Abstract
We describe a unique, versatile bioreactor consisting of two plates and a modified commercial porous membrane suitable for in vitro analysis of the liver sinusoid. The modular bioreactor allows i) excellent control of the cell seeding process; ii) cell culture under controlled shear stress stimulus, and; iii) individual analysis of each cell type upon completion of the experiment. The advantages of the bioreactor detailed here are derived from the modification of a commercial porous membrane with an elastomeric wall specifically moulded in order to define the cell culture area, to act as a gasket that will fit into the bioreactor, and to provide improved mechanical robustness. The device presented herein has been designed to simulate the in vivo organization of a liver sinusoid and tested by co-culturing endothelial cells (EC) and hepatic stellate cells (HSC). The results show both an optimal morphology of the endothelial cells as well as an improvement in the phenotype of stellate cells, most probably due to paracrine factors released from endothelial cells. This device is proposed as a versatile, easy-to-use co-culture system that can be applied to biomedical research of vascular systems, including the liver.
Introduction
In recent years, a variety of in vitro 3D cell culture methods have been designed to study either the interaction of multiple cell types or the effect of certain drugs on tissue- or organ-specific microarchitecture [1]–[3]. Advances in microfabrication and polymer processing technologies have enabled the development of highly complex systems where a variety of cell types can be co-cultured in a controlled environment, thereby establishing a new multidisciplinary scientific field known as organ on a chip [4]–[11].
Organ on a chip devices have been developed to study the pathophysiology of a variety of organs, including lung [12], liver [13], gut [14] and kidney [15]. The main interest in developing such systems is to culture cells under real world conditions. For that, microfluidic structures allowing cell stimulation with culture media are needed. This is especially relevant when analyzing the behavior of endothelial cells which are continuously stimulated by blood flow-derived shear stress inside the human body. Therefore, shear stress must be applied over cultured endothelium in order to mimic the cell behavior in human vascular systems.
The liver, in particular, has attracted much of the research in organ on a chip due to its central role in drug metabolism, toxicity control, and the impact of clinical diseases [16]. In order to properly study the pathophysiology of liver diseases, the unique hepatic microcirculatory architecture should be considered. The liver sinusoid, mainly composed by endothelial cells and stellate cells, plays an essential role in most liver diseases since it represents the sieve plate by which oxygen and nutrients, but also toxicants and viruses, enter the parenchyma. In the specific scenario of liver cirrhosis, the hepatic sinusoid is considered a major contributor to the progression, aggravation, and also regression upon treatment of cirrhosis. Phenotypic changes in sinusoidal cells lead to de-regulated paracrine interactions that markedly contribute to parenchymal damage and, more importantly, determine the aggravation of cirrhosis due to the development of portal hypertension, and its complications [17]. Therefore, organ on a chip devices designed to mimic the liver should incorporate these two types of sinusoidal cells, and importantly culture them in a sinusoidal-like architectural distribution. More specifically, endothelial cells should be exposed to blood-flow derived shear stress, below hepatic stellate cells placed in the “Space of Disse”.
A variety of hepato-microfluidic bioreactors, mostly aimed at the study hepatocytes integrity, can be found in the literature [13], [16]. Although dynamic cultures are necessary to maintain the liver-specific functions [18], hepatocytes themselves should be protected from the direct influence of shear stress. This has traditionally been accomplished by the creation of scaffolds, by embedding cells in 3D hydrogels [19], [20], by means of nanoporous membranes [21], or by culturing them on grooved substrates [22]. Another strategy consists of maintaining the hepatocytes within cord-like microstructures that mimic the endothelial barrier [23]–[26]. Finally, more complex devices to maintain liver tissues under the optimal culture conditions have been also developed [27], [28].
However, none of the previously cited liver models have sufficient in vivo organization to co-culture and individually analyze sinusoidal cells, necessary to study their integrity and their paracrine communications. In the present work, a microporous membrane separates two cell culture microfluidic chambers. This permits biomechanical stimulation in the upper compartment, paracrine interactions through the membrane, and static culture in the bottom compartment. Although previous studies have used a similar design [12], [15], [29]–[32], in those studies the membrane was glued to the bioreactor, resulting in a unique and compact device that compromises the individual analysis of the different cell types after being co-cultured. Moreover, in the aforementioned systems, cells must be seeded directly inside the bioreactor, making it difficult to control the exact culture area and to monitor cell viability during the assay. Furthermore, any alterations during the cell culture may compromise the function of the device, which cannot be re-used.
In our work, a bioreactor integrating a modified commercial membrane that separates two cell culture chambers is proposed to facilitate the study of the liver sinusoid. In particular, this bioreactor simulates the architecture and microcirculation in the liver in order to properly analyze the paracrine interactions between cells.
Materials and Methods
Concept and design of the bioreactor
This novel bioreactor is a modular system that comprises two transparent plates and a porous membrane that can be easily mounted and dismounted. To do that, the membrane is modified by adding an elastomeric wall (figure 1). The wall provides mechanical stability to the membrane, defines the cell culture area, and seals the assembly into the bioreactor. Then, it is possible to seed cells on the membrane outside the bioreactor, in static conditions following the well-known standards that are used in the laboratory.
Figure 1. Schematic of the two-plate bioreactor integrating the home-modified membrane, with detail of the upper plate where the flow paths are shown in red (top).
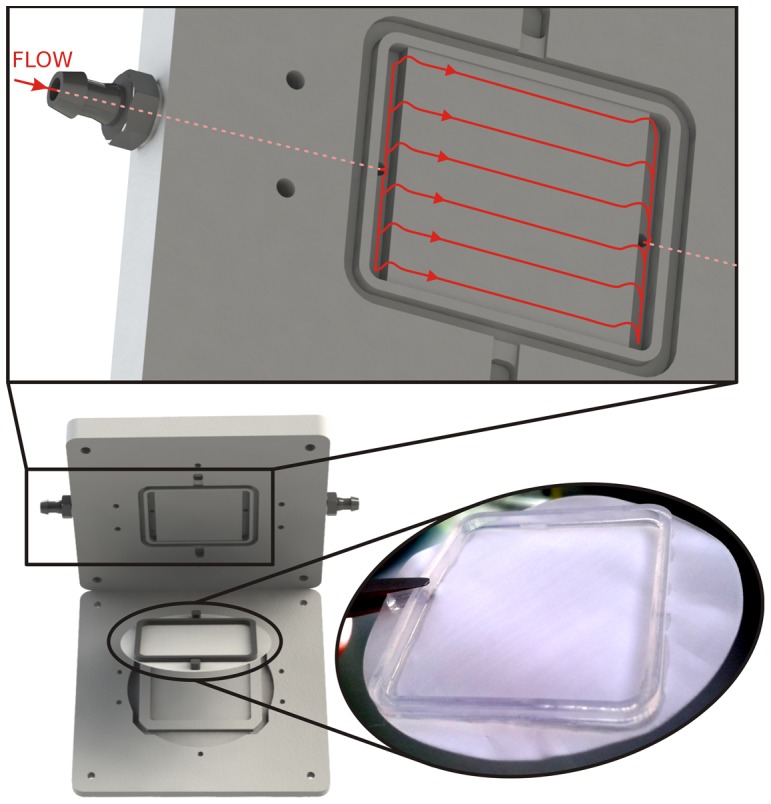
The upper plate includes trenches where the elastomeric wall manufactured on the membrane will fit and a channel to perfuse the medium over the endothelial cell culture. To ensure a well-defined, laminar and homogeneous fluid flow along the whole channel area, and over the membrane, a parallel-plate flow chamber configuration was used [33]. In particular, small canals that connect the external microfluidic set-up with the inner channel fall into a couple of reservoirs that are placed along the width of the channel. While the cells placed below these reservoirs would not be under uniform flow conditions, it can be assumed that more than the 95% of the area of the channel is under the same flow conditions [34]. Therefore, the shear stress sensed by the cells cultured on the membrane can be assumed to be uniform.
The lower plate of the bioreactor contains a rectangular pool with no fluidic connection where a second cell type can be seeded while being protected from the shear stress during the cell-culture. This pool has the same area as that delimited by the elastomeric wall on the membrane. Moreover, an external pool was also designed to receive the excess of liquid from the inner pool once the bioreactor is closed.
Fabrication
The present bioreactor aims to include a culture area large enough to allow optimal cell seeding and culture, along with multipurpose, post-experimental analysis. Although some molecular studies can be done in a single-cell, most biomedical research requires a decent amount of cells to cooperate and communicate such that a large range of molecular assays can be performed. For that, commercial hydrophilic microporous membrane filters (47 mm in diameter, 100 um thick, and 1 µm of pore size, Omnipore, Millipore, USA) were chosen.
To manufacture the 2 mm×1.5 mm elastomeric wall on the membrane encompassing an area of 969 mm2 (34×28.5 mm), a mould with the negative of the wall-structure was fabricated in Teflon by CNC machining. Next, the mold was filled with a UV-curable silicon-based elastomeric adhesive (Loctite 5055, Henkel). Then, the membrane was placed between the mold and a polymethilmetacrylate (PMMA) cover. After a few seconds, the full-sandwiched system was placed under a UV Curing Light Lamp System (PC-5000, Dymax) with irradiance of 62 mW cm−2 at the wavelength of 365 nm for 60 s in order to cure the silicone adhesive. Finally, the membrane attached with the cured elastomeric wall was gently peeled off from the mold.
The fabrication of the bioreactor was performed by CNC machining on a 5 mm (for the lower plate) and 8 mm thick (for the top plate where microfluidic connections are included) PMMA plates. In particular, the cell-culture pool on the lower plate had a depth of 1 mm, a distance fully adequate for paracrine interactions since it is much smaller than commonly used cell culture inserts. Meanwhile, the perfusion channel micromachined in the upper plate was 500 µm deep.
In order to place the second cell type on the top of the lower plate, the PMMA surface needs to be modified [35]. For that, hydrophilization by means of a corona discharge treatment was applied to it. The same treatment was applied to the bottom of the upper plate to facilitate fluid flow and to minimize the generation of bubbles that can occur on hydrophobic surfaces.
Cell culture
The hepatic sinusoid was mimicked using endothelial cells and hepatic stellate cells previously validated as gold standard methods for vascular and hepatic research [36], [37]. Primary human umbilical vein endothelial cells were plated at a confluency of 85% on the gelatin-coated membrane. Human activated hepatic stellate cells (LX-2) were plated at a confluency of 80% on the lower plate. Cells were separately seeded and cultured until assembly of the bioreactor. On the day of the experiment, cells exhibited 95% confluency.
Assembly and perfusion
The bioreactor was assembled, as shown in figure 2, and connected to the perfusion system using barbed connectors. Silicone tubing was used to connect the recirculating microfluidic set-up with a commercial peristaltic pump (Minipuls 3, Gilson). The pump injects the perfusion media (M199 medium with 10% FBS, 100 U/mLPen/Strep, 2 mM Glutamine, and 2% Dextran) from a flask to the bioreactor. A commercial, 5 µm pore-size membrane filter with an integrated venting system (Speedflow Kids, GVS) was placed in front of the bioreactor inlet to guarantee total safety against air bubbles.
Figure 2. Cross-section schematic of the mounted bioreactor with the endothelial cells culture on top of the home-modified membrane and the HSC culture on top of the lower plate.
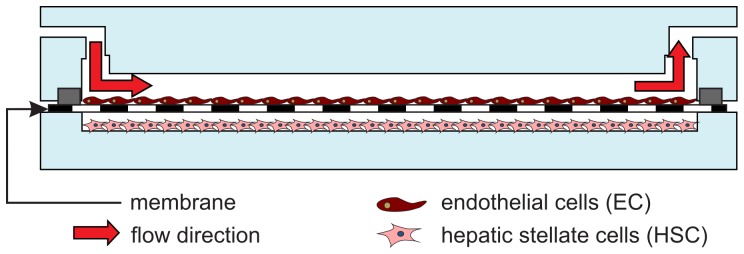
In the current set-up, the peristaltic pump was placed outside the incubator to preserve its integrity. Then, the perfusion media was held at ambient conditions on its way from the flask to the bioreactor. This avoids bubble generation, especially if the perfusion media is stirred during preparation. To further avoid bubble generation problems, the media was degasified in a vacuum chamber before use and left in an incubator overnight. Once the media was stabilized under the desired conditions, (37°C, 5% CO2) the full system was connected and the peristaltic pump switched on.
The fluid flow through the channel above the membrane where the endothelial cells were cultured was chosen taking into account the formula for the shear stress τ via perfusion in a parallel-plate flow chamber [38]:
where μ is the media viscosity, Q is the flow rate and w and h are the channel width and height respectively. In this study, a shear stress sufficient to stimulate the endothelial population of 3 dyn/cm2 was applied for 24 h [39]–[41]. The bioreactor was then disassembled and the two cell types were separated and analyzed individually. By design, the hepatic stellate cells on the lower plate were cultured under static conditions, as it happens in the hepatic sinusoid.
Cells phenotype analysis
Endothelial cell morphology and the production of nitric oxide were analyzed in real time by membrane and nuclei staining using Image-IT kit LIVE (Invitrogen), and by staining with DAF-FM (Invitrogen), respectively. Nuclei and membrane staining is necessary to evaluate the morphological state, the bonding and the alignment of the cells after shear stress stimulation. Similarly, the production of nitric oxide (NO) represents an excellent functional test to validate the improvement of the endothelial phenotype due to biomechanical stimulus (shear stress). Measurement of NO in real time has been described in depth elsewhere [42], [43]. Briefly, cells are washed with PBS, incubated 20 min with phenol red-free medium with 10 uM DAF-FM, and photographed using a fluorescent microscope equipped with a digital camera (Olympus BX51). 10 pictures per experimental condition are analyzed using ImageJ software (NIH). In each experiment, a control condition was cultured in static conditions in the same incubator. Specificity controls for DAF-staining were included (cells co-incubated with the NO synthase inhibitor L-NAME 1.5 mM) [44].
For HSC phenotype characterization, two major markers of their phenotype, alpha-smooth muscle actin (α-SMA) and pro-collagen I, were analyzed using qPCR [37]. Previous work from this team and others validated the analysis of these two genes as a way to analyze the phenotype of HSC [37], [45].
Results and Discussion
The bioreactor presented in this work improves the usability of the reported bioreactors. In particular, the elastomeric structure that is manufactured over a porous membrane facilitates the experimental procedure as it defines the cell culture area and provides significant mechanical robustness to often delicate membranes. Then, cell seeding over the membrane can be performed outside the bioreactor, allowing the use of well-established protocols under static conditions. This results in improved test reliability since the viability of the cell culture will no longer affect the outcome.
A final, but no less important, advantage of the device presented in this work is the possibility to separate cell types after the experiment. Since the cell cultures are placed on different substrates, they can easily be separated (membrane and lower plate), and tested independently as it was done in order to present the following results.
Endothelial cells phenotype after culture within the bioreactor
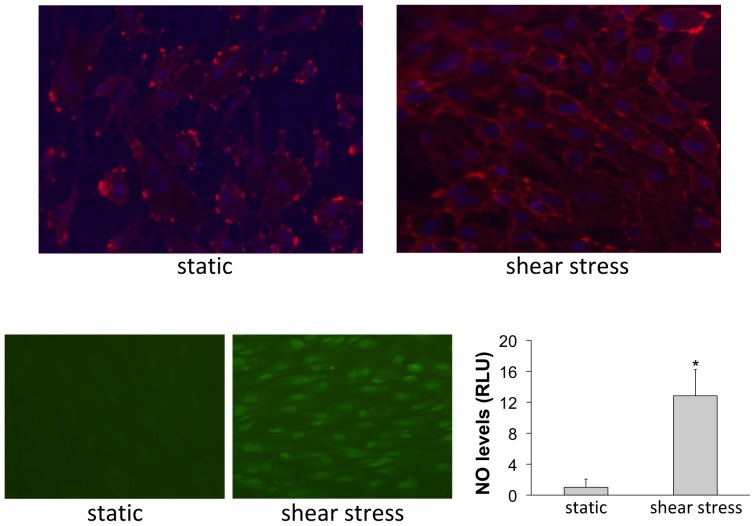
As shown in figure 3, endothelial cells cultured under shear stress stimulation maintained the confluence established before starting the shear stress and showed a correct morphology of a stretched cell, oriented in the direction of the flow. This is not observed in cells cultured under static conditions. Additionally, endothelial cells cultured under biomechanical stimulation showed markedly higher production of NO in comparison to those cells cultured in static conditions. This demonstrates that the application of shear stress markedly improves the stimulation of the endothelium. Although future experiments will validate the system using other magnitudes of shear stress, and/or, in other types of endothelial cells, the experiments herein reported demonstrate an optimal endothelial stimulation conferred by the system.
Figure 3. Representative images of endothelial cells cultured in the present system under continuous perfusion (shear stress) and static conditions (static).
Top, Cell membrane staining (red) and nuclei (blue). Bottom, Real-time production of nitric oxide (green) and fluorescence quantification (data come from n = 3 experiments; and fluorescence intensity was divided by the total number of cultured cells; *p<0.05 vs. static t-test).
Hepatic stellate cells phenotype after co-culture within the bioreactor
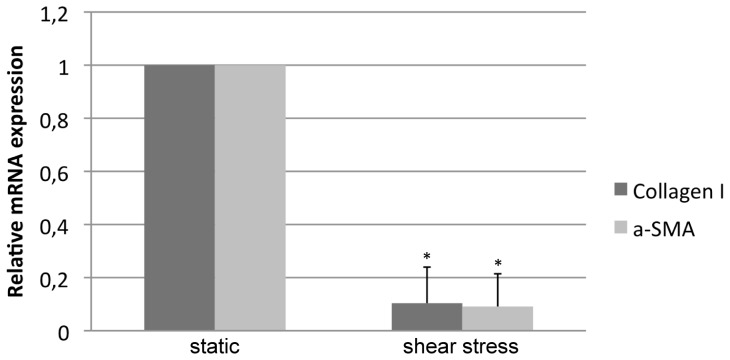
Hepatic stellate cells co-cultured in the bioreactor with shear-stress stimulated endothelial cells exhibited a much lower expression of both phenotypic markers, α-SMA and pro-collagen I, than did cells co-cultured without biomechanical stimulation. This suggests that significant amelioration in the phenotype was due to paracrine signaling from stimulated endothelial cells (Figure 4). Considering our previous report demonstrating the amelioration of HSC phenotype due to paracrine release of NO from simvastatin-treated endothelial cells [37], we propose a shear stress-derived endothelial NO-dependent mechanism to explain the improvement in HSC status.
Figure 4. mRNA expression of two activation markers (a-SMA and Collagen I) in hepatic stellate cells co-cultured in the bioreactor with endothelial cells under shear stress stimulus or under static conditions.
The reduction in both markers indicates an improvement in the stellate cells phenotype, most probably derived from the nitric oxide produced by the endothelial cells stimulated with shear stress (data from n = 3 experiments; * p<0.01 vs. static t-test).
Figure 4 is particularly relevant as it demonstrates that the bioreactor presented here allows paracrine interactions between co-cultured cells. This is a significant improvement over the previous method of using common cell culture inserts under static conditions since our bioreactor simulates the in vivo situation of the hepatic vasculature. The improved communication of hepatic cells and the modification in their phenotype validate the bioreactor design described here. Indeed, the effects of endothelial cells on hepatic stellate cells phenotype in a physiological-like environment is much prominent than those observed using ordinary co-culture assays [37].
Conclusions
The successful demonstration of the bioreactor described here enables improved study of the structure and function of the liver sinusoid. Permitting different cell types to be easily co-cultured under physiological conditions and separately analyzed afterwards simplifies and improves the reliability of clinical work on liver sinusoids. Importantly, our report demonstrates the improvement of hepatic stellate cells phenotype due to paracrine factors released from endothelial cells cultured under biomechanical stimulation. This has not been described in the literature to the best of our knowledge due to the unavailability of an easy-to-use bioreactor that allows cell co-culture under uniform and controlled shear stress stimulation.
In addition, these successful results point out the feasibility of using the proposed bioreactor for basic organ on a chip experimentation, as different cell types can be easily co-cultured in an in vivo environment. This is because of the excellent control of the seeding processes and the post-experimentation analysis possibilities; therefore, the applicability of this bioreactor in other vascular systems different from the liver is promising but will require further study.
Acknowledgments
Authors are indebted to Bio-Model (www.bio-model.com) for its technical assistance and to Dr. Erik Koep for the language support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: CSIC (PIE 201450E116) (RV); Instituto de Salud Carlos III (FIS PI11/00235) (JGS); Ministerio de Economía y Competitividad (SAF2012-31238) (CP); European Union (Fondos FEDER, “una manera de hacer Europa”) (JGS and CP); European Community's Seventh Framework Programme (EC FP7/2007-2013, grant agreement 229673) (JGS); CIBER-EHD (funded by Instituto de Salud Carlos III) (JGS and CP); and CIBER-BBN (funded by Instituto de Salud Carlos III) (RV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kim L, Toh Y-C, Voldman J, Yu H (2007) A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip 7: 681–694 10.1039/B704602B [DOI] [PubMed] [Google Scholar]
- 2. Morimoto Y, Takeuchi S (2013) Three-dimensional cell culture based on microfluidic techniques to mimic living tissues. Biomater Sci 1: 257–264 10.1039/C2BM00117A [DOI] [PubMed] [Google Scholar]
- 3. Inamdar NK, Borenstein JT (2011) Microfluidic cell culture models for tissue engineering. Curr Opin Biotechnol 22: 681–689 10.1016/j.copbio.2011.05.512 [DOI] [PubMed] [Google Scholar]
- 4. Baker M (2011) Tissue models: A living system on a chip. Nature 471: 661–665 10.1038/471661a [DOI] [PubMed] [Google Scholar]
- 5. Huh D, Hamilton GA, Ingber DE (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol 21: 745–754 10.1016/j.tcb.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meer AD van der, Berg A van den (2012) Organs-on-chips: breaking the in vitro impasse. Integr Biol 4: 461–470 10.1039/C2IB00176D [DOI] [PubMed] [Google Scholar]
- 7. Ghaemmaghami AM, Hancock MJ, Harrington H, Kaji H, Khademhosseini A (2012) Biomimetic tissues on a chip for drug discovery. Drug Discov Today 17: 173–181 10.1016/j.drudis.2011.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stolpe A van de, Toonder J den (2013) Workshop meeting report Organs-on-Chips: human disease models. Lab Chip 13: 3449–3470 10.1039/C3LC50248A [DOI] [PubMed] [Google Scholar]
- 9. Yum K, Hong SG, Healy KE, Lee LP (2014) Physiologically relevant organs on chips. Biotechnol J 9: 16–27 10.1002/biot.201300187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capulli AK, Tian K, Mehandru N, Bukhta A, Choudhury SF, et al. (2014) Approaching the in vitro clinical trial: engineering organs on chips. Lab Chip. Available: http://pubs.rsc.org/en/content/articlelanding/2014/lc/c4lc00276h. Accessed 15 May 2014. [DOI] [PMC free article] [PubMed]
- 11. Polini A, Prodanov L, Bhise NS, Manoharan V, Dokmeci MR, et al. (2014) Organs-on-a-chip: a new tool for drug discovery. Expert Opin Drug Discov 9: 335–352 10.1517/17460441.2014.886562 [DOI] [PubMed] [Google Scholar]
- 12. Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, et al. (2010) Reconstituting Organ-Level Lung Functions on a Chip. Science 328: 1662–1668 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Materne E-M, Tonevitsky AG, Marx U (2013) Chip-based liver equivalents for toxicity testing – organotypicalness versus cost-efficient high throughput. Lab Chip 13: 3481–3495 10.1039/c3lc50240f [DOI] [PubMed] [Google Scholar]
- 14. Kim HJ, Huh D, Hamilton G, Ingber DE (2012) Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12: 2165–2174 10.1039/C2LC40074J [DOI] [PubMed] [Google Scholar]
- 15. Jang K-J, Suh K-Y (2010) A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 10: 36–42 10.1039/b907515a [DOI] [PubMed] [Google Scholar]
- 16. Ebrahimkhani MR, Neiman JAS, Raredon MSB, Hughes DJ, Griffith LG (2014) Bioreactor technologies to support liver function in vitro. Adv Drug Deliv Rev 69–70: 132–157 10.1016/j.addr.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. García-Pagán J-C, Gracia-Sancho J, Bosch J (2012) Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol 57: 458–461 10.1016/j.jhep.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 18. LeCluyse EL, Bullock PL, Parkinson A (1996) Strategies for restoration and maintenance of normal hepatic structure and function in long-term cultures of rat hepatocytes. Adv Drug Deliv Rev 22: 133–186 10.1016/S0169-409X(96)00418-8 [DOI] [Google Scholar]
- 19. Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, et al. (2002) A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng 78: 257–269 10.1002/bit.10143 [DOI] [PubMed] [Google Scholar]
- 20. Sung JH, Shuler ML (2009) A micro cell culture analog (μCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 9: 1385–1394 10.1039/B901377F [DOI] [PubMed] [Google Scholar]
- 21. Carraro A, Hsu W-M, Kulig KM, Cheung WS, Miller ML, et al. (2008) In vitro analysis of a hepatic device with intrinsic microvascular-based channels. Biomed Microdevices 10: 795–805 10.1007/s10544-008-9194-3 [DOI] [PubMed] [Google Scholar]
- 22. Park J, Berthiaume F, Toner M, Yarmush ML, Tilles AW (2005) Microfabricated grooved substrates as platforms for bioartificial liver reactors. Biotechnol Bioeng 90: 632–644 10.1002/bit.20463 [DOI] [PubMed] [Google Scholar]
- 23. Lee PJ, Hung PJ, Lee LP (2007) An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng 97: 1340–1346 10.1002/bit.21360 [DOI] [PubMed] [Google Scholar]
- 24. Zhang MY, Lee PJ, Hung PJ, Johnson T, Lee LP, et al. (2008) Microfluidic environment for high density hepatocyte culture. Biomed Microdevices 10: 117–121 10.1007/s10544-007-9116-9 [DOI] [PubMed] [Google Scholar]
- 25. Goral VN, Hsieh Y-C, Petzold ON, Clark JS, Yuen PK, et al. (2010) Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants. Lab Chip 10: 3380–3386 10.1039/C0LC00135J [DOI] [PubMed] [Google Scholar]
- 26. Meissner R, Eker B, Kasi H, Bertsch A, Renaud P (2011) Distinguishing drug-induced minor morphological changes from major cellular damage via label-free impedimetric toxicity screening. Lab Chip 11: 2352–2361 10.1039/C1LC20212J [DOI] [PubMed] [Google Scholar]
- 27. Domansky K, Inman W, Serdy J, Dash A, Lim MHM, et al. (2010) Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10: 51–58 10.1039/B913221J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Midwoud PM, Groothuis GMM, Merema MT, Verpoorte E (2010) Microfluidic biochip for the perifusion of precision-cut rat liver slices for metabolism and toxicology studies. Biotechnol Bioeng 105: 184–194 10.1002/bit.22516 [DOI] [PubMed] [Google Scholar]
- 29. Epshteyn AA, Maher S, Taylor AJ, Holton AB, Borenstein JT, et al. (2011) Membrane-integrated microfluidic device for high-resolution live cell imaging. Biomicrofluidics 5: 046501 10.1063/1.3647824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griep LM, Wolbers F, Wagenaar B de, Braak PM ter, Weksler BB, et al. (2013) BBB ON CHIP: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices 15: 145–150 10.1007/s10544-012-9699-7 [DOI] [PubMed] [Google Scholar]
- 31. Booth R, Kim H (2012) Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 12: 1784–1792 10.1039/C2LC40094D [DOI] [PubMed] [Google Scholar]
- 32.Kang YB, Sodunke TR, Cirillo J, Bouchard MJ, Noh H (2013) Liver on a chip: Engineering the liver sinusoid. 2013 Transducers Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS EUROSENSORS XXVII). pp.301–304. doi:10.1109/Transducers.2013.6626762.
- 33. Brown TD (2000) Techniques for mechanical stimulation of cells in vitro: a review. J Biomech 33: 3–14 10.1016/S0021-9290(99)00177-3 [DOI] [PubMed] [Google Scholar]
- 34. Gemmiti CV, Guldberg RE (2006) Fluid Flow Increases Type II Collagen Deposition and Tensile Mechanical Properties in Bioreactor-Grown Tissue-Engineered Cartilage. Tissue Eng 12: 469–479 10.1089/ten.2006.12.469 [DOI] [PubMed] [Google Scholar]
- 35. Chu PK, Chen JY, Wang LP, Huang N (2002) Plasma-surface modification of biomaterials. Mater Sci Eng R Rep 36: 143–206 10.1016/S0927-796X(02)00004-9 [DOI] [Google Scholar]
- 36. Gracia-Sancho J, Villarreal G, Zhang Y, Yu JX, Liu Y, et al. (2010) Flow Cessation Triggers Endothelial Dysfunction During Organ Cold Storage Conditions: Strategies for Pharmacologic Intervention: Transplant J. 90: 142–149 10.1097/TP.0b013e3181e228db [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marrone G, Russo L, Rosado E, Hide D, García-Cardeña G, et al. (2013) The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial–stellate cell deactivation induced by statins. J Hepatol 58: 98–103 10.1016/j.jhep.2012.08.026 [DOI] [PubMed] [Google Scholar]
- 38. Bacabac RG, Smit TH, Cowin SC, Van Loon JJWA, Nieuwstadt FTM, et al. (2005) Dynamic shear stress in parallel-plate flow chambers. J Biomech 38: 159–167 10.1016/j.jbiomech.2004.03.020 [DOI] [PubMed] [Google Scholar]
- 39.Kabirian F, Amoabediny G, Haghighipour N, Salehi-Nik N, Zandieh-Doulabi B (2014) Nitric oxide secretion by endothelial cells in response to fluid shear stress, aspirin, and temperature. J Biomed Mater Res A: n/a–n/a. doi:10.1002/jbm.a.35233. [DOI] [PubMed]
- 40. Vion A-C, Ramkhelawon B, Loyer X, Chironi G, Devue C, et al. (2013) Shear Stress Regulates Endothelial Microparticle Release. Circ Res 112: 1323–1333 10.1161/CIRCRESAHA.112.300818 [DOI] [PubMed] [Google Scholar]
- 41. Vozzi F, Bianchi F, Ahluwalia A, Domenici C (2014) Hydrostatic pressure and shear stress affect endothelin-1 and nitric oxide release by endothelial cells in bioreactors. Biotechnol J 9: 146–154 10.1002/biot.201300016 [DOI] [PubMed] [Google Scholar]
- 42. Gracia-Sancho J, Laviña B, Rodríguez-Vilarrupla A, García-Calderó H, Fernández M, et al. (2008) Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology 47: 1248–1256 10.1002/hep.22166 [DOI] [PubMed] [Google Scholar]
- 43. Rosado E, Rodríguez-Vilarrupla A, Gracia-Sancho J, Monclús M, Bosch J, et al. (2012) Interaction between NO and COX pathways modulating hepatic endothelial cells from control and cirrhotic rats. J Cell Mol Med 16: 2461–2470 10.1111/j.1582-4934.2012.01563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hide D, Ortega-Ribera M, Fernandez-Iglesias A, Fondevila C, Salvado MJ, et al. (2014) A novel form of the human manganese superoxide dismutase protects rat and human livers undergoing ischemia and reperfusion injuries. Clin Sci Lond Engl 1979. doi:10.1042/CS20140125. [DOI] [PubMed]
- 45. Hennenberg M, Trebicka J, Kohistani Z, Stark C, Nischalke H-D, et al. (2011) Hepatic and HSC-specific sorafenib effects in rats with established secondary biliary cirrhosis. Lab Invest 91: 241–251 10.1038/labinvest.2010.148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.