Abstract
Bionic devices electrically activate neural populations to partially restore lost function. Of fundamental importance is the functional integrity of the targeted neurons. However, in many conditions the ongoing pathology can lead to continued neural degeneration and death that may compromise the effectiveness of the device and limit future strategies to improve performance. The use of drugs that can prevent nerve cell degeneration and promote their regeneration may improve clinical outcomes. In this paper we focus on strategies of delivering neuroprotective drugs to the auditory system in a way that is safe and clinically relevant for use in combination with a cochlear implant. The aim of this approach is to prevent neural degeneration and promote nerve regrowth in order to improve outcomes for cochlear implant recipients using techniques that can be translated to the clinic.
Keywords: drug delivery, cell-based therapy bionics, neural protection, neurotrophins, electrical stimulation
Introduction
Medical bionics and Neurodegeneration
Medical bionics is a rapidly expanding field combining biology and electronics for the development of neural prostheses that can be applied to treat neurological disorders. Specifically, this involves interfacing engineered bionic devices with the body to treat pathological conditions of the nervous system. To date, medical bionic devices have been successfully implemented to manage chronic neurological pain, through spinal cord stimulation; to treat movement disorders such as, Parkinson's disease, tremor and dystonia, through deep brain stimulation; and to enable some hearing for people with severe-profound hearing loss, through the cochlear implant (CI). The CI, in particular, has been highly successful, restoring some hearing function to over 220,000 people, with about two-fifths of those being children [1]. Similar technologies are now being utilised for the development of a bionic eye to restore some visual function to blind recipients.
Neural prostheses typically stimulate neural tissue that has undergone degenerative changes as a result of the underlying pathological condition. Therefore, technologies designed to minimise ongoing neural degeneration and improve the electrode-neural interface are important for improving device efficacy and may enable new strategies for activating nerve populations with electrical stimulation.
While there are numerous neural conditions for which medical bionics are currently used, and many others for which there exist potential future applications, this paper will focus on the auditory system, and the development of cell-based strategies for drug delivery to the inner ear to preserve and regrow the neurons of the cochlea, commonly called spiral ganglion neurons (SGNs). However, many of the strategies designed to deliver therapeutics in combination with a CI are also likely to be relevant to pathologies in other systems that can be treated with a bionic device.
Sensorineural Hearing Loss
Deafness is one of the most common health conditions in developed countries. In 2004, the World Health Organisation estimated that over 275 million people globally had moderate-to-profound hearing impairment, and as the population ages and people live longer, the incidence of hearing loss is going to increase. Sensorineural hearing loss (SNHL) is the most common form of deafness, accounting for approximately 80% of cases of hearing loss. SNHL typically occurs following damage to, or loss of, cochlear hair cells (HCs), with a complete loss of HCs leading to profound SNHL. For patients with a profound SNHL, the only therapeutic option to restore some hearing function is a CI (Figure 1).
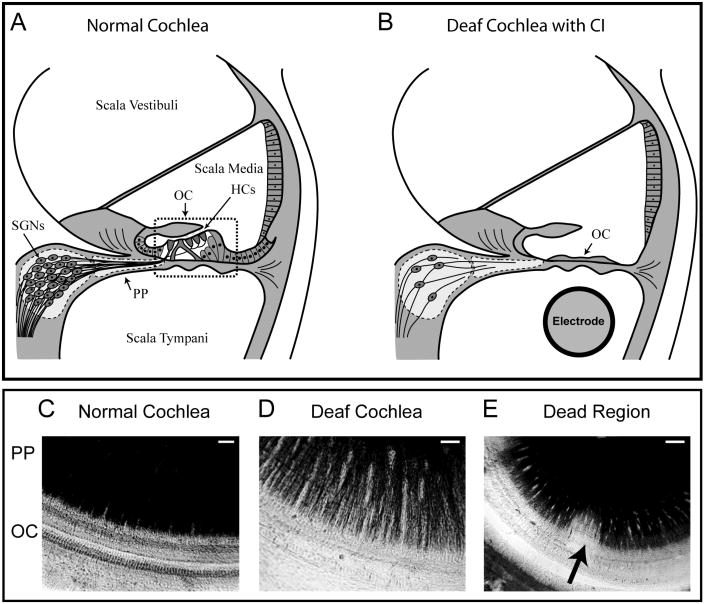
Figure 1.
Upper panel; Schematic diagram of a cross-section through a normal cochlea (A) showing the three fluid filled compartments of the cochlea – the scalae vestibuli, media and tympani. The sensory hair cells (HCs) are located in the organ of Corti (OC) and the cell bodies of the spiral ganglion neurons (SGNs) are located centrally. The SGN peripheral processes (PP) innervate the sensory HCs. In a deafened cochlea (B), cells within the OC are severely damaged, and a cochlear electrode array can be implanted to electrically activate SGNs. However, the PP continue to retract, ultimately leading to death of the SGNs. Lower panel: surface preparations, providing a top-down view of the OC and PP, show the PP stained black and the OC in a normal hearing guinea pig cochlea (C), a cochlea that has been deafened with aminoglycoside drugs (D), and a deafened cochlea that exhibits a localised region (‘dead region’) of greater loss of SGNs and their PP (E – arrow), (scale bar 20 μm C and D, 40 μm E).
Cochlear Implants
Cochlear implants bypass the damaged or missing HCs to directly electrically stimulate SGNs in order to provide the auditory cues necessary for sound perception and speech recognition. The CI has an electrode array that is inserted into the scala tympani compartment of the tonotopically organised cochlea (Figure 1) and typically delivers charge-balanced biphasic pulses to each electrode contact in a monopolar configuration with a return electrode located outside the cochlea. Electrical stimuli are delivered to different electrode contacts in order to activate different regions of the cochlea and provide cues relating to the spectral composition of incoming sound. Sound intensity is encoded by electrical stimulus intensity and the temporal properties of speech are encoded by the dynamics of the electrical stimulus envelope [2,3].
One of the significant limitations of contemporary CIs is the manner in which charge is delivered to the SGNs. The positioning of the array in the scala tympani, which is filled with perilymph (essentially a conductive saline solution), with a return electrode located outside the cochlea, leads to significant spread of electrical charge, resulting in relatively broad activation profiles [4,5,6]. This limits the spatial resolution of the device, due to overlap of stimulating currents and thus limits CI performance [7,8]. Therefore much attention has been concentrated on stimulation strategies designed to minimise spread of current in order to limit channel interactions [9] and increase the precision with which spectral and temporal information is presented via the CI.
To address this limitation, stimulation strategies which use the intracochlear electrode contacts as return electrodes have been developed, to ‘focus’ currents in order to activate more discrete populations of SGNs and provide greater spatial resolution [9]. However, such focussing strategies have not led to the expected improvements in clinical CI performance [10]. Complicating the matter is the fact that perceptual thresholds are higher and more variable for individual electrode contacts, meaning that relatively greater stimulation intensities are required to activate the SGNs in the perceptual dynamic range compared to conventional stimulation configurations [11,12,13].
In order to limit the effects of channel interactions caused by current spread, contemporary CIs use strategies that ensure that channels are not activated simultaneously whereby electrical stimuli are delivered by individual electrode contacts with non-overlapping stimulation [14,15]. Although stimulation strategies used in contemporary devices limit channel interactions, no stimulation strategy is able to provide fine temporal information. Temporal fine structure is most important for pitch perception [3] and the loss of this information compromises CI performance in noisy environments and the comprehension of speech in tonal languages that are rich in pitch information. However, the use of focussing strategies to deliver more spatially precise and independent channels of activation would enable the use of synchronous stimulation strategies that may enhance temporal fine structure.
A significant limitation constraining strategies designed to improve spatial resolution in cochlear implant recipients is the variability in neural responses to electrical stimulation [16]. Variation in SGN survival within the cochlea can lead to a poor electrode-nerve interface through localised neuronal loss, or ‘dead regions’, with poor nerve survival [12] (Figure 1E). Increased thresholds in regions with poor SGN survival would lead to higher current amplitudes and thus broader activation profiles with less precise neural activation [17]. The end result is that focussing strategies have not yielded the expected improvements in CI performance. Poor electrode-nerve interface is arguably less of a problem in conventional (monopolar) stimulation strategies, as the broader current spread means that SGN loss has much less of an influence on the selectivity of neural activation. Indeed, studies have failed to show a correlation between overall SGN survival and CI performance using contemporary stimulation strategies [18,19].
Therefore, factors such as SGN loss and distance of the electrode to the neural population are all significant limitations to the effectiveness of new stimulation strategies designed to improve the spatial and temporal resolution of CIs. Compounding these limitations is the fact that SGN degeneration is progressive over time. Degeneration is characterised by retraction of the peripheral processes away from the electrode array and towards the centrally located cell bodies of the SGN [20] (Figure 1B). Other changes, such as cell shrinkage, might also compromise SGN responses to electrical stimulation [20]. Therefore, there has been strong interest in research that aims to prevent or reverse SGN degeneration using therapeutics delivered to the cochlea in combination with a CI. If we can develop drug delivery strategies that improve SGN survival, and possibly promote SGN regrowth to improve the electrode-nerve interface, then it might be possible to combine this approach with new stimulation strategies to improve CI function.
Neurotrophins and the Auditory System
One approach that has received considerable research attention is the administration of neurotrophins (NTs) into the deafened cochlea in order to prevent SGN degeneration. Neurotrophins are naturally occurring proteins that are involved in the development and maintenance of the nervous system. The NTs brain derived neurotrophic factor (BDNF) and neurotrophin-3 (NT3) are produced by the hair cells [21,22] and supporting cells [23,24] of the organ of Corti within the cochlea, and are key to the development and ongoing survival of SGNs; loss of this endogenous neurotrophic support is a key factor in SGN degeneration.
It is well established that the exogenous application of NTs via mini-osmotic pumps [25,26,27,28,29,30,31,32,33,34], bolus injections [35] or slow release systems [36], rescues SGNs from deafness-induced degeneration. When the application of NTs has been combined with chronic electrical stimulation from a CI the SGN survival effects were enhanced over either treatment alone [31], and there were functional improvements in terms of reduced electrically-evoked auditory brainstem response thresholds [37,38,39]. Lower electrical thresholds would be advantageous for reducing current spread and minimising power consumption using current focussing strategies.
However, the supply of NTs using pump-based devices is finite, and the survival-promoting effects of NT delivery have not been shown to persist beyond two to four weeks following NT cessation [28,40,41]. The implication is that long term NT delivery is likely to be required for the survival effects to be maintained over time. Furthermore, the significant risk of infection associated with implantable pump devices, compounded with the likely need to refill the pump once the NTs are exhausted, preclude the use of pumps as a clinically relevant delivery method [42]. This has led to the development of drug delivery strategies that can be combined with a CI and translated to the clinic.
Drug delivery – Clinically viable therapeutic options
There are a number of options in terms of potential clinically viable methods for drug delivery to the cochlea (see [42,43]). These include drug-eluting polymers which can provide slow-release drug delivery based upon changes in the surrounding environment. For example, polypyrrole is an electroactive polymer that can be loaded with therapeutic compounds such as NTs. The release of these NTs can be induced and controlled via electrical stimulation, and can elicit nerve survival and regrowth in the inner ear [44]. However, there is a maximum amount of NT with which these polymers can be loaded and therefore the amount of NT available for release will diminish over time. Although diminished NT supply may compromise nerve survival, there is evidence that chronic electrical stimulation from a cochlear implant can potentiate the effects of NTs [31,45]. Therefore, it might be possible to maintain nerve survival when the NT supply is reduced, or even exhausted, with concurrent electrical stimulation from a cochlear implant [46].
The use of nanoporous particles as biocarriers for NT delivery may also have clinical application in the treatment of damaged nerves, with BDNF-loaded poly (L-glutamic acid) (PGA) nanoparticles eliciting survival effects on SGNs in an in vivo model of deafness [47]. Although the release profile of BDNF from these PGA particles indicated NT release for up to 70 days, most of the BDNF was released within the first month of treatment with lower levels released subsequently [47]. Further research is required to engineer particles that provide more even and sustained NT delivery over many months.
Cell-based therapies for preserving spiral ganglion neurons
Cell-based therapies using neuronal or sensory stem cells to repair or replace lost neural or sensory cell populations may one day restore lost function in CI recipients [48,49]. In addition to cell replacement, cell-based therapies may also be employed to deliver NTs to the cochlea. Such cell-based therapies involve the transplantation of cells which naturally, or via genetic manipulation, secrete therapeutic agents in a safe, efficient, consistent and physiologically relevant manner. We have previously reported that Schwann cells can be genetically modified to over-express BDNF, and that these BDNF-expressing Schwann cells support SGN survival in vitro [50]. For clinical application in deafness, cell-based therapies are likely to require the use of encapsulation technologies which encase the transplanted cells in a biocompatible, semi-permeable membrane (Figure 2A). The semi-permeable membrane allows for outward diffusion of the therapeutic proteins and other cellular metabolites, and inward diffusion of nutrients essential for cell survival. In addition, the encapsulation membrane provides an immunological barrier that protects allogeneic or xenogeneic cells from the host immune system. Furthermore, and particularly relevant in the case of the fluidfilled cochlea, encapsulation prevents cellular dispersal away from the site of implantation, as can occur following injection of cell suspensions [51].
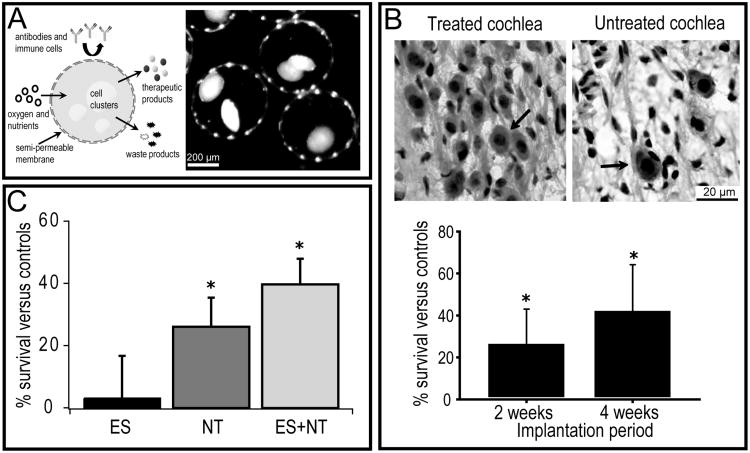
Figure 2.
(A) Schematic diagram illustrating the general concept of encapsulated cell-based therapies. Cells which secrete therapeutic products such as neurotrophins (NTs) are encapsulated in a biocompatible, semi-permeable membrane which allows for outward diffusion of the therapeutics as well as waste products, and inward diffusion of oxygen and nutrients for survival of the transplanted cells. The membrane also provides an immunoprotective barrier to the transplanted cells. The second panel in (A) shows an example of cells encapsulated in an alginate membrane; these are clusters of BDNF-expressing Schwann cells. (B) Photomicrographs showing spiral ganglion neurons (SGNs) (arrows) in the deaf guinea pig cochlea after implantation of BDNF-expressing Schwann cells (treated) or empty control capsules (untreated). Cell-based NT delivery using modified Schwann cells for a two and four week treatment period resulted in enhanced SGN survival in the treated cochlea compared to the control (modified from [52]). (C) Cell-based NT delivery using choroid plexus cells (cells that naturally produce and release neurotrophins), alone (NT) or in combination with chronic electrical stimulation (ES+NT) from cochlear implant, also enhanced SGN survival over a long-term (6 months) treatment period in the deafened cat (modified from [45]).
Importantly, BDNF-expressing Schwann cells encapsulated in an alginate membrane enhanced SGN survival in profoundly deaf guinea pigs (Figure 2B) [52]. Guinea pigs that were bilaterally deafened were implanted with either encapsulated BDNF-Schwann cells or empty control capsules, and the density of surviving SGNs (see upper panel Figure 2B) was quantified at two or four weeks post-implantation. At both time points (two and four weeks treatment), there was significantly greater SGN survival in the cochleae that received the encapsulated BDNF-Schwann cell implants as compared to those receiving the control capsules [52]. We have also reported that encapsulated choroid plexus cells (NTCell), which naturally secrete NTs, can be implanted long-term (6 months) in the deafened cat to promote SGN survival. These survival effects were enhanced with concurrent chronic electrical stimulation from a cochlear implant [45]. Furthermore, minimal inflammatory reactions were observed following these chronic cell implantations providing evidence that encapsulation technology is well tolerated in vivo [45]. In addition, other studies which have implanted NT-expressing cells into the rat brain have shown no adverse effects on the target or surrounding tissue following implantation for over 12 months [53]. These results provide evidence that cell-based therapies are safe for clinical application.
Cell-based therapies for preserving the peripheral processes
Previous studies have shown that NT treatment delivered by pump-based systems can protect SGN peripheral processes against deafness-induced retraction [32,34]. Consistent with these studies, we have shown that cell-based NT delivery was also effective in protecting peripheral processes within the osseous spiral lamina (OSL) of the cochlea (Figure 3A-D). This effect was enhanced with chronic electrical stimulation from a CI. Figure 3 shows examples of the peripheral processes in cross-sections taken through the OSL (at a location indicated by the dotted line in Figure 3C). There was a significantly greater density of processes in NT treated cochleae compared to the deafened control (contralateral and untreated) cochleae. There was no difference in peripheral process density in cochleae that received electrical stimulation on its own. When cell-based NT was provided with concurrent chronic electrical stimulation from a CI the survival promoting effect on the peripheral processes was enhanced.
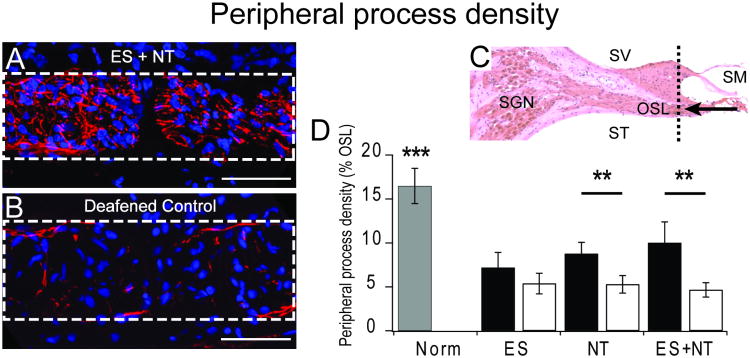
Figure 3.
(A and B) example images of cross sections through the osseous spiral lamina (OSL – dotted white box) showing peripheral processes stained with antibodies for neurofilament proteins (red) and cell nuclei stained with DAPI (blue; scale bar 50 μm). Greater process density was observed in the cochleae that received cell-based NT delivery in combination with chronic electrical stimulation from a CI (ES+NT) compared to the deafened control cochlea. (C) Sections were collected in the periphery of the cochlea (dotted black line) and are viewed from the direction of the arrow (SV – scala vestibuli; SM – scala media; ST – scala tympani; OSL – osseous spiral lamina). (D) Means (± standard errors) for peripheral process density within the OSL (dotted box in A and B) of normal hearing cochleae (grey bar), treated cochleae (black bars) and the untreated deaf, contralateral control cochleae (white bars) for each experimental cohort. NT treatment resulted in significantly greater density of peripheral processes compared to the deafened control cochleae. The effect was enhanced with chronic electrical stimulation (NT + ES). There was no effect of electrical stimulation on its own (ES).
In terms of implications for CI function, significant and ongoing peripheral process retraction limits the possible site of action potential initiation to a central location at, or near, the cell body. Animal and modelling studies indicate that a central location for spike initiation sites reduces the dynamic range and capacity to provide selective activation compared to a peripheral site of activation [54]. Lower thresholds of activation were observed when the stimulating electrode was placed close to the peripheral processes, and a shallower gradient in the input/output growth function for electrical stimulation was also observed [55]. An electrode array close to the peripheral processes is preferred [55] as it would be expected to provide more spatially restricted activation of SGNs and thus help to limit the broad activation profiles that normally occur with contemporary CI stimulation strategies. Therefore, drug delivery techniques that prevent retraction of the peripheral processes may also improve electrical thresholds, excitation growth functions and spatial selectivity of the CI.
Cell-based therapies for regrowth of peripheral processes
The SGN peripheral processes have an inherent capacity to regrow following damage to the organ of Corti [56,57] and this can be enhanced with NTs [32]. We have observed peripheral process regrowth following cochlear implantation and NT treatment with cell-based therapies [45]. Processes were observed to regrow into the scala tympani compartment (Figure 4A) and also within the scala media compartment (Figure 4B) of the cochlea. In the scala tympani, regrowing processes were incorporated into the fibrous tissue matrix that was associated with the tissue response to the intracochlear electrode (see schematic Figure 4C). The fibrotic tissue response is likely to provide a tissue matrix which supports the regrowth of the peripheral processes. Importantly, the regrowing processes were in close proximity to the electrode array, raising the possibility of harnessing regrowth using novel drug delivery techniques in order to bring the neurons closer to the electrode array and improve the electrode-nerve interface. Regrowth of peripheral processes towards the electrode array may lead to functional improvements in CI performance and may be a factor contributing to the reduced electrical thresholds observed with NT treatment [31,34,39].
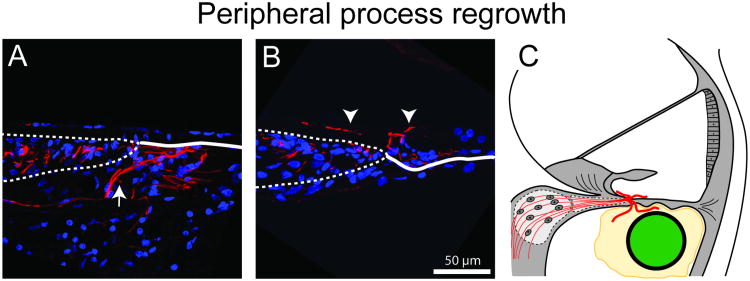
Figure 4.
Regrowth of the peripheral processes was observed following cell-based NT delivery. Ectopic processes were observed in the scala tympani (A arrow) and scala media (B arrow heads) compartments of the cochlea as depicted schematically (C). Interestingly, resprouting processes in the scala tympani were observed only in regions of the cochlea where there was a fibrotic tissue response surrounding the implanted electrode array. Presumably there was a dependence on a tissue scaffold provided by the tissue response to provide a matrix in which the processes could regrow. (Adapted from [45]).
However, several questions remain. It is not known whether the regrowing processes possess the neural machinery required to elicit functional responses to electrical stimulation. While there is evidence that resprouting processes can become remyelinated [34], we do not know if these processes express the ion channels required for action potential initiation or if they have nodes of Ranvier necessary for saltatory conduction. Therefore, regrowing peripheral processes may have different functional responses to electrical stimulation from an implant. NT-induced changes in peripheral process size, myelination, ion channel density and function is likely to influence the response characteristics to electrical stimulation from the CI. Furthermore, contrary to the potential benefit that peripheral process regrowth may provide, uncontrolled and extensive regrowth extending along the electrode array may potentially reduce the specificity of the cochlear implant. Extensive ectopic re-sprouting, particularly if processes project longitudinally along the electrode array, may act to reduce the precision of electrical stimulation delivered by a cochlear implant. Therefore, a key requirement of regrowing SGN peripheral processes to improve CI performance is to control the direction and extent of regrowth.
Gene therapy for the localised production of neurotrophins
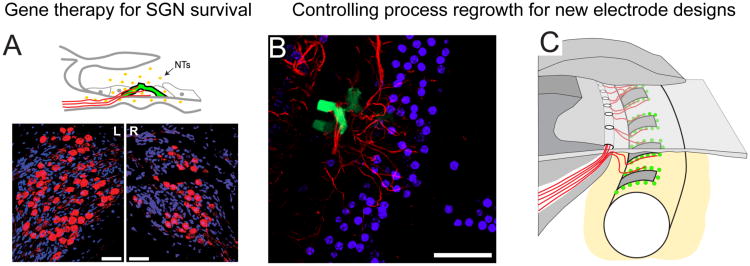
NT gene therapy has the potential to provide long-term NTs to protect SGNs from deafness-induced degeneration, and also to harness and control peripheral process regrowth, via a single application. By administering vectors containing the genes for NT production into the cochlea it is possible to transfect cochlear cells so that they produce and release NTs in a way that mimics their normal production and release. The aim is to produce a localised, cellular source of NTs in regions of the cochlea that are accessible to regrowing peripheral processes so that the processes grow towards, and possibly make contact with these NT-producing cells.
We have injected adenoviral vectors containing genes for NTs and the marker green fluorescent protein (GFP) into the scala media compartment of the ototoxically deafened guinea pig cochlea in order to transfect remaining supporting cells with genes for NT production (see schematic in Figure 5A). Using this approach we have shown increased SGN survival in the treated cochleae compared to the untreated contralateral cochlea (Figure 5A) [45,58]. NT gene therapy also has the potential to provide localised cues that have the capacity to attract and control regrowing peripheral processes. We found a significantly greater density of peripheral processes around cells that were transfected with genes for NT production compared to cells transfected with the GFP control vector (Figure 5B) [58] (see also [59]).
Figure 5.
(A) Schematic diagram illustrating supporting cells (pillar cells) transfected with NT genes (green GFP labelled) in the ototoxically damaged organ of Corti. The transfected cells produce and release NTs (yellow dots) that are accessible to the SGNs and their peripheral processes (red). Lower panels: NT gene therapy resulted in greater SGN survival in the treated (L) cochlea compared to the untreated contralateral cochlea (R), as indicated by a significantly greater density of SGNs in Rosenthal's canal (scale bar 50 μm). (B) A transfected pillar cell (green) in the organ of Corti (top down view). The peripheral processes (red) grew towards cells transfected with NT genes. There was a significantly greater density of processes around NT expressing cells compared to cells expressing the control (GFP only) vector. (C) Schematic illustration depicting the potential of using cell or gene therapy to provide localised sources of NTs in order to promote SGN survival and attract regrowing peripheral processes in a controlled manner. An electrode with this capability might have a greater density of electrode contacts to enable higher resolution and provide greater pitch and temporal information. (Figure 5A adapted from [62] and Figure 5B from [58]).
Viral vectors have the capacity to provide stable, long-term gene expression following a single application, and regulatable gene expression systems, in which transgene expression can be switched off if required, is an added advantage of this technology. While mutagenic integration and immune responses are current limitations [60], immunogenic responses to newer generation viral vectors are less severe [58,61,62], and the ongoing demonstration of the safety and efficacy of viral vectors through current clinical trials (see http://www.abedia.com/wiley/index.html) will continue to expand the possibilities for use in other systems and for other neurodegenerative conditions.
The possibility of using drug delivery therapies that can provide localised sources of NTs at physiologically relevant levels for increased SGN survival and are also capable of promoting targeted regrowth of the SGN may lead to new developments in CIs. New electrode arrays may incorporate localised cellular sources of NTs in order to promote regrowth towards the electrode array and improve the electrode-nerve interface. This may act to reduce the spread of SGN activation that is problematic for strategies aiming to increase spectral and temporal resolution. Furthermore, BDNF and NT3 are known to be differentially expressed along the length of the cochlea and exposure to these different NTs may lead to changes in the response characteristics of SGNs [63]. For example, when early postnatal (postnatal day 3 - day 8) SGNs that originate in the apex of the cochlea were exposed to BDNF they exhibited responses that were like SGNs that originate in the base of the cochlea. If NTs were to be incorporated into an electrode array then the production of different NTs along the array may lead to improvements in SGN function that are relevant to the particular region of the cochlea in which they reside, and therefore to improved functional outcomes.
Conclusion
Ongoing degeneration and loss of SGNs in the deafened cochlea can lead to a deterioration of the neural signals provided to the brain from a cochlear implant. Nerve cell loss can potentially compromise the efficacy and impede the development of new stimulation strategies that are designed to deliver focussed electrical stimulation in order to improve the spatial and temporal resolution of the device. The delivery of neuroprotective factors, such as NTs, to the cochlea can prevent SGN degeneration and promote regrowth of their peripheral fibres. However, clinically safe and effective techniques for drug delivery are yet to be established. Delivery strategies that use cells, both endogenous and exogenous, to produce and release NTs over extended periods of time offer an attractive means by which therapeutics can be delivered to the cochlea in combination with a cochlear implant.
Of considerable interest to the cochlear implant field is the potential of drug delivery technology that can be employed to preserve any residual hearing that a cochlear implant recipient may have before cochlear implantation. Clinically there is a significant risk of losing some (or all) residual hearing following implantation and therefore there is a need to develop strategies to prevent this loss. One potential cause of loss of residual hearing is an inflammatory response due to the presence of the cochlear electrode array. Future cochlear implants may combine drug delivery that not only prevents SGNs loss but also prevents the loss of any remaining hearing function, possibly by limiting the local inflammatory response to the electrode array.
While there is considerable effort to develop clinically relevant strategies to provide NTs for SGN survival, we believe these techniques also have significant potential for application in other neurodegenerative diseases. For example, cell- or gene-based therapies may be applied as a source of long-term NT delivery in Amyotrophic Lateral Sclerosis, Parkinson's disease or Alzheimer's disease, to prevent neural degeneration. Furthermore, the development of a retinal implant (bionic eye) for retinal degenerative and dystrophic diseases may benefit from combined electrical stimulation and application of NTs to support the survival of retinal ganglion cells. These techniques are not restricted to the delivery of NTs, and in fact have the potential to be used to deliver a variety of therapeutic substances.
Of fundamental importance to the effectiveness of a bionic device is to deliver electrical charge to a specific target neural population. Unwanted activation of non-targeted neurons can reduce device effectiveness and may lead to unwanted side effects. The delivery of drugs to protect the target neurons and to improve the electrode-nerve interface is likely to be an important strategy leading to improvements in outcomes for medical bionics. The development of clinically viable drug delivery methods which can be used in conjunction with medical bionics devices therefore has the potential to have a major impact on the treatment of a variety of neurodegenerative conditions for which there are limited or no current treatment options. Considering the social and economic impact of these conditions, the scope of potential benefits to be obtained from the combined application of medical bionics and drug delivery therapies is significant.
Acknowledgments
The authors would like to acknowledge the contributions of Dr James Fallon, Dr Rachael Richardson, Professor Robert Shepherd and Living Cells Technologies Limited (LCT). Funding was provided by the National Institutes of Health (HHS-N-263-2007-00053-C), The Garnett Passe and Rodney Williams Memorial Foundation, and the National Health and Medical Research Council of Australia. The Bionics Institute would like to acknowledge the support from the State Government of Victoria's Operational Infrastructure Program.
References
- 1.Cosetti MK, Waltzman SB. Cochlear implants: current status and future potential. Expert Rev Med Devices. 2011;8:389–401. doi: 10.1586/erd.11.12. [DOI] [PubMed] [Google Scholar]
- 2.McKay CM, Remine MD, McDermott HJ. Loudness summation for pulsatile electrical stimulation of the cochlea: effects of rate, electrode separation, level, and mode of stimulation. J Acoust Soc Am. 2001;110:1514–1524. doi: 10.1121/1.1394222. [DOI] [PubMed] [Google Scholar]
- 3.Smith ZM, Delgutte B, Oxenham AJ. Chimaeric sounds reveal dichotomies in auditory perception. Nature. 2002;416:87–90. doi: 10.1038/416087a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black RC, Clark GM, Tong YC, Patrick JF. Current distributions in cochlear stimulation. Ann N Y Acad Sci. 1983;405:137–145. doi: 10.1111/j.1749-6632.1983.tb31626.x. [DOI] [PubMed] [Google Scholar]
- 5.van den Honert C, Stypulkowski PH. Single fiber mapping of spatial excitation patterns in the electrically stimulated auditory nerve. Hear Res. 1987;29:195–206. doi: 10.1016/0378-5955(87)90167-5. [DOI] [PubMed] [Google Scholar]
- 6.Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J Neurophysiol. 2002;87:478–492. doi: 10.1152/jn.00212.2001. [DOI] [PubMed] [Google Scholar]
- 7.Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant listeners. J Acoust Soc Am. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- 8.Fu QJ, Nogaki G. Noise susceptibility of cochlear implant users: the role of spectral resolution and smearing. J Assoc Res Otolaryngol. 2005;6:19–27. doi: 10.1007/s10162-004-5024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Honert C, Kelsall DC. Focused intracochlear electric stimulation with phased array channels. J Acoust Soc Am. 2007;121:3703–3716. doi: 10.1121/1.2722047. [DOI] [PubMed] [Google Scholar]
- 10.Pfingst BE, Franck KH, Xu L, Bauer EM, Zwolan TA. Effects of electrode configuration and place of stimulation on speech perception with cochlear prostheses. J Assoc Res Otolaryngol. 2001;2:87–103. doi: 10.1007/s101620010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litvak LM, Spahr AJ, Emadi G. Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners. J Acoust Soc Am. 2007;122:967–981. doi: 10.1121/1.2749414. [DOI] [PubMed] [Google Scholar]
- 12.Goldwyn JH, Bierer SM, Bierer JA. Modeling the electrode-neuron interface of cochlear implants: effects of neural survival, electrode placement, and the partial tripolar configuration. Hear Res. 2010;268:93–104. doi: 10.1016/j.heares.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landsberger DM, Padilla M, Srinivasan AG. Reducing current spread using current focusing in cochlear implant users. Hear Res. 2012;284:16–24. doi: 10.1016/j.heares.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott H. An advanced multiple channel cochlear implant. IEEE Trans Biomed Eng. 1989;36:789–797. doi: 10.1109/10.32112. [DOI] [PubMed] [Google Scholar]
- 15.McDermott HJ, McKay CM, Vandali AE. A new portable sound processor for the University of Melbourne/Nucleus Limited multielectrode cochlear implant. J Acoust Soc Am. 1992;91:3367–3371. doi: 10.1121/1.402826. [DOI] [PubMed] [Google Scholar]
- 16.Azadpour M, McKay CM. A psychophysical method for measuring spatial resolution in cochlear implants. J Assoc Res Otolaryngol. 2012;13:145–157. doi: 10.1007/s10162-011-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z, Tang Q, Zeng FG, Guan T, Ye D. Cochlear-implant spatial selectivity with monopolar, bipolar and tripolar stimulation. Hear Res. 2012;283:45–58. doi: 10.1016/j.heares.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadol JB, Jr, Eddington DK. Histopathology of the inner ear relevant to cochlear implantation. Adv Otorhinolaryngol. 2006;64:31–49. doi: 10.1159/000094643. [DOI] [PubMed] [Google Scholar]
- 19.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 21.Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, et al. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- 22.Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 23.Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, et al. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci. 2012;32:405–410. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 26.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 27.Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, et al. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 28.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15:1121–1125. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- 30.McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- 33.Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, et al. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507:1602–1621. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- 34.Leake PA, Hradek GT, Hetherington AM, Stakhovskaya O. Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol. 2011;519:1526–1545. doi: 10.1002/cne.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson RT, O'Leary S, Wise A, Hardman J, Clark G. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res. 2005;204:37–47. doi: 10.1016/j.heares.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Noushi F, Richardson RT, Hardman J, Clark G, O'Leary S. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol. 2005;26:528–533. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, et al. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, et al. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycindeafened guinea pigs. J Neurosci Res. 2004;78:75–86. doi: 10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- 39.Landry TG, Wise AK, Fallon JB, Shepherd RK. Spiral ganglion neuron survival and function in the deafened cochlea following chronic neurotrophic treatment. Hear Res. 2011;282:303–313. doi: 10.1016/j.heares.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agterberg MJ, Versnel H, van Dijk LM, de Groot JC, Klis SF. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol. 2009;10:355–367. doi: 10.1007/s10162-009-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fransson A, Maruyama J, Miller JM, Ulfendahl M. Post-treatment effects of local GDNF administration to the inner ears of deafened guinea pigs. J Neurotrauma. 2010;27:1745–1751. doi: 10.1089/neu.2009.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettingill LN, Richardson RT, Wise AK, O'Leary SJ, Shepherd RK. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng. 2007;54:1138–1148. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Leary SJ, Richardson RR, McDermott HJ. Principles of design and biological approaches for improving the selectivity of cochlear implant electrodes. J Neural Eng. 2009;6:055002. doi: 10.1088/1741-2560/6/5/055002. [DOI] [PubMed] [Google Scholar]
- 44.Richardson RT, Wise AK, Thompson BC, Flynn BO, Atkinson PJ, et al. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 2009;30:2614–2624. doi: 10.1016/j.biomaterials.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wise AK, Fallon JB, Neil AJ, Pettingill LN, Geaney MS, et al. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics. 2011;8:774–787. doi: 10.1007/s13311-011-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242:100–109. doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan J, Wang Y, Yip X, Glynn F, Shepherd RK, et al. Nanoporous peptide particles for encapsulating and releasing neurotrophic factors in an animal model of neurodegeneration. Adv Mater. 2012;24:3362–3366. doi: 10.1002/adma.201200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunewardene N, Dottori M, Nayagam BA. The Convergence of Cochlear Implantation with Induced Pluripotent Stem Cell Therapy. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9320-0. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490:278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pettingill LN, Minter RL, Shepherd RK. Schwann cells genetically modified to express neurotrophins promote spiral ganglion neuron survival in vitro. Neuroscience. 2008;152:821–828. doi: 10.1016/j.neuroscience.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coleman B, Hardman J, Coco A, Epp S, de Silva M, et al. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant. 2006;15:369–380. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettingill LN, Wise AK, Geaney MS, Shepherd RK. Enhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pig. PLoS One. 2011;6:e18733. doi: 10.1371/journal.pone.0018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winn SR, Lindner MD, Lee A, Haggett G, Francis JM, et al. Polymer-encapsulated genetically modified cells continue to secrete human nerve growth factor for over one year in rat ventricles: behavioral and anatomical consequences. Exp Neurol. 1996;140:126–138. doi: 10.1006/exnr.1996.0123. [DOI] [PubMed] [Google Scholar]
- 54.Miller CA, Abbas PJ, Nourski KV, Hu N, Robinson BK. Electrode configuration influences action potential initiation site and ensemble stochastic response properties. Hear Res. 2003;175:200–214. doi: 10.1016/s0378-5955(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 55.Shepherd RK, Hatsushika S, Clark GM. Electrical stimulation of the auditory nerve: the effect of electrode position on neural excitation. Hear Res. 1993;66:108–120. doi: 10.1016/0378-5955(93)90265-3. [DOI] [PubMed] [Google Scholar]
- 56.Webster DB, Webster M. Multipolar spiral ganglion neurons following organ of Corti loss. Brain Res. 1982;244:356–359. doi: 10.1016/0006-8993(82)90097-x. [DOI] [PubMed] [Google Scholar]
- 57.Terayama Y, Kaneko K, Tanaka K, Kawamoto K. Ultrastructural changes of the nerve elements following disruption of the organ of Corti. II. Nerve elements outside the organ of Corti. Acta Otolaryngol. 1979;88:27–36. doi: 10.3109/00016487909137136. [DOI] [PubMed] [Google Scholar]
- 58.Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, et al. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–1122. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibata SB, Cortez SR, Beyer LA, Wiler JA, Di Polo A, et al. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp Neurol. 2010;223:464–472. doi: 10.1016/j.expneurol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seow Y, Wood MJ. Biological gene delivery vehicles: beyond viral vectors. Mol Ther. 2009;17:767–777. doi: 10.1038/mt.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson RT, Wise AK, Andrew JK, O'Leary SJ. Novel drug delivery systems for inner ear protection and regeneration after hearing loss. Expert Opin Drug Deliv. 2008;5:1059–1076. doi: 10.1517/17425247.5.10.1059. [DOI] [PubMed] [Google Scholar]
- 62.Wise AK, Tu T, Atkinson PJ, Flynn BO, Sgro BE, et al. The effect of deafness duration on neurotrophin gene therapy for spiral ganglion neuron protection. Hear Res. 2011;278:69–76. doi: 10.1016/j.heares.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci. 2002;22:1385–1396. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]