Fruit Orchard in Northern Greece. J Econ Entomol 94: 971–978. [DOI] [PubMed] [Google Scholar]
- 5. Gasperi G, Bonizzoni M, Gomulski L, Murelli V, Torti C, et al. (2002) Genetic differentiation, gene flow and the origin of infestations of the medfly, Ceratitis capitata. Genetica 116: 125–135. [DOI] [PubMed] [Google Scholar]
- 6. Carey JR (1996) The Incipient Mediterranean Fruit Fly Population in California: Implications for Invasion Biology. Ecology 77: 1690–1697 10.2307/2265775 [DOI] [Google Scholar]
- 7.Jang EB (2007) Fruit flies and their impact on agriculture in Hawaii. Available: http://scholarspace.manoa.hawaii.edu/handle/10125/1297. Accessed 21 November 2011.
- 8.APHIS (1992) Risk assessment, Mediterranean fruit fly. Washington D.C.: Planning and Risk Analysis Systems. Policy and Program Development. Animal and Plant Health Inspection Service USDA.
- 9.Cross E (2004) Mediterranean fruit flies attempt to sneak in—again. Cust Bord Prot TODAY.
- 10. Siebert JB, Cooper T (1995) If medfly infestation triggered a trade ban: Embargo on California produce would cause revenue, job loss. Calif Agric 49: 7–12 10.3733/ca.v049n04p7 [DOI] [Google Scholar]
- 11. Enkerlin W, Mumford J (1997) Economic Evaluation of Three Alternative Methods for Control of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Israel, Palestinian Territories, and Jordan. J Econ Entomol 90: 1066–1072. [Google Scholar]
- 12.IAEA (2013) Tephritid Workers Database. Available: http://nucleus.iaea.org/sites/naipc/twd/Pages/default.aspx.
- 13.EPPO (2001) PQR - EPPO database on quarantine pests. Available: http://www.eppo.int.
- 14.EPPO (2009) Data Sheets on Quarantine Pests: Ceratitis capitata. Available: http://www.eppo.int/QUARANTINE/insects/Ceratitis_capitata/CERTCA_ds.pdf.
- 15.CABI (2000) Ceratitis capitata. Distribution Maps of Plant Pests. Available: http://www.cabi.org/isc/?compid=5&dsid=12367&loadmodule=datasheet&page=481&site=144.
- 16. De Meyer M, Robertson MP, Peterson AT, Mansell MW (2008) Ecological niches and potential geographical distributions of Mediterranean fruit fly (Ceratitis capitata) and Natal fruit fly (Ceratitis rosa). J Biogeogr 35: 270–281 10.1111/j.1365-2699.2007.01769.x [DOI] [Google Scholar]
- 17. Vera MT, Rodriguez R, Segura DF, Cladera JL, Sutherst RW (2002) Potential Geographical Distribution of the Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae), with Emphasis on Argentina and Australia. Environ Entomol 31: 1009–1022 10.1603/0046-225X-31.6.1009 [DOI] [Google Scholar]
- 18. Papadopoulos NT, Carey JR, Katsoyannos BI, Kouloussis NA (1996) Overwintering of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Northern Greece. Ann Entomol Soc Am 89: 526–534. [Google Scholar]
- 19.Gutierrez AP, Ponti L (2011) Assessing the invasive potential of the Mediterranean fruit fly in California and Italy. Biol Invasions. Available: http://www.springerlink.com/content/x148rk17p78t62w3/. Accessed 2011 Oct 10.
- 20. Li B, Ma J, Hu X, Liu H, Zhang R (2009) Potential Geographical Distributions of the Fruit Flies Ceratitis capitata, Ceratitis cosyra, and Ceratitis rosa in China. J Econ Entomol 102: 1781–1790 10.1603/029.102.0508 [DOI] [PubMed] [Google Scholar]
- 21. Escudero-Colomar LA, Vilajeliu M, Batllori L (2008) Seasonality in the occurrence of the Mediterranean fruit fly [Ceratitis capitata (Wied.)] in the north-east of Spain. J Appl Entomol 132: 714–721 10.1111/j.1439-0418.2008.01372.x [DOI] [Google Scholar]
- 22. Harris EJ, Vargas RI, Gilmore JE (1993) Seasonality in Occurrence and Distribution of Mediterranean Fruit Fly (Diptera: Tephritidae) in Upland and Lowland Areas on Kauai, Hawaii. Environ Entomol 22: 404–410. [Google Scholar]
- 23. Harris EJ, Olalquiaga G (1991) Occurrence and Distribution Patterns of Mediterranean Fruit Fly (Diptera: Tephritidae) Desert Areas in Chile and Peru. Environ Entomol 20: 174–178. [Google Scholar]
- 24. Israely N, Yuval B, Kitron U, Nestel D (1997) Population Fluctuations of Adult Mediterranean Fruit Flies (Diptera: Tephritidae) in a Mediterranean Heterogeneous Agricultural Region. Environ Entomol 26: 1263–1269. [Google Scholar]
- 25. Katsoyannos BI, Kouloussis NA, Carey JR (1998) Seasonal and Annual Occurrence of Mediterranean Fruit Flies (Diptera: Tephritidae) on Chios Island, Greece: Differences Between Two Neighboring Citrus Orchards. Ann Entomol Soc Am 91: 43–51. [Google Scholar]
- 26. Maelzer DA, Bailey PT, Perepelicia N (2004) Factors supporting the non-persistence of fruit fly populations in South Australia. Aust J Exp Agric 44: 109 10.1071/EA01128 [DOI] [Google Scholar]
- 27. Martínez-Ferrer MT, Navarro C, Campos JM, Marzal C, Fibla JM, et al. (2010) Seasonal and annual trends in field populations of Mediterranean fruit fly, Ceratitis capitata, in Mediterranean citrus groves: comparison of two geographic areas in eastern Spain. Span J Agric Res 8: 757–765. [Google Scholar]
- 28. Mavrikakis PG, Economopoulos AP, Carey JR (2000) Continuous Winter Reproduction and Growth of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Heraklion, Crete, Southern Greece. Environ Entomol 29: 1180–1187 10.1603/0046-225X-29.6.1180 [DOI] [Google Scholar]
- 29. Papadopoulos NT, Katsoyannos BI, Carey JR (1998) Temporal Changes in the Composition of the Overwintering Larval Population of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Northern Greece. Ann Entomol Soc Am 91: 430–434. [Google Scholar]
- 30. Abdel-Galil FA, Amro MA, Abdel-Moniem ASH, El-Fandary OO (2009) Population fluctuations and interspecific competition between Tephritid flies attacking fruit crops in the New Valley oases, Egypt. Arch Phytopathol Plant Prot 43: 647–659 10.1080/03235400802021272 [DOI] [Google Scholar]
- 31.Aguiar EL, Menezes EB (1996) Population dynamics of fruit flies in Itaguai County, State of Rio de Janeiro, Brazil. I. Survey of the species. Available: http://agris.fao.org/agris-search/search/display.do?f=1996%2FUS%2FUS96199.xml%3BUS9613307. Accessed 2012 June 1.
- 32. Aguiar-Menezes EL, Menezes EB, Silva PS, Bittar AC, Cassino PCR (2001) Native Hymenopteran Parasitoids Associated with Anastrepha spp. (Diptera: Tephritidae) in Seropedica City, Rio de Janeiro, Brazil. Fla Entomol 84: 706–711 10.2307/3496405 [DOI] [Google Scholar]
- 33.Aguirre CL (1997) Distribución y registros de las principales especies de moscas de las frutas(diptera. IICA Biblioteca Venezuela. 72 p.
- 34. Alemany A, Miranda MA, Alonso R, Martın Escorza C (2004) Efectividad del trampeo masivo de hembras de Ceratitis capitata (Diptera: Tephritidae) a base de atrayentes alimentarios.“‘Efecto-borde’”y papel de los frutales abandonados como potenciadores de la plaga. Bol San Veg Plagas 30: 255–264. [Google Scholar]
- 35. Aluja M, Sivinski J, Rull J, Hodgson PJ (2005) Behavior and predation of fruit fly larvae (Anastrepha spp.)(Diptera: Tephritidae) after exiting fruit in four types of habitats in tropical Veracruz, Mexico. Environ Entomol 34: 1507–1516. [Google Scholar]
- 36. Argov Y, Gazit Y (2008) Biological control of the Mediterranean fruit fly in Israel: Introduction and establishment of natural enemies. Biol Control 46: 502–507 10.1016/j.biocontrol.2008.04.021 [DOI] [Google Scholar]
- 37.Augier L, Gastaminza, Villagrán ME, Villagrán MF, Zaia G, et al. (2007) Fruit flies adults monitoring in lemon orchards. Tucumán, Argentina: Estacion Experimental Agroindustrial Obispo Columbres (EEAOC).
- 38. Avery JW, Chambers DL, Cunningham RT, Leonhardt BA (1994) Use of ceralure and trimedlure in Mediterranean fruit fly (Diptera: Tephritidae) mass-trapping tests. J Entomol Sci 29: 543–556. [DOI] [PubMed] [Google Scholar]
- 39. Baker RHA, Sansford CE, Jarvis CH, Cannon RJC, MacLeod A, et al. (2000) The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agric Ecosyst Environ 82: 57–71 10.1016/S0167-8809(00)00216-4 [DOI] [Google Scholar]
- 40.Bariani R, Casarini V, Canobbio G, Fredditori M, Inversini M, et al. (n.d.)Osservazioni sulla biologia e sul comportamento della Ceratitis capitata nell'Oltrepo pavese e zone limitrofe.
- 41. Barr NB (2009) Pathway Analysis of Ceratitis capitata (Diptera: Tephritidae) Using Mitochondrial DNA. J Econ Entomol 102: 401–411 10.1603/029.102.0153 [DOI] [PubMed] [Google Scholar]
- 42. Batllori L, Escudero A, Vilajeliu M, Garcia F, Benejam J (2008) Area-wide mass trapping to control Ceratitis capitata (Wied.) on stone fruits in Girona, NE of Spain. Integrated Plant Protection in Stone Fruit. IOBCwprs Bull 37: 73–82. [Google Scholar]
- 43.Bjeliš M, Radunić D, Masten T, Kotlar A (2007) Spatial distribution and temporal outbreaks of medfly Ceratitis capitata Wied.(Diptera, Tephritidae) in Republic of Croatia. 8th Slovenian conference on plant protection.
- 44.Bjeliš M, Ljubetić V, Novosel N (2006) Control of Medfly by SIT in the Nereva River Valley. International symposium on fruit flies of economic importance: from basic to applied knowledge; Salvador, BA (Brazil).
- 45.Bjeliš M, Pelicarić V (2004) Tephritid fruit fly pests in Croatia: an overview of damage and current control strategies. Isteg Scientific Publications. pp. 325–329.
- 46.Bloem K, Chambers D, Bloem S, Muniz E (1993) Relative effectiveness of Jackson and McPhail traps: a year-long comparison in coffee in Guatemala. Springer Verlag.
- 47. Bohonak AJ, Davies N, Villablanca FX, Roderick GK (2001) Invasion genetics of New World medflies: testing alternative colonization scenarios. Biol Invasions 3: 103–111. [Google Scholar]
- 48. Bonizzoni M, Guglielmino CR, Smallridge CJ, Gomulski M, Malacrida a R, et al. (2004) On the origins of medfly invasion and expansion in Australia. Mol Ecol 13: 3845–3855 10.1111/j.1365-294X.2004.02371.x [DOI] [PubMed] [Google Scholar]
- 49. Borge MN-R, Basedow T (1997) A Survey on the Occurrence and Flight Periods of Fruit Fly Species (Diptera: Tephritidae) in a Fruit Growing Area in Southwest Nicaragua, 1994/95. Bull Entomol Res 87: 405–412 10.1017/S000748530003741X [DOI] [Google Scholar]
- 50. Cáceres C, Ramírez E, Wornoayporn V, Mohammad Islam S, Ahmad S (2007) A protocol for storage and long-distance shipment of Mediterranean fruit fly (DipteraL Tephritidae) eggs. I. Effect of temperature, embryo age, and storage time on survival and quality. Fla Entomol 90: 103–109 10.1653/0015-4040(2007)90103:APFSAL2.0.CO2 [DOI] [Google Scholar]
- 51.Callejas C, Ochando MD (2004) SOD sequences in Ceratitis capitata (Diptera: Tephritidae) samples from different geographic origins. Isteg Scientific Publications. pp. 435–438.
- 52. Carey (1991) Establishment of the Mediterranean fruit fly in California. Science 253: 1369–1373 10.1126/science.1896848 [DOI] [PubMed] [Google Scholar]
- 53. Carey JR (October) The future of the Mediterranean fruit fly Ceratitis capitata invasion of California: A predictive framework. Biol Conserv 78: 35–50 10.1016/0006-3207(96)00016-X [DOI] [Google Scholar]
- 54. Carey JR (2011) Biodemography of the Mediterranean fruit fly: Aging, longevity and adaptation in the wild. Exp Gerontol 46: 404–411 10.1016/j.exger.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carey JR, Papadopoulos NT, Müller H, Katsoyannos BI, Kouloussis NA, et al. (2008) Age structure changes and extraordinary lifespan in wild medfly populations. Aging Cell 7: 426–437 10.1111/j.1474-9726.2008.00390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cayol JP, Rössler Y, Weiss M, Bahdousheh M, Omari M, et al. (2004) Fruit fly control and monitoring in the Near East: shared concern in a regional transboundary problem. Isteg Scientific Publications pp. 155–171. [Google Scholar]
- 57. Cayol JP, Causse R (2009) Mediterranean fruit fly Ceratitis capitata Wiedemann (Dipt., Trypetidae) back in Southern France. J Appl Entomol 116: 94–100 10.1111/j.1439-0418.1993.tb01172.x [DOI] [Google Scholar]
- 58. Celedonio-Hurtado H, Aluja M, Liedo P (1995) Adult Population Fluctuations of Anastrepha Species (Diptera: Tephritidae) in Tropical Orchard Habitats of Chiapas, Mexico. Environ Entomol 24: 861–869. [Google Scholar]
- 59. Cohen Y, Cohen A, Hetzroni A, Alchanatis V, Broday D, et al. (2008) Spatial decision support system for Medfly control in citrus. Comput Electron Agric 62: 107–117 10.1016/j.compag.2007.12.005 [DOI] [Google Scholar]
- 60. Copeland RS, Wharton RA, Luke Q, De Meyer M (2002) Indigenous Hosts of Ceratitis capitata (Diptera:Tephritidae) in Kenya. Ann Entomol Soc Am 95: 672–694 10.1603/0013-8746(2002)0950672:IHOCCD2.0.CO2 [DOI] [Google Scholar]
- 61. Dantas L, Pereira R, Silva N, Rodrigues A, Costa R (2004) The SIT control programme against Medfly on Madeira Island. Isteg Scientific Publications pp. 127–130. [Google Scholar]
- 62. Davies N, Villablanca FX, Roderick GK (1999) Bioinvasions of the Medfly Ceratitis Capitata: Source Estimation Using DNA Sequences at Multiple Intron Loci. Genetics 153: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. De Lima C (2007) Area Wide Management of Mediterranean Fruit Fly in Australia. VIII International Symposium on Modelling in Fruit Research and Orchard Management 803 pp. 51–60. [Google Scholar]
- 64. Demirel N (2007) Behavior Paradigms in the Mediterranean Fruit Fly. J Entomol 4: 129–135. [Google Scholar]
- 65. Diamantidis AD, Carey JR, Nakas CT, Papadopoulos NT (2011) Ancestral populations perform better in a novel environment: domestication of Mediterranean fruit fly populations from five global regions. Biol J Linn Soc 102: 334–345 10.1111/j.1095-8312.2010.01579.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dominiak BC, Daniels D (2012) Review of the past and present distribution of Mediterranean fruit fly (Ceratitis capitata Wiedemann) and Queensland fruit fly (Bactrocera tryoni Froggatt) in Australia. Aust J Entomol 51: 104–115 10.1111/j.1440-6055.2011.00842.x [DOI] [Google Scholar]
- 67. Duyck P, David P, Quilici S, Duyck P, David P, et al. (2006) Climatic niche partitioning following successive invasions by fruit flies in La Réunion, Climatic niche partitioning following successive invasions by fruit flies in La Réunion. J Anim Ecol J Anim Ecol 75 75: 518 –526 10.1111/j.1365-2656.2006.01072.x,10.1111/j.1365-2656.2006.01072.x [DOI] [PubMed] [Google Scholar]
- 68. Duyck PF, Quilici S (2002) Survival and Development of Different Life Stages of Three Ceratitis Spp. (Diptera: Tephritidae) Reared at Five Constant Temperatures. Bull Entomol Res 92: 461–469 10.1079/BER2002188 [DOI] [PubMed] [Google Scholar]
- 69. El Keroumi A, Naamani K, Dahbi A, Luque I, Carvajal A, et al. (2010) Effect of ant predation and abiotic factors on the mortality of medfly larvae, Ceratitis capitata, in the Argan forest of Western Morocco. Biocontrol Sci Technol 20: 751–762 10.1080/09583151003734651 [DOI] [Google Scholar]
- 70. El-Hamalawii AS (2004) The Population Dynamics of the Mediterranean Fruit Fly, Ceratitis capitata Wied. Diptera: Tephritidae in Some Fruit Orchards in Gaza Strip. -Najah Univ J Res 18: 249–265. [Google Scholar]
- 71. Eskafi F (1990) Parasitism of fruit flies Ceratitis capitata and Anastrepha SPP. [Diptera: Tephritidae] in Guatemala. BioControl 35: 355–362 10.1007/BF02375259 [DOI] [Google Scholar]
- 72. Eskafi F, Kolbe M (1990) Infestation Patterns of Commonly Cultivated, Edible Fruit Species by Ceratitis-Capitata and Anastrepha Spp (diptera, Tephritidae) in Guatemala and Their Relationship to Environmental-Factors. Environ Entomol 19: 1371–1380. [Google Scholar]
- 73. Eskafi F, Kolbe M (1990) Predation on Larval and Pupal Ceratitis-Capitata (diptera, Tephritidae) by the Ant Solenopsis-Geminata (Hymenoptera, Formicidae) and Other Predators in Guatemala. Environ Entomol 19: 148–153. [Google Scholar]
- 74.Fimiani P (1989) Mediterranean region. Fruit Flies Their Biol Nat Enemies Control Amst Elsevier: 39–50.
- 75.Fischer-Colbrie P, Busch-Petersen E (1989) Temperate Europe and West Asia. In: Robinson AS, Hooper G, editors. Fruit Flies: their Biology, Natural Enemies and Control. New York: Elsevier. pp. 91–100.
- 76. Gasparich GE, Silva JG, Han H-Y, McPheron BA, Steck GJ, et al. (1997) Population Genetic Structure of Mediterranean Fruit Fly (Diptera: Tephritidae) and Implications for Worldwide Colonization Patterns. Ann Entomol Soc Am 90: 790–797. [Google Scholar]
- 77. Gevrey M, Worner SP (2006) Prediction of Global Distribution of Insect Pest Species in Relation to Climate by Using an Ecological Informatics Method. J Econ Entomol 99: 979–986 10.1603/0022-0493-99.3.979 [DOI] [PubMed] [Google Scholar]
- 78. Grout TG, Stoltz KC (2007) Developmental Rates at Constant Temperatures of Three Economically Important Ceratitis spp. (Diptera: Tephritidae) From Southern Africa. Environ Entomol 36: 1310–1317 10.1603/0046-225X(2007)361310:DRACTO2.0.CO2 [DOI] [PubMed] [Google Scholar]
- 79. He M, Haymer DS, He M, Haymer DS (1999) Genetic relationships of populations and the origins of new infestations of the Mediterranean fruit fly, Genetic relationships of populations and the origins of new infestations of the Mediterranean fruit fly. Mol Ecol Mol Ecol 8 8: 1247 –1257 10.1046/j.1365-294X.1999.00685.x,10.1046/j.1365-294X.1999.00685.x [DOI] [PubMed] [Google Scholar]
- 80. Hedström I (1993) Population dynamics and host relationships of neotropical fruit flies (Diptera: Tephritidae) in seasonal and non-seasonal environments. Int J Pest Manag 39: 400–410 10.1080/09670879309371831 [DOI] [Google Scholar]
- 81. Hunt MK, Nicholls CJ, Wood RJ, Rendon AP, Gilburn AS (2004) Sexual selection for symmetrical male medflies (Diptera: Tephritidae) confirmed in the field. Biol J Linn Soc 81: 347–355 10.1111/j.1095-8312.2003.00300.x [DOI] [Google Scholar]
- 82. Israely N, Ritte U, Oman SD (2004) Inability of Ceratitis capitata (Diptera: Tephritidae) to Overwinter in the Judean Hills. J Econ Entomol 97: 33–42 10.1603/0022-0493-97.1.33 [DOI] [PubMed] [Google Scholar]
- 83. Israely N, Ziv Y, Oman SD (2005) Spatiotemporal Distribution Patterns of Mediterranean Fruit Fly (Diptera: Tephritidae) in the Central Region of Israel. Ann Entomol Soc Am 98: 77–84 10.1603/0013-8746(2005)0980077:SDPOMF2.0.CO2 [DOI] [Google Scholar]
- 84. Israely N, Oman SD (2005) Effect of Combined Insecticide Sprays and Sanitation Techniques on Population Dynamics of Ceratitis capitata (Diptera: Tephritidae) in the Central Mountains of Israel. J Econ Entomol 98: 739–748 10.1603/0022-0493-98.3.739 [DOI] [PubMed] [Google Scholar]
- 85. Katsoyannos BI, Heath RR, Papadopoulos NT, Epsky ND, Hendrichs J (1999) Field Evaluation of Mediterranean Fruit Fly (Diptera: Tephritidae) Female Selective Attractants for Use in Monitoring Programs. J Econ Entomol 92: 583–589. [Google Scholar]
- 86.Korneyev VA (2003) New and Little-Known Tephritidae (Diptera, Cyclorrhapha) from Europe. Нoвыеи малoизвестные Tephritidae (Diptera, Cyclorrhapha) из Еврoпы. Available: http://194.44.242.245:8080/handle/123456789/3781. Accessed 2012 April 26.
- 87. Katsoyannos BI, Papadopoulos NT, Kouloussis NA, Hendrichs J (2004) Effect of citrus peel substances on male Mediterranean fruit fly behaviour. Isteg Scientific Publications pp. 13–17. [Google Scholar]
- 88. Kourti A, Loukas M, Sourdis J (2011) Dispersion pattern of the medfly from its geographic centre of origin and genetic relationships of the medfly with two close relatives. Entomol Exp Appl 63: 63–69 10.1111/j.1570-7458.1992.tb02420.x [DOI] [Google Scholar]
- 89. Kwasi W (2009) Assessment of Fruit Fly Damage and Implications for the Dissemination of Management Practices for Mango Production in the Upper West Region of Ghana. J Dev Sustain Agric 3: 117–134. [Google Scholar]
- 90. Lanzavecchia SB, Cladera JL, Faccio P, Petit Marty N, Vilardi JC, et al. (2008) Origin and Distribution of Ceratitis capitata Mitochondrial DNA Haplotypes in Argentina. Ann Entomol Soc Am 101: 627–638 10.1603/0013-8746(2008)101627:OADOCC2.0.CO2 [DOI] [Google Scholar]
- 91. Leza MM, Juan A, Capllonch M, Alemany A (2008) Female-biased mass trapping vs. bait application techniques against the Mediterranean fruit fly, Ceratitis capitata (Dipt., Tephritidae). J Appl Entomol 132: 753–761 10.1111/j.1439-0418.2008.01370.x [DOI] [Google Scholar]
- 92. Larcher-Carvalho A, Mumford J (2004) Cost-benefit analysis for the suppression of the Mediterranean fruit fly in the Algarve using the sterile insect technique. Isteg Scientific Publications pp 143–153. [Google Scholar]
- 93. Livingston MJ (2007) The Mediterranean Fruit Fly: Efficient Dynamic and Static Phytosanitary Measures, Information Values, and Current Policy. 2007 Annual Meeting, July 29-August 1, 2007, Portland, Oregon TN [Google Scholar]
- 94. Lysandrou M (2009) Fruit flies in the mediterranean and Arab world: how serious a threat are they and how can we minimize their impact. Arab J Plant Prot 27: 236–239. [Google Scholar]
- 95. Malacrida AR, Marinoni F, Torti C, Gomulski LM, Sebastiani F, et al. (1998) Genetic Aspects of the Worldwide Colonization Process of Ceratitis Capitata. J Hered 89: 501–507 10.1093/jhered/89.6.501 [DOI] [PubMed] [Google Scholar]
- 96. Malacrida AR, Gomulski LM, Bonizzoni M, Bertin S, Gasperi G, et al. (2006) Globalization and fruitfly invasion and expansion: the medfly paradigm. Genetica 131: 1–9 10.1007/s10709-006-9117-2 [DOI] [PubMed] [Google Scholar]
- 97.McPheron BA, Steck GJ (1996) Fruit Fly Pests: A World Assessment of Their Biology and Management. CRC Press. 612 p.
- 98. Meats A, Smallridge CJ (2007) Short- and long-range dispersal of medfly, Ceratitis capitata (Dipt., Tephritidae), and its invasive potential. J Appl Entomol 131: 518–523 10.1111/j.1439-0418.2007.01168.x [DOI] [Google Scholar]
- 99.Medeiros A, Oliveira L, Garcia P (2007) Suitability as Medfly Ceratitis capitata (Diptera, Tephritidae) hosts, of seven fruit species growing on the island of São Miguel, Azores. Arquipél Ciênc Biológicas E Mar: 33–40.
- 100. Meixner MD, McPheron BA, Silva JG, Gasparich GE, Sheppard WS (2002) The Mediterranean fruit fly in California: evidence for multiple introductions and persistent populations based on microsatellite and mitochondrial DNA variability. Mol Ecol 11: 891–899 10.1046/j.1365-294X.2002.01488.x [DOI] [PubMed] [Google Scholar]
- 101. Michelakis SE (1992) Phenology of the Mediterranean fruit fly, Ceratitis capitata Wiedemann in Crete. Isr J Entomol 25: 177–180. [Google Scholar]
- 102. Merz B, Dawah HA (2005) Fruit flies (Diptera, Tephritidae) from Saudi Arabia, with descriptions of a new genus and six new species. Rev Suisse Zool 112: 983–1028. [Google Scholar]
- 103.Midgarden D, Lira E (2006) Ecological relationship of medfly and coffee in Guatemala and Mexico. Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance. pp. 10–15.
- 104. Mirsardoo S, Mafi PS, Barari H (2010) Preliminary investigation on the geographical distribution of Mediterranean fruit fly, Ceratitis capitata (Wiedemann)(Dip., Tephritidae), in Mazandaran province, Iran. J Entomol Res 2: 143–154. [Google Scholar]
- 105. Mkize N, Hoelmer KA, Villet MH (2008) A survey of fruit-feeding insects and their parasitoids occurring on wild olives, Olea europaea ssp. cuspidata, in the Eastern Cape of South Africa. Biocontrol Sci Technol 18: 991–1004 10.1080/09583150802450154 [DOI] [Google Scholar]
- 106. Mohamed SA, Wharton RA, Mérey G von, Schulthess F (2006) Acceptance and suitability of different host stages of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) and seven other tephritid fruit fly species to Tetrastichus giffardii Silvestri (Hymenoptera: Eulophidae). Biol Control 39: 262–271 10.1016/j.biocontrol.2006.08.016 [DOI] [Google Scholar]
- 107. Morales P, Cermeli M, Godoy F, Salas B (n.d.) A list of Mediterranean fruit fly Ceratitis capitata Wiedemann (Diptera: Tephritidae) host plants based on the records of INIA-CENIAP Museum of Insects of Agricultural Interest. Entomotropica 19: 51–54. [Google Scholar]
- 108. Müller H-G, Wu S, Diamantidis AD, Papadopoulos NT, Carey JR (2009) Reproduction is adapted to survival characteristics across geographically isolated medfly populations. Proc R Soc B Biol Sci 276: 4409–4416 10.1098/rspb.2009.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nyamukondiwa C, Terblanche JS (2009) Thermal tolerance in adult Mediterranean and Natal fruit flies (Ceratitis capitata and Ceratitis rosa): Effects of age, gender and feeding status. J Therm Biol 34: 406–414 10.1016/j.jtherbio.2009.09.002 [DOI] [Google Scholar]
- 112.Nestel D, Katsoyannos B, Nemny-Lavy E, Mendel Z, Papadopoulos N (2004) Spatial analysis of Medfly populations in heterogeneous landscapes. Isteg Scientific Publications. pp. 35–43.
- 113. Oroño LE, Albornoz-Medina P, Núñez-Campero S, Van Nieuwenhove GA, Bezdjian LP, et al. (2006) Update of host plant list of Anastrepha fraterculus and Ceratitis capitata in Argentina. Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance pp. 10–15. [Google Scholar]
- 114.Oukil S, Bues R, Causse R, Toubon JF (1997) Etude de la variabilite genetique chez 6 populations de Ceratitis capitata dans differentes zones geographiques du bassin Mediterraneen. Bull OILBSROP 20.
- 115. Ovruski S, Schliserman P, Aluja M (2003) Native and Introduced Host Plants of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in Northwestern Argentina. J Econ Entomol 96: 1108–1118 10.1603/0022-0493-96.4.1108 [DOI] [PubMed] [Google Scholar]
- 116. Ovruski SM, Schliserman P, Oroño LE, Nuñéz-Campero SR, Albornoz-Medina P, et al. (2008) Natural Ocurrence of Hymenopterous Parasitoids Associated with Anastrepha fraterculus (Diptera: Tephritidae) in Myrtaceae Species in Entre Rios, Northeastern Argentina. Fla Entomol 91: 220–227 10.1653/0015-4040(2008)91220:NOOHPA2.0.CO2 [DOI] [Google Scholar]
- 117. Ovruski SM, Schliserman P, Aluja M (2004) Indigenous parasitoids (Hymenoptera) attacking Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in native and exotic host plants in Northwestern Argentina. Biol Control 29: 43–57 10.1016/S1049-9644(03)00127-0 [DOI] [Google Scholar]
- 118. Ovruski SM, Schliserman P, Van Nieuwenhove GA, Bezdjian LP, Núñez-Campero S, et al. (2010) Occurrence of Ceratitis capitata and Anastrepha fraterculus (Diptera: Tephritidae) on Cultivated, Exotic Fruit Species in the Highland Valleys of Tucuman in Northwest Argentina. Fla Entomol 93: 277–282 10.1653/024.093.0219 [DOI] [Google Scholar]
- 119. Paini DR, Worner SP, Cook DC, De Barro PJ, Thomas MB (2010) Threat of invasive pests from within national borders. Nat Commun 1: 115 10.1038/ncomms1118 [DOI] [PubMed] [Google Scholar]
- 120. Pires AO, Selivon D, Perondini ALP (2004) Variation in symmetrical patterns of development in Anastrepha grandis and Ceratitis capitata (Diptera: Tephritidae). Isteg Scientific Publications pp. 259–263. [Google Scholar]
- 121.Papadopoulos NT, Katsoyannos BI (n.d.) Development of Ceratitis capitata (Diptera: Tephritidae) in three apple varieties in the laboratory. In: Barnes B, editor. Proceedings of 6th International Fruit Fly Symposium, 6–10 May, Stellenbosch, South Africa 2002. Irene, South Africa: Isteg Scientific Publication. pp. 19–22.
- 122. Reyes A, Ochando MD (1998) Use of Molecular Markers for Detecting the Geographical Origin of Ceratitis capitata (Diptera: Tephritidae) Populations. Ann Entomol Soc Am 91: 222–227. [Google Scholar]
- 123. Schliserman P, Ovruski SM (2004) Incidencia de moscas de la fruta de importancia económica sobre Citrus aurantium (Rutaceae) en Tucumán, Argentina. Rev Manejo Integrado Plagas Agroecol Costa Rica 72: 52–61. [Google Scholar]
- 124. Reboulakis C, Mavrikakis PG, Economopoulos AP, Ragoussis N (2004) Orange fruit volatiles are Medfly species- and male-specific attractants. Isteg Scientific Publications pp. 291–294. [Google Scholar]
- 125. Ros JP, Escobar I, Garcia Tapia FJ, Aranda G (2000) Pilot experiment to control Medfly, Ceratitis capitata (Wied.) (Diptera: Tephritidae) using mass trapping technique in a custard apple (Annona cherimola Mill.) orchard. Penerbit Universiti Sains Malaysia pp. 639–643. [Google Scholar]
- 126. Yuval B, Kaspi R, Shloush S, Warburg MS (1998) Nutritional reserves regulate male participation in Mediterranean fruit fly leks. Ecol Entomol 23: 211–215 10.1046/j.1365-2311.1998.00118.x [DOI] [Google Scholar]
- 127. Papadopoulos NT, Katsoyannos BI, Carey JR (2002) Demographic Parameters of the Mediterranean Fruit Fly (Diptera: Tephritidae) Reared in Apples. Ann Entomol Soc Am 95: 564–569 10.1603/0013-8746(2002)0950564:DPOTMF2.0.CO2 [DOI] [Google Scholar]
- 128. Papadopoulos NT, Katsoyannos BI, Nestle D (2003) Spatial Autocorrelation Analysis of a Ceratitis capitata (Diptera: Tephritidae) Adult Population in a Mixed Deciduous Fruit Orchard in Northern Greece. Environ Entomol 32: 319–326 10.1603/0046-225X-32.2.319 [DOI] [Google Scholar]
- 129. Peck SL, McQuate GT (2000) Field Tests of Environmentally Friendly Malathion Replacements to Suppress Wild Mediterranean Fruit Fly (Diptera: Tephritidae) Populations. J Econ Entomol 93: 280–289 10.1603/0022-0493-93.2.280 [DOI] [PubMed] [Google Scholar]
- 130. Peñarrubia-María E, Avilla J, Escudero-Colomar LA (2012) Survival of Wild Adults of Ceratitis capitata (Wiedemann) under Natural Winter Conditions in North East Spain. Psyche J Entomol 2012: 1–6 10.1155/2012/497087 [DOI] [Google Scholar]
- 131. Puche H, Midgarden DG, Ovalle O, Kendra PE, Epsky ND, et al. (2005) Effect of Elevation and Host Availability on Distribution of Sterile and Wild Mediterranean Fruit Flies (Diptera: Tephritidae). Fla Entomol 88: 83–90 10.1653/0015-4040(2005)0880083:EOEAHA2.0.CO2 [DOI] [Google Scholar]
- 132. Radonjić S, Garcia-Marí F (2006) The Mediterranean fruit fly Ceratitis capitata Wiedemann (Diptera, Tephritidae), a new pest in Montenegro. International Organization for Biological and Integrated Control of Noxious Animals and Plants (OIBC/OILB), West Palaearctic Regional Section (WPRS/SROP) Vol. 29 pp. 217–224 Available: http://www.cabdirect.org/abstracts/20063147058.html;jsessionid=E226CCD2816DAAD41D29EA6A25153612?freeview=true Accessed 2012 May 9. [Google Scholar]
- 133. Rengifo JA, Garcia JG, Rodriguez JF, Wyckhuys KAG (2011) Host Status of Purple Passionfruit for the Mediterranean Fruit Fly (Diptera: Tephritidae). Fla Entomol 94: 91–96 10.1653/024.094.0112 [DOI] [Google Scholar]
- 134.Rigamonti IE (2004) Contributions to the knowledge of Ceratitis capitata Wied. (Diptera, Tephritidae) in Northern Italy. I. Observations on the biology. Available: http://hdl.handle.net/2434/27431. Accessed 2012 May 3.
- 135. Rivnay E (1950) The Mediterranean Fruit Fly in Israel. Bull Entomol Res 41: 321–341 10.1017/S0007485300027656 [DOI] [Google Scholar]
- 136. Sciarretta A, Trematerra P (2011) Spatio-temporal distribution of Ceratitis capitata population in a heterogeneous landscape in Central Italy. J Appl Entomol 135: 241–251 10.1111/j.1439-0418.2010.01515.x [DOI] [Google Scholar]
- 137.Segura DF, Vera MT, Cladera JL, Barnes BN (2004) Host utilization by the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Isteg Scientific Publications. pp. 83–90. Available: http://www.cabdirect.org/abstracts/20063113999.html;jsessionid=E0A45EF3B6AF8858E271E938AC95A0CC. Accessed 2012 May 7.
- 138. Segura DF, Vera MT, Cladera JL (2004) Fluctuación estacional en la infestación de diversos hospedadores por la mosca del Mediterráneo, Ceratitis capitata (Diptera: Tephritidae), en la provincia de Buenos Aires. Ecol Austral 14: 3–17. [Google Scholar]
- 139. Segura DF, Vera MT, Cagnotti CL, Vaccaro N, De Coll O, et al. (2006) Relative Abundance of Ceratitis capitata and Anastrepha fraterculus (Diptera: Tephritidae) in Diverse Host Species and Localities of Argentina. Ann Entomol Soc Am 99: 70–83 10.1603/0013-8746(2006)0990070:RAOCCA2.0.CO2 [DOI] [Google Scholar]
- 140. Shoukry A, Hafez M (1979) Studies on the biology of the Mediterranean fruit fly Ceratitis capitata. Entomol Exp Appl 26: 33–39 10.1007/BF02996633 [DOI] [Google Scholar]
- 141. Silva JG, Meixner MD, Mcpheron BA, Steck GJ, Sheppard WS (2003) Recent Mediterranean Fruit Fly (Diptera: Tephritidae) Infestations in Florida—A Genetic Perspective. J Econ Entomol 96: 1711–1718 10.1603/0022-0493-96.6.1711 [DOI] [PubMed] [Google Scholar]
- 142. Souza SAS, Resende ALS, Strikis PC, Costa JR, Ricci MSF, et al. (2005) Natural infestation by frugivorous flies (Diptera: Tephritoidea) in shaded and unshaded arabic coffee under organic management in Valença, RJ, Brazil. Neotrop Entomol 34: 639–648 10.1590/S1519-566X2005000400015 [DOI] [Google Scholar]
- 143. Souza-Filho ZA, De Araujo EL, Guimarães JA, Silva JG (2007) Endemic Parasitoids Associated with Anastrepha spp. (Diptera: Tephritidae) Infesting Guava (Psidium Guajava) in Southern Bahia, Brazil. Fla Entomol 90: 783–785 10.1653/0015-4040(2007)90783:EPAWAS2.0.CO2 [DOI] [Google Scholar]
- 144. Souza-Filho MF, Raga A, Azevedo-Filho JA, Strikis PC, Guimarães JA, et al. (2009) Diversity and seasonality of fruit flies (Diptera: Tephritidae and Lonchaeidae) and their parasitoids (Hymenoptera: Braconidae and Figitidae) in orchards of guava, loquat and peach. Braz J Biol 69: 31–40 10.1590/S1519-69842009000100004 [DOI] [PubMed] [Google Scholar]
- 145. Staub CG, De Lima F, Majer JD (2008) Determination of host status of citrus fruits against the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Aust J Entomol 47: 184–187 10.1111/j.1440-6055.2008.00646.x [DOI] [Google Scholar]
- 146. Suckling DM, Jang EB, Holder P, Carvalho L, Stephens AE (2008) Evaluation of lure dispensers for fruit fly surveillance in New Zealand. Pest Manag Sci 64: 848–856 10.1002/ps.1578 [DOI] [PubMed] [Google Scholar]
- 147. Sugayama RL, Branco ES, Malavasi A, Kovaleski A, Nora I (1997) Oviposition behavior of Anastrepha fraterculus in apple and diel pattern of activities in an apple orchard in Brazil. Entomol Exp Appl 83: 239–245 10.1023/A:1002913018833 [DOI] [Google Scholar]
- 148. Terblanche JS, Nyamukondiwa C, Kleynhans E (2010) Thermal variability alters climatic stress resistance and plastic responses in a globally invasive pest, the Mediterranean fruit fly (Ceratitis capitata). Entomol Exp Appl 137: 304–315 10.1111/j.1570-7458.2010.01067.x [DOI] [Google Scholar]
- 149. Tormos J, Beitia F, Alonso M, Asís JD, Gayubo SF (2010) Assessment of Ceratitis capitata (Diptera, Tephritidae) pupae killed by heat or cold as hosts for rearing Spalangia cameroni (Hymenoptera: Pteromalidae). Ann Appl Biol 156: 179–185 10.1111/j.1744-7348.2009.00377.x [DOI] [Google Scholar]
- 150.Tóth M, Tabilio R, Franco F di (2009) Developing semiochemical tools for monitoring, mass trapping and integrated control of potential pests of citrus and Mediterranean fruit crops. Prot Delle Colt: 34–39.
- 151. Uchôa-Fernandes MA, Oliveira ID, Molina RMS, Zucchi RA (2002) Species Diversity of Frugivorous Flies (Diptera: Tephritoidea) from Hosts in the Cerrado of the State of Mato Grosso do Sul, Brazil. Neotrop Entomol 31: 515–524 10.1590/S1519-566X2002000400002 [DOI] [Google Scholar]
- 152.Vadora ML, Salaverrey L, Rodriguez L (1993) Prediction of Ceratitis capitata captures based on climatic factors. Springer Verlag.
- 153.Vargas RI, Nishida T (1989) Spatial Distribution of Mediterranean Fruit Fly (Diptera: Tephritidae) throughout West Oahu: Development of Eradication Strategies. Available: http://scholarspace.manoa.hawaii.edu/handle/10125/11243. Accessed 2012 April 26.
- 154. Vargas RI, Piñero JC, Mau RFL, Jang EB, Klungness LM, et al. (2010) Area-Wide Suppression of the Mediterranean Fruit Fly, Ceratitis capitata, and the Oriental Fruit Fly, Bactrocera dorsalis, in Kamuela, Hawaii. J Insect Sci 10: 1–17 10.1673/031.010.13501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Vayssières J-F, Korie S, Ayegnon D (2009) Correlation of fruit fly (Diptera Tephritidae) infestation of major mango cultivars in Borgou (Benin) with abiotic and biotic factors and assessment of damage. Crop Prot 28: 477–488 10.1016/j.cropro.2009.01.010 [DOI] [Google Scholar]
- 156. Villablanca FX, Roderick GK, Palumbi SR (1998) Invasion genetics of the Mediterranean fruit fly: variation in multiple nuclear introns. Mol Ecol 7: 547–560 10.1046/j.1365-294x.1998.00351.x [DOI] [PubMed] [Google Scholar]
- 157. Weldon CW, Terblanche JS, Chown SL (2011) Time-course for attainment and reversal of acclimation to constant temperature in two Ceratitis species. J Therm Biol 36: 479–485 10.1016/j.jtherbio.2011.08.005 [DOI] [Google Scholar]
- 158. Worner SP, Gevrey M (2006) Modelling global insect pest species assemblages to determine risk of invasion. J Appl Ecol 43: 858–867 10.1111/j.1365-2664.2006.01202.x [DOI] [Google Scholar]
- 159.Zaborowski A, Tison G (2008) Evaluation d'une nouvelle methode de lutte contre la mouche des fruits Ceratitis capitata Wied. sur agrumes en Corse: la chemosterilisation a base de lufenuron. Syngenta Agro SAS - 1 av des pres - 78286 Guyancourt, France. Association Francaise de Protection des Plantes (AFPP) Association Francaise de Protection des Plantes (AFPP).
- 160. Zeki C, Er H, Özdem A, Bozkurt V (2008) Distribution and infestation of Mediterranean fruit fly (Ceratitis capitata Wied.)(Diptera: Tephritidae) on pome and stone fruits in Isparta and Burdur provinces (Turkey). Munis Entomol Zool 3: 231–238. [Google Scholar]
- 161. Ros JP, Gomila J, Reurer M, Pons P, Castillo E, et al. (2004) The use of mass-trapping against Medfly (Ceratitis capitata (Wied.)) in a sustainable agriculture system on Minorca Island, Spain. Isteg Scientific Publications pp. 361–364 Available: http://www.cabdirect.org/abstracts/20063113974.html;jsessionid=60D58E0E3DDC46BD96846D1CDFBBE378 Accessed 2012 June 14. [Google Scholar]
- 162. Hay SI, Sinka ME, Okara RM, Kabaria CW, Mbithi PM, et al. (2010) Developing Global Maps of the Dominant Anopheles Vectors of Human Malaria. PLoS Med 7: e1000209 10.1371/journal.pmed.1000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, et al. n.d. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors 4: 89–89 10.1186/1756-3305-4-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, et al. (2010) The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors 3: 117 10.1186/1756-3305-3-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, et al. (2010) The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic précis. Parasit Vectors 3: 72 10.1186/1756-3305-3-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.APHIS (1999) The Mediterranean Fruit Fly - Factsheet PPQ. USDA APHIS.
- 167. Honěk A, Kocourek F (1990) Temperature and development time in insects: a general relationship between thermal constants. Zool Jahrb Abt Für Syst Ökol Geogr Tiere 117: 401–439. [Google Scholar]
- 168. Ricalde MP, Nava DE, Loeck AE, Donatti MG (2012) Temperature-Dependent Development and Survival of Brazilian Populations of the Mediterranean Fruit Fly, Ceratitis capitata, from Tropical, Subtropical and Temperate Regions. J Insect Sci 12 Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3471798/ Accessed 2014 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Gjullin CM (1931) Probable distribution of the Mediterranean fruit fly (Ceratitis capitata Weid.) in the United States. Ecology 12: 248–258. [Google Scholar]
- 170. Back EA, Pemberton CE (1915) Susceptibility of citrus fruits to the attack of the Mediterranean fruit fly. J Agric Res 3: 311–330. [Google Scholar]
- 171. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25: 1965–1978. [Google Scholar]
- 172. Hay SI, Tatem AJ, Graham AJ, Goetz SJ, Rogers DJ (2006) Global Environmental Data for Mapping Infectious Disease Distribution. Adv Parasitol 62: 37–77 10.1016/S0065-308X(05)62002-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, et al. (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17: 43–57 10.1111/j.1472-4642.2010.00725.x [DOI] [Google Scholar]
- 174. Elith J, Graham CH, Anderson RP, Dudık M, Ferrier S, et al. (2006) Novel methods improve prediction of species' distributions from occurrence data. Ecography 29: 129–151. [Google Scholar]
- 175. Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190: 231–259. [Google Scholar]
- 176. Phillips SJ, Dudík M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31: 161–175 10.1111/j.0906-7590.2008.5203.x [DOI] [Google Scholar]
- 177. Elith J, Leathwick JR, Hastie T (2008) A working guide to boosted regression trees. J Anim Ecol 77: 802–813 10.1111/j.1365-2656.2008.01390.x [DOI] [PubMed] [Google Scholar]
- 178. Elith J, Kearney M, Phillips S (2010) The art of modelling range-shifting species. Methods Ecol Evol 1: 330–342 10.1111/j.2041-210X.2010.00036.x [DOI] [Google Scholar]
- 179. Anderson RP, Lew D, Peterson AT (2003) Evaluating predictive models of species' distributions: criteria for selecting optimal models. Ecol Model 162: 211–232 10.1016/S0304-3800(02)00349-6 [DOI] [Google Scholar]
- 180.Trapping Guidelines for Area-Wide Fruit Fly Programmes (2003) Non-serial Publications. Vienna: IAEA/FAO-TG/FFP. Available: http://www-pub.iaea.org/books/IAEABooks/6916/Trapping-Guidelines-for-Area-Wide-Fruit-Fly-Programmes.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curves. Red lines represent mean AUC and mean +/− one standard deviation (blue field). The lines show the “fit” of the model to the training and test data. Black line represents random prediction. The red line can be considered to show the real test of the models predictive power [175].
(DOC)
Medfly occurrence dataset.
(XLSX)