Abstract
The development of drug resistance by cancer cells is recognized as a major cause for drug failure and disease progression. PI3K/Akt/mTOR pathway is aberrantly stimulated in many cancer cells and thus it has emerged as a target for therapy. However, mTORC1 and S6K also mediate potent negative feedback loops that attenuate signaling via insulin/IGF receptor and other tyrosine kinase receptors. Suppression of these feedback loops causes over-activation of upstream pathways, including PI3K, Akt and ERK that potentially oppose the anti-proliferative effects of mTOR inhibitors and lead to drug resistance. A corollary of this concept is that release of negative feedback loops and consequent compensatory over-activation of pro-mitogenic pathways in response to signal inhibitors can circumvent the mitogenic block imposed by targeting only one pathway. Consequently, the elucidation of the negative feedback loops that regulate the outputs of signaling networks has emerged as an area of fundamental importance for the rational design of effective anticancer combinations of inhibitors. Here, we review pathways that undergo compensatory over-activation in response to inhibitors that suppress feedback inhibition of upstream signaling and underscore the importance of unintended pathway activation in the development of drug resistance to clinically relevant inhibitors of mTOR, Akt, PI3K or PI3K/mTOR.
Keywords: Rapamycin, Active-site mTOR inhibitors, Dual PI3K/mTOR inhibitors, Metformin, MEK inhibitors, Ras/Raf/ MEK/ERK
INTRODUCTION
Multicellular organisms have developed highly efficient mechanisms of receptor-mediated cell communication to integrate and coordinate the function and proliferation of individual cell types. In this context, the phosphoinositide 3-kinase (PI3K)/Akt/ mammalian target of rapamycin (mTOR) pathway plays a critical role in regulating multiple normal and abnormal biological processes, including metabolism, migration, survival, autophagy, lysosome biogenesis and growth (1). In response to different stimuli, including ligands of G protein-coupled receptors (GPCRs) and tyrosine kinase receptors (TRKs), PI3K catalyzes the formation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), a membrane lipid second messenger that coordinates the localization and activation of a variety of downstream effectors the most prominent of which are the isoforms of the Akt family (2). The Akts possess a PH domain and conserved residues (Thr308 and Ser473 in Akt1, the most commonly expressed isoform in normal cells) which are critical for Akt activation. Specifically, Akt translocated to the plasma membrane in response to products of PI3K, is activated by phosphorylation at Thr308 in the kinase activation loop and at Ser473 in the hydrophobic motif (1). The components of the PI3K pathway and the role of this pathway in disease have been reviewed (1,3).
mTOR functions as a catalytic subunit in two structurally distinct multiprotein complexes, mTORC1 and mTORC2 (1,4). mTORC1, a complex of mTOR, the substrate binding subunit Raptor, GβL, and PRAS40, senses nutrients and growth factors. mTORC1 phosphorylates and controls at least two regulators of protein synthesis, the 40S ribosomal protein subunit S6 kinase (S6K) and the inhibitor of protein synthesis 4E-binding protein 1, referred as 4EBP1 which promote translation of cell growth proteins, including c-Myc and cyclin D. mTORC1 also plays a critical role in the regulation of cellular metabolism (5). The heterodimer of the tumor suppressor tuberous sclerosis complex 2 (TSC2; tuberin) and TSC1 (hamartin) represses mTORC1 signaling by acting as the GTPase-activator protein for the small G protein Rheb (Ras homolog enriched in brain), a potent activator of mTORC1 signaling in its GTP-bound state. Phosphorylation of TSC2 by Akt and/or ERK/p90RSK uncouples TSC1/TSC2 from Rheb, leading to Rheb-GTP accumulation and mTORC1 activation. The Rag GTPases activate mTORC1 in response to amino acids, by promoting mTORC1 translocation to lysosomal membrane that contains Rheb-GTP (4). Ras-like (Ral) small GTPases, in their GTP-bound state, also promote mTORC1 activation through a pathway parallel to Rheb (6). Phosphatase and tensin homologue (PTEN) opposes PI3K by degrading PIP3 to PIP2 thereby inactivating Akt and mTOR signaling (7).
The PI3K/Akt/mTORC1 module is aberrantly activated in many human cancers, and plays a pivotal role in insulin/IGF receptor signaling. Inactivation of p53, as seen during the progression of ~50% of human malignancies, potently up-regulates the insulin/IGF-1/mTORC1 pathway (8). Consequently, mTORC1 and the upstream components of the cascade have emerged as attractive therapeutic targets in a variety of common malignancies (9).
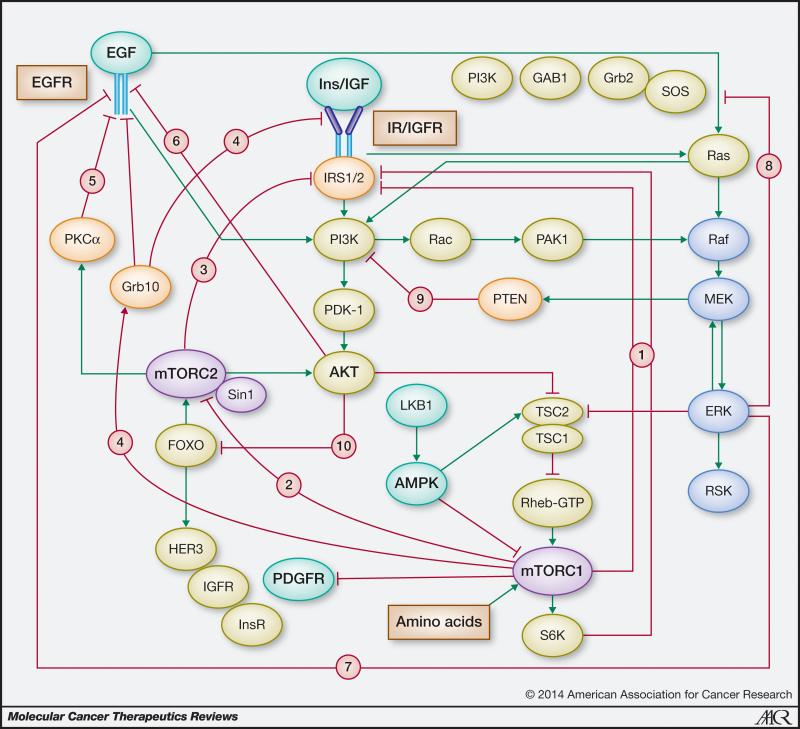
Mounting evidence indicates that the mTORC1/S6K axis not only promotes growth-promoting signaling but also mediates potent negative feedback loops that restrain upstream signaling through insulin/IGF receptor and other tyrosine kinase receptors in both normal and oncogene-transformed cells (Fig. 1). Suppression of these feedback loops by inhibitors of mTORC1/S6K causes compensatory over-activation of upstream signaling nodes, including PI3K, Akt and ERK that potentially oppose the anti-proliferative effects of the inhibitors and lead to drug resistance. To realize the therapeutic potential of targeting mTOR, it is necessary to elucidate the full spectrum of feedback loops that are unleashed by suppression of the PI3K/Akt/mTOR pathway. The detailed understanding of these feedback mechanisms will allow the design of rational combinations of therapeutic agents to overcome drug resistance produced by compensatory activation of upstream pathways and the identification of biomarkers to predict which patient will respond to them. The purpose of this article is to review negative feedback mechanisms that restrain signaling via upstream elements of the PI3K/Akt/mTOR pathway as well as mechanisms leading to the compensatory activation of other pro-oncogenic pathways, including MEK/ERK. The studies discussed here underscore the importance of unintended pathway activation in the development of drug resistance to clinically relevant inhibitors of mTOR, Akt, PI3K or PI3K/mTOR.
Figure 1.
Signaling through PI3K/mTOR and Ras/Raf/MEK/ERK pathways is controlled by negative feedback loops. Feedback loops emanate from distal elements of the same pathway (intrinsic negative loops) or from other pathways (extrinsic loops) and restrain the activity of upstream signaling nodes thereby fine-tuning the output of the signaling network. These potent negative feedback loops are indicated by the red lines and identified with numbers. Stimulatory connections are in green. See text for detailed description.
a) mTORC1 and mTORC2 mediate negative feedback of PI3K/Akt activation through inhibition and degradation of IRS-1
The insulin receptor substrate (IRS) docking proteins, including IRS-1 and IRS-2, play a key role in insulin/IGF signaling through PI3K. These proteins are phosphorylated by these receptors at multiple Tyr residues that play a critical role in downstream signaling, including PI3K activation. The IRS family is also phosphorylated at multiple serine and threonine residues that attenuate signaling and promote degradation. As illustrated in Fig. 1, loop 1, activation of the mTORC1/S6K cascade inhibits IRS-1 function, including PI3K/Akt activation, following its phosphorylation at multiple residues, including Ser636/639 by mTORC1 and Ser270/307/636/1001 by S6K (10). Accordingly, suppression of mTORC1 activity by rapamycin (sirolimus) and its analogs (rapalogs) prevents inhibitory phosphorylations mediated by mTORC1/S6K (11). Rapalogs, (e.g. RAD001/ everolimus) which act as allosteric inhibitors of mTORC1 via FKBP-12 were the first generation of mTOR inhibitors to be tested as anticancer agents.
A prominent consequence of mTORC1/S6K inhibition by rapalogs in cells, preclinical cancer models and clinical trials has been a striking increase in Akt phosphorylation at Thr308 by PDK1 and at Ser473 by mTORC2 (11-13). In this context, loss of PTEN expression which can potentiate feedback Akt phosphorylation in response to rapamycin is actually a marker of rapalog resistance rather than a biomarker for the use of mTORC1 inhibitors in human bladder cancer cells (14). mTORC2-mediated phosphorylation of Akt at Ser473 in response to rapamycin can be further enhanced by eliminating negative crosstalk from mTORC1/S6K (Fig. 1, loop 2). Specifically, Liu et. al. reported that phosphorylation of Sin1, a specific component of mTORC2 also known as mitogen-activated protein kinase-associated protein 1, at Thr86 and Thr398 suppresses mTORC2 kinase activity by dissociating Sin1 from mTORC2 (15). Sin1 phosphorylation, mediated by S6K in epithelial cells (Akt can also phosphorylate Sin1 in mesenchymal cells), inhibits not only insulin- or IGF-1-mediated, but also PDGF- or EGF-induced Akt phosphorylation by mTORC2 (15). Thus, these findings reveal a novel negative feedback loop connecting mTORC1 and mTORC2 and an additional mechanism by which exposure to rapamycin enhances Akt phosphorylation at Ser473.
A recent study by Jacinto et. al. (16) revealed that mTORC2 can also regulate the cellular level of IRS-1. These investigators found that despite phosphorylation at the mTORC1-mediated serine sites, inactive IRS-1 accumulated in mTORC2-disrupted cells. Defective IRS-1 degradation was due to diminished expression and phosphorylation of the ubiquitin ligase substrate-targeting subunit, Fbw8 (16). mTORC2 stabilizes Fbw8 by phosphorylation at Ser86, allowing the insulin-induced translocation of Fbw8 to the cytosol where it mediates IRS-1 degradation. Thus, mTORC2 negatively feeds back to IRS-1 via control of Fbw8 stability and localization (Fig. 1, loop 3). These findings indicate that mTORC1 and mTORC2 cooperate in promoting IRS-1 degradation and imply that the potential therapeutic benefit of inhibiting mTORC1 with rapamycin is opposed by release of feedback inhibition of PI3K/Akt activation (11,13), resulting in disease progression. Although rapalogs have been demonstrated to prolong overall survival of patients with metastatic renal cell carcinoma, the clinical antitumor activity of rapamycin analogues in many types of cancer has been rather limited. In some cases, mutated cancer genes can serve as biomarkers of response to targeted agents. So far, the use of PTEN, PI3K mutations and Akt phosphorylation as biomarkers for predicting rapalog sensitivity has not been successful in clinical settings. In fact, as mentioned above, loss of PTEN expression may be a marker of rapalog resistance, at least in some cancer cells. It is likely that treatment with rapalogs not only interferes with feedback loops that restrain PI3K/Akt activation but also with other signaling pathways that can promote drug resistance, as discussed below.
b) Rapamycin-induced ERK over-activation
In addition to the feedback loop that restrains PI3K/Akt activation, inmunohistochemical analysis of biopsies of breast cancer patients that were treated with the rapalog RAD001 (everolimus) revealed that there was a marked increase in ERK activation, i.e. ERK phosphorylated on the activation loop residues Thr202 and Tyr204 (17). These results indicated that anticancer therapy with allosteric mTORC1 inhibitors can lead to activation of the ERK pathway, thus adding a new level of complexity to the previously described negative feedback loop involving mTORC1/PI3K/Akt. Based on experiments using inhibitors of PI3K (LY294002) and a dominant-negative form of Ras (RasN17), Carracedo et. al. (17) concluded that ERK over-activation in response to rapamycin depended on the function of PI3K/Ras but the mechanism(s) was not defined. In all the experiments presented, the cells were exposed to the rapamycin for at least 24h (17). Thus it is not clear whether the putative PI3K-dependent pathway is an acute effect of unleashing a rapid feedback loop or a slow feedback loop involving a transcriptional response (see below).
A recent study with breast cancer cells harboring PI3KCA mutant or HER2 amplification but without RAS mutations suggest a possible mechanism by which PI3K can lead to ERK pathway activation (18). Specifically, PI3K-mediated PIP3 accumulation increased the activity of Rac1 (Rac1-GTP) via PIP3-dependent Rac exchanger 1 (P-Rex1) and of its effector PAK1 leading to phosphorylation of Raf1 at the activating Ser338. These findings imply that robust PI3K-mediated Rac/PAK1 can enhance Raf stimulation and thereby promote MEK/ERK over-activation (Fig. 1). It will be of interest to determine whether rapamycin-induced ERK is correlated to P-Rex1 expression, Rac-GTP and Raf Ser338 phosphorylation. A putative alternative pathway of PIP3-dependent ERK activation involves the recruitment of the adaptor Grb2-associated binder 1 (GAB1) which in turn recruits Grb2–SOS, leading to Ras/Raf activation (19). In this context, it is also relevant that long-term exposure to rapamycin also initiates transcriptional up-regulation of PI3K subunits, e.g p85α and p110δ(20), potentially reinforcing the PI3K- mediated signaling to Rac/PAK1 and/or GAB1/Grb2/SOS which can lead to MEK/ERK over-activation in response to rapamycin.
Another mTORC1-mediated negative feedback loop restrains the expression of platelet-derived growth factor receptor (PDGFR). Activation of PI3K or Akt, or deletion of PTEN in mouse embryonic fibroblasts suppresses PDGFR expression whereas rapamycin increases PDGFR expression (21). In hepatocellular carcinoma cells prolonged (>6 h) treatment with rapamycin induced ERK signaling through increased expression and phosphorylation of PDGFRβ (22). The role of this PDGFRβ-dependent loop leading to ERK signaling in other cancer cells requires further experimental work. The remodeling of the signaling network in response to rapalogs is illustrated in Fig. 2A.
Figure 2.
A) Compensatory over-activation of signal transduction pathways induced by rapamycin-mediated suppression of negative feedback loops. Rapamycin triggers PI3K activation and Akt phosphorylation at Thr308 and Ser473 via suppression of mTORC1/S6K-mediated phosphorylation of IRS-1 and Sin1 (loop 1). mTORC2-mediated phosphorylation of Akt at Ser473 in response to rapamycin can be further enhanced by eliminating negative crosstalk from S6K (loop 2). Furthermore, mTORC2 negatively feeds back to IRS-1 via control of its stability (loop 3). In some cancer cells, rapalogs also induce MEK/ERK via PI3K- dependent pathway that could involve Rac/PAK1 and/or PDGFR. B) Compensatory over-activation of signal transduction pathways induced by active-site mTOR inhibitors. Active-site mTOR inhibitors which block both mTORC1 and mTORC2, also eliminate feedback loops that restrain PI3K/PDK1 activation. Specifically, these agents disable feedback loops 1, 2, 3, 4, 5, 6 and 10. Active-site mTOR inhibitors enhance Akt phosphorylation at the activation loop (Thr308) and consequently these agents do not completely block Akt activity. Acute exposure of a variety of cell types to active-site mTOR inhibitors induced a striking over-activation of MEK/ERK pathway via a PI3K-independent pathway. Chronic exposure to these agents also disables the negative influence of Akt on FOXO thereby promoting expression of tyrosine kinase receptors and adaptors. For additional details see text. Inhibitory connections are in red. Stimulatory connections are in green. Pathways activated by suppression of negative feedback loops are highlighted in yellow.
c) Compensatory activation of PI3K and ERK signaling in response to active-site mTOR inhibitors and dual PI3K/mTOR inhibitors
As discussed above, the potential anti-cancer activity of rapamycin (or analogs) can be counterbalanced by release of feedback inhibition of PI3K/Akt and ERK activation. Furthermore, rapamycin incompletely inhibits 4E-BP1 phosphorylation (23,24). Specifically, most cells display a high basal level of 4E-BP1 phosphorylation at Thr37/46 that is not further increased by growth factor stimulation nor inhibited by rapamycin (25). However, cell stimulation reduced the mobility of 4E-BP1 in SDS/PAGE, a response suggestive of increased phosphorylation at other sites. Indeed, growth factor stimulation of pancreatic cancer cells markedly stimulated 4EBP1phosphorylation on Thr70, a response blocked by treatment with rapamycin (25). These results revealed an unappreciated regulation of 4E-BP1 phosphorylation on different residues in response to external signals and demonstrate that rapamycin inhibits inducible but not constitutive 4E-BP1 phosphorylations. More studies are needed to determine whether 4E-BP1 is subject to constitutive and inducible phosphorylations at different sites in different cancer cells.
In an effort to target the mTOR pathway more effectively, novel ATP-competitive inhibitors of mTOR that act at its catalytic active site (active-site mTOR inhibitors) have been identified, including PP242 (26), Torin (27), KU63794 (28) and its analogue AZD8055 (29). These compounds inhibit 4E-BP1 phosphorylation at rapamycin-resistant sites (e.g. Thr37/46) and block Akt phosphorylation at Ser473, in line with the notion that mTORC2 is the major protein kinase that phosphorylates Akt at this residue. Active-site inhibitors proved more effective inhibitors of cell proliferation than rapamycin in a variety of model systems. However, active-site mTOR inhibitors also eliminate negative feedback loops that restrain PI3K activation and consequently, their therapeutic effectiveness can also be diminished by activation of upstream pathways that oppose their anti-proliferative effects (Fig. 2B). Specifically, active-site mTOR inhibitors enhance PI3K/PDK-dependent Akt phosphorylation at its activation loop (Thr308) and consequently these agents do not completely block Akt activity (30).
Surprisingly, short-term exposure of a variety of cell types, including human pancreatic cancer cells (25) and multiple myeloma cells (31), to active-site mTOR inhibitors, such as KU63794 or PP242, induced a striking over-activation of ERK. The mTOR inhibitors also induced MEK over-activation, as scored by assessing the phosphorylation of Ser217 and Ser221 in the MEK activation loop, and MEK inhibitors abrogated the over-activation of MEK. In contrast, treatment with rapamycin at concentrations that completely prevented the mTORC1/S6K axis, as scored by phosphorylation of S6K on Thr389 did not cause any change in ERK phosphorylation in cells harboring RAS mutations (25,31). These results indicated that first and second generations of mTOR inhibitors promote over-activation of different upstream prooncogenic pathways in a cell-context dependent manner.
Further evidence supporting that active-site inhibitors enhance ERK over-activation through a PI3K-independent feedback loop was obtained by determining the effect of KU63794 or PP242 on ERK activity in multiple myeloma cells treated with wortmannin (31) or pancreatic cancer cells treated with A66, a selective inhibitor of the p110α catalytic subunit of PI3K (25). Inhibition of PI3K did not prevent enhancement of ERK activation in response to active-site mTOR inhibitors. These results identified a PI3K-independent feedback loop regulating the crosstalk between the mTOR and MEK/ERK pathways which is different from the loop previously identified with rapamycin (17). The remodeling of the signaling network in response to active-site mTOR inhibitors is illustrated in Fig. 2B.
The fact that PI3K and mTOR have high homology in their kinase domains has made possible the development of dual active-site inhibitors (PI3K/TOR-KIs), including NPV-BEZ235(32), PKI-587 (33) and GDC-0980 (34). Additional PI3K/TOR-KIs that are being tested in preclinical and clinical trials include XL765, NVP-BKM120, XL147, SF1126, GSK2126458, VS-5584 and PF-04691502. As mentioned above, over-activation of the ERK pathway induced by active-site mTOR inhibitors is mediated through a PI3K-independent pathway (25,31). Therefore, it could be expected that dual PI3K and mTOR inhibitors also promote ERK over-activation. In line with this prediction, our current studies demonstrate ERK over-activation in pancreatic cancer cells treated with multiple clinically relevant PI3K/TOR-KIs, including NPV-BEZ235, PKI-587 and GDC-0980 (Soares, Sinnett-Smith and Rozengurt, manuscript in preparation). These results with dual PI3K and mTOR catalytic kinase inhibitors provide conclusive evidence identifying a novel PI3K-independent feedback loop that restrains the activity of the MEK/ERK pathway. The remodeling of signaling in response to active-site PI3K/mTOR inhibitors is illustrated in Fig. 3A.
Figure 3.
A) Compensatory over-activation of signal transduction pathways induced by dual PI3K/mTOR inhibitors. Dual PI3K/mTOR inhibitors disable feedback loops 1, 2, 3, 4, 5, 6 and 10. Acute exposure of a variety of cell types to dual PI3K/mTOR inhibitors induced over-activation of MEK/ERK pathway via a PI3K-independent pathway, probably involving Grb2/SOS-mediated Ras activation as a result of tyrosine kinase receptor and/or IRS activation. Chronic exposure to these agents also promotes FOXO-mediated expression of tyrosine kinase receptors and adaptors. B) Compensatory over-activation of signal transduction pathways induced by MEK inhibitors. These inhibitors disable feedback loops 7, 8 and 9 leading to Ras/Raf and PI3K/Akt over-activation. See text for detailed description. Inhibitory connections are in red. Stimulatory connections are in green. Pathways activated by suppression of negative feedback loops are highlighted in yellow.
d) Mechanism(s) by which mTOR and PI3K/mTOR inhibitors stimulate MEK/ERK signaling
A plausible mechanism by which active-site mTOR inhibitors or dual PI3K/mTOR inhibitors relieve a negative feedback on receptor tyrosine kinases that leads to Raf/MEK/ERK has been suggested by recent phosphoproteomic studies demonstrating that mTORC1 directly phosphorylates the adaptor protein growth factor receptor-bound protein 10 (Grb10) at multiple sites (35,36). Grb10 is known to suppress signaling induced by insulin and IGFs (37) and mice lacking Grb10 are larger than normal and exhibit enhanced insulin sensitivity (38). In addition to inhibiting insulin/IGF1 receptor tyrosine kinase activity by direct binding, Grb10 also mediates degradation of these receptors through ubiquitination (39). The phosphorylation of Grb10 by mTORC1 enhances its stability and capacity to inhibit insulin/IGF signaling. The sites phosphorylated by mTORC1 were mapped to Ser104, Ser150, Thr155, Ser428, and Ser476 (35). While active-site mTOR inhibitors blocked phosphorylation of Grb10 at all five sites, rapamycin only prevented Grb10 phosphorylation at Ser476 (35). Therefore, Grb10, like 4E-BP1, is an mTORC1 substrate with both rapamycin-sensitive and -insensitive sites. Since the phosphorylation of Grb10 potentiates its inhibitory activity on insulin/IGF receptor signaling, acute suppression of Grb10 phosphorylation at all sites (by direct mTOR inhibitors or dual PI3K/mTOR inhibitors) eliminates its ability to attenuate insulin/IGF signaling (Fig. 1, loop 4) thereby leading to MEK/ERK activation.
Another potential mechanism by which active-site mTOR or dual PI3K/mTOR inhibitors could promote MEK/ERK signaling is via enhanced EGFR activity. The EGFR tyrosine kinase activity and affinity for its ligand is known to be negatively regulated by PKCα via phosphorylation at Thr654 (40). Recent studies indicated that mTORC2 mediates PKCα phosphorylation of both the turn and hydrophobic motifs (41,42). Interestingly, the mTORC2-dependent phosphorylation of PKCα plays an important role in its maturation, stability and signaling (41,42). It is plausible, therefore, that suppression of mTORC2-mediated post-translational processing of PKCαinterferes with negative feedback of PKCα on EGFR thereby leading to hyper-activation of EGFR and over-activation of ERK signaling in response to EGFR agonists or G protein-coupled receptor transactivation (43), as illustrated in Fig 1, loop 5.
A recent study examining the role of Akt in EGFR trafficking elucidated a novel negative feedback mechanism (44). Specifically, EGF-induced activation of Akt promotes progression of internalized EGFR through the early endosomes and EGFR degradation. In cells treated with inhibitors of PI3K or Akt, EGFR trafficking was impaired and accumulated in the early endosomes, resulting in increased ERK activation (44). It is conceivable that Akt inhibition interferes with this negative feedback loop (Fig. 1, loop 6) thereby promoting EGFR accumulation and enhanced ERK signaling in cells with a hyperactive EGFR signaling system. Accordingly, it will be of interest to determine whether EGFR inhibitors potentiate the inhibitory effects of Akt inhibitors.
In addition to MEK/ERK, dual PI3K/mTOR inhibitors can also induce compensatory activation of other signaling pathways that mediate resistance to these drugs. In triple-negative breast cancer (TNBC), a clinically aggressive subtype of breast cancer defined by lack of expression of estrogen and progesterone receptors and HER2 amplification, PI3K/mTOR inhibition induced feedback activation of JAK2/STAT5 and secretion of IL-8 in cell lines and primary breast tumors (45). In TNBC, inhibition of JAK2 abrogated this feedback loop and combined PI3K/mTOR and JAK2 inhibition synergistically reduced cancer cell number and tumor growth (45).
e) Mechanism(s) by which MEK inhibitors stimulate PI3K/Akt signaling
It is pertinent that crosstalk between PI3K/Akt/mTOR and MEK/ERK pathways also functions in the opposite direction. Specifically, MEK inhibitors have been shown to induce PI3K/Akt activation via EGFR (46), thus revealing a negative feedback loop mediated by ERK phosphorylation of EGFR (e.g. at Thr669) that restrains PI3K/Akt activation and PIP3 accumulation (47). This negative feedback (Fig. 1, loop 7) could also underlie the different responses of colon and melanoma cancer cells (both with BRAF V600E mutations) to BRAF inhibitors (48,49). While melanomas are highly sensitive to BRAF inhibitors, colon cancers harboring the identical BRAF V600E mutation are resistant to these agents. Two elegant studies demonstrated that the drug resistance of colon cancer cells is due to increased expression and signaling of EGFR in these cells (48,49). Previously, we mentioned that EGFR signaling is negatively regulated by PKCα (40). Interestingly, PKCα is expressed in melanoma (50) but decreased in most colorectal cancers (51), suggesting another mechanism by which EGFR signaling could be stronger in colon cancers following release of feedback inhibition. Consequently, release of feedback inhibition by BRAF inhibitors induces stronger EGFR activation in colon cells thereby recovering ERK pathway activation via alternative pathways (CRAF instead BRAF) as well as enhancing EGFR-induced PI3K/Akt signaling.
Another feedback loop could also involve the ERK-regulated p90RSK which has been shown to phosphorylate (at Ser1101) and inhibit IRS-1 (52) and ERK-mediated feedback of Ras/Raf activation, e.g. via phosphorylation of SOS (Fig. 1, loop 8). More recent work has demonstrated that MEK1 as an essential regulator of the lipid/protein phosphatase PTEN, through which restrains PIP3 accumulation and Akt signaling. MEK1 has been shown to be required for PTEN membrane recruitment as part of a ternary complex containing the multidomain adaptor MAGI1 (53). Complex formation depends on phosphorylation of MEK1 at Thr292 by activated ERK. Consequently, ERK inhibition by MEK inhibitors prevents PTEN membrane recruitment, increasing PIP3 accumulation and Akt activation (Fig. 1, loop 9).
Reciprocal feedback loops connecting PI3K/Akt/mTOR and MEK/ERK pathways provide further impetus for developing combination of inhibitors that co-target both pathways. Indeed, phase 1 clinical trials co-targeting these pathways with MEK inhibitors plus PI3K/mTOR inhibitors are ongoing (e.g. Clinicaltrial.gov identifiers NCT01347866; NCT01390818). The remodeling of the signaling network in response to MEK inhibitors is illustrated in Fig. 3B.
f) Chronic exposure to PI3K/PDK1/Akt inhibitors suppresses a feedback loop that mediates repression of tyrosine kinase receptor and survival protein expression
In addition to ERK and Akt over-activation in response to acute mTOR pathway inhibition, a number of studies demonstrated that long-term treatment with PI3K, Akt or PI3K/mTOR inhibitors induces a transcriptional response that also leads to drug resistance (54-59). The forkhead box O (FoxO) transcription factors, which include FoxO1, 3, 4, and 6 in mammals, are major downstream targets of the Akts. The phosphorylation of FoxO by Akt creates docking sites for 14-3-3 proteins. The binding of 14-3-3 to FoxO promotes its translocation from the nucleus to the cytoplasm. Reciprocally, inhibition of Akt activity releases a feedback loop that promotes nuclear localization of the FoxO transcription factors (Fig. 1, loop 10). Nuclear FoxO family members stimulate transcription of several tyrosine kinase receptors, including EGFR, IGFR and insulin receptor in a spectrum of tumor cells (54). Furthermore, a recent study showed that FoxOs up-regulated the expression of Rictor, thereby enhancing mTORC2 and Akt phosphorylation at Ser473 (60), thus creating an amplification loop. At least in some cancer cells, FoxO-mediated transcription cooperates with enhanced cap-independent translation mediated by Pim-1 (58).
Accordingly, recent pre-clinical and clinical studies revealed that suppression of PI3K, Akt or PI3K/mTORC1 initiates transcriptional responses that lead to the over-expression of tyrosine kinase receptors or adaptor proteins, including HER3, IGFR, FGFR and in some cases, consequent enhancement of ERK. Dimerization of HER3 with EGFR or HER2 then promotes resistance to a number of inhibitors of PI3K signaling (61). Chronic exposure of HER2-positive breast cancer models to NPV-BEZ235 induced activation of HER family of receptors and adaptors leading to ERK over-activation, as shown by increased expression of HER3 and phosphorylation of HER2 and HER3 (56). In these breast cancer cells, ERK over-activation was completely prevented by inhibitors of MEK or HER2, suggesting clear combinatorial strategies to circumvent resistance to PI3K/Akt inhibition. Compensatory activation of HER3 and ERK has been corroborated in clinical samples following treatment with GDC-0068, an inhibitor of Akt catalytic activity (59). In contrast, treatment with a PI3K inhibitor (XL147) promoted expression of several tyrosine kinase receptors but did not stimulate ERK over-activation (55). In ovarian cancer cells, NVP-BEZ235 also induces a program leading to expression of receptors and survival proteins, at least in part due to enhanced cap-independent translation, but do not appear to stimulate ERK signaling (57). In conclusion, treatment of a variety of tumor cells with inhibitors that block the PI3K/Akt/mTOR pathway at each level induces a concerted transcriptional response mediated, at least in part by FoxO family members that oppose the anticancer effects of these agents. This gene expression loop should be distinguished from the rapid MEK/ERK overactivation induced by mTOR and dual PI3K/mTOR inhibitors in other cell types (25,31). The FOXO-mediated transcriptional response on signaling in response to active-site mTOR inhibitors and dual PI3K/mTOR inhibitors are highlighted in Figs. 2 and 3.
g) Metformin inhibits mTORC1 but does not elicit Akt or ERK over-activation: role of AMPK
Metformin (1,1-dimethylbiguanide hydrochloride) is the most widely prescribed drug for treatment of type 2 diabetes mellitus (T2DM) worldwide but its mechanism of action remains incompletely understood. At the cellular level, metformin indirectly stimulates AMP–activated protein kinase (AMPK) activation via inhibition of mitochondrial complex I (62), though other cellular mechanisms of action have been proposed, especially at high concentrations (63,64). AMPK is a conserved regulator of the cellular response to low energy, and it is activated when the ATP concentration decreases and 5’-AMP and ADP concentrations increase. AMPK, a potent inhibitor of anabolic metabolism, is also implicated in the regulation of epithelial cell polarity (65). The tumor suppressor LKB-1/STK11 (Liver kinase B1/serine–threonine kinase 11) is the major kinase phosphorylating the AMPK activation loop at Thr172.
Recent epidemiological studies are linking administration of metformin with reduced incidence, recurrence and mortality of a variety of cancers in T2DM patients (66). Although epidemiological associations do not establish causation, they provide an important line of evidence that supports the need for mechanistic studies. The protective effects of metformin in human cancers could be mediated by direct suppression of mitogenic signaling through AMPK-dependent and/or AMPK-independent pathways. It is well established that metformin inhibits mTORC1 activation in a variety of cancer cell types (67). At low concentrations of metformin, the inhibitory effect on mTORC1 is prominent in cells incubated in medium containing physiological concentrations of glucose (68). Studies in vitro demonstrated that AMPK inhibits mTORC1 activation at several levels: 1) AMPK stimulates TSC2 function via phosphorylation on Ser1345 (69), leading to accumulation of Rheb-GDP (the inactive form) and thereby to inhibition of mTORC1; 2) AMPK directly phosphorylates Raptor (on Ser722 and Ser792), which disrupts its association with mTOR, leading to dissociation of mTORC1 (70); 3) Metformin has also been proposed to inhibit mTORC1 via AMPK-independent pathways, targeting Rag GTPases or cyclin D1 but these effects were elicited at very high concentrations. Direct effects of metformin at clinically relevant doses are of great significance because they imply that this drug will be a useful anticancer agent not only for T2DM patients but also for non-diabetic patients.
Although metformin inhibits the mTORC1/S6K axis, its effects on feedback loops regulating Akt and ERK activation are very different from rapalogs, active-site mTOR inhibitors and dual PI3K/mTOR inhibitors (25). For example, metformin, in contrast to rapamycin, did not over-stimulate Akt phosphorylation on Ser473 although both rapamycin and metformin strongly inhibited the mTORC1/S6K axis. Although the precise mechanism explaining this difference is not fully understood, it is relevant that AMPK directly phosphorylates IRS-1 on Ser794, a site that interferes with PI3K activation (71,72). In addition, mTORC2 phosphorylates Akt not only at Ser473 but also at the turn motif site (Thr450 ) of Akt required for its proper folding (41,42). A recent study demonstrated that a high level of cellular ATP levels is required to maintain the integrity of mTORC2-mediated phosphorylation of Akt on the turn motif Thr450 site (73). Because metformin inhibits mitochondrial ATP production, it is conceivable that a small decline in ATP levels induced by this biguanide interferes with the integrity of mTORC2 and with its ability to phosphorylate Akt at Ser473, another mechanism that would prevent Akt over-activation even when the mTORC1/S6K axis is suppressed.
An important difference between the effects of metformin and mTOR inhibitors is that metformin inhibited rather than over-activated MEK/ERK in response to growth factors (25). A plausible mechanism underlying the inhibitory effect metformin on MEK/ERK activation is suggested by a recent study showing that AMPK phosphorylates BRAF at Ser729 (74). The phosphorylation of this site promotes the association of BRAF with 14-3-3 proteins and disrupts its interaction with the KSR1 scaffolding protein leading to decrease in the activity of the MEK-ERK pathway. Interestingly, ERK signaling was not decreased by AMPK in BRAF mutant tumors (74). Collectively, these studies in vitro imply that metformin has considerable advantages in promoting mTOR inhibition without unleashing feedback loops that oppose its antiproliferative effects, though these effects are likely depend on cell context and oncogenic mutations. A number of clinical trials in progress, combining metformin with established anticancer agents, will determine whether metformin is useful in cancer therapeutics. Since AMPK appears to prevent tumor development rather than to inhibit advanced malignancies, it is conceivable that metformin will be more useful in chemoprevention rather than in a therapeutic setting.
Concluding remarks and clinical implications
One of the first indications that cells can be stimulated to reinitiate DNA synthesis through different molecular pathways that act in a combinatorial manner was obtained from studies using multiple growth factors in Swiss 3T3 fibroblasts (75). Subsequent studies substantiated the concept that multiple parallel pathways that crosstalk and converge on key signaling nodes lead to proliferation of both normal and cancer cells. A corollary of this concept is that release of negative feedback loops and consequent compensatory over-activation of pro-mitogenic pathways in response to signal inhibitors can circumvent the mitogenic block imposed by targeting only one pathway. Consequently, the elucidation of the network of feedback loops that regulate signal transduction outputs of complex signaling networks has emerged as an area of fundamental importance for the rational design of effective anticancer combinations of inhibitors. In recent years, it has become apparent that most signaling pathways are controlled by negative feedback loops that fine-tune the signaling network and that in many cases, the success of therapies targeting one pathway is thwarted by the compensatory over-activation of upstream pathways that remodeled the network.
Here, we discussed that inhibition of mTOR or PI3K/mTOR induces rapid signaling in a variety of cancer cells through compensatory over-activation of prooncogenic and pro-survival pathways mediated by unleashing feedback inhibition of upstream signaling (Figs. 2, 3). Specifically, rapamycin triggers PI3K activation and Akt phosphorylation at Thr308 and Ser473 via suppression of mTORC1/S6K phosphorylation of IRS-1 and Sin1. In some cancer cells, rapalogs also induce MEK/ERK via PI3K-dependent pathway that could involve Rac/PAK1. Active-site mTOR inhibitors induce PI3K activation and Akt phosphorylation at Thr308 but not at Ser473 most likely via suppression of Grb10-mediated feedback inhibition of insulin/IGF receptors and/or suppression of PKCα negative regulation of EGFR. In turn, dual PI3K/mTOR inhibitors promote robust over-activation of the MEK/ERK pathway, most likely via suppression of Grb10- and mTORC2-mediated feedback loops.
Chronic exposure to the same agents initiates a program of FOXO-mediated transcriptional de-repression leading to increased expression of multiple tyrosine kinase receptors, including HER3, IGFR and insulin receptor. The distinction between short-term and long-term consequences in response to inhibitors is important for defining strategies to overcome drug resistance, including the dosing schedule of the drug and the pathways that should be co-targeted for optimal response. It is reasonable to propose that rapid over-activation of ERK signaling will be important in mediating resistance when strong but intermittent inhibition of mTOR is used. In this case, inhibition of mTOR should be associated with MEK inhibitors. Reciprocally, slow FOXO-mediated transcriptional de-repression of tyrosine kinase receptor expression is likely to be important when mTOR or dual PI3K/mTOR inhibitors are administrated to produce constant inhibition of these targets. In this case, inhibitors of EGFR and HER2 (e.g. lapatinib) and/or IGFR might be the drugs of choice for combinatory therapy (i.e. mTOR or dual PI3K/mTOR inhibitors with either lapatinib or IGFR inhibitor).
Although metformin inhibits stimulation of the mTORC1/S6K axis in vitro, its effects on feedback loops regulating Akt and ERK activation are very different from rapalogs, active-site mTOR inhibitors or dual PI3K/mTOR inhibitors. Metformin, in contrast to rapamycin, did not over-stimulate Akt phosphorylation on Ser473. An important difference between the effects of metformin and active-site mTOR inhibitors is that metformin inhibited rather than over-activated the MEK/ERK pathway in several cell types in response to growth factors. Collectively, these studies imply that metformin has considerable advantages in promoting mTOR inhibition without unleashing feedback loops that oppose its anti-proliferative effects, though these effects are likely to depend on cell context and oncogenic mutations.
In conclusion, the elucidation of the feedback loops that regulate the outputs of signaling networks has emerged as an area of fundamental importance for the rationale design of effective anticancer combinations of inhibitors. Here, we review pathways in cancer cells that undergo compensatory over-activation in response to inhibitors that suppress feedback inhibition of upstream signaling. Developing appropriate strategies or regimens that maximize the effect on tumor cells and spare normal cells will be also therapeutically important. This article highlights the importance of discovering signaling feedbacks to anticipate mechanisms of tumor resistance to new drugs.
Acknowledgments
The work in the laboratory of ER is supported by National Institutes of Health Grants P30DK41301, P01CA163200 and R01DK55003, Department of Veterans Affair Grant 1I01BX001473 and funds from the endowed Ronald S. Hirschberg Chair of Pancreatic Cancer Research. (http://www.nih.gov/). The funders had no role in data analysis, decision to publish, or preparation of the manuscript
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Laplante M, Sabatini David M. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–88. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 4.Jewell JL, Guan KL. Nutrient signaling to mTOR and cell growth. Trends Biochem Sci. 2013;38:233–42. doi: 10.1016/j.tibs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–64. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin TD, Chen XW, Kaplan RE, Saltiel AR, Walker CL, Reiner DJ, et al. Ral and Rheb GTPase Activating Proteins Integrate mTOR and GTPase Signaling in Aging, Autophagy, and Tumor Cell Invasion. Mol Cell. 2014;53:209–20. doi: 10.1016/j.molcel.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 8.Feng Z, Levine AJ. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010;20:427–34. doi: 10.1016/j.tcb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polivka J, Jr., Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142:164–75. doi: 10.1016/j.pharmthera.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol. 2009;9:753–62. doi: 10.1016/j.coph.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Lane HA, Breuleux M. Optimal targeting of the mTORC1 kinase in human cancer. Curr Opin Cell Biol. 2009;21:219–29. doi: 10.1016/j.ceb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seront E, Pinto A, Bouzin C, Bertrand L, Machiels JP, Feron O. PTEN deficiency is associated with reduced sensitivity to mTOR inhibitor in human bladder cancer through the unhampered feedback loop driving PI3K/Akt activation. Br J Cancer. 2013;109:1586–92. doi: 10.1038/bjc.2013.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P, Gan W, Inuzuka H, Lazorchak AS, Gao D, Arojo O, et al. Sin1 phosphorylation impairs mTORC2 complex integrity and inhibits downstream Akt signalling to suppress tumorigenesis. Nat Cell Biol. 2013;15:1340–50. doi: 10.1038/ncb2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, DeStefano MA, Oh WJ, Wu CC, Vega-Cotto NM, Finlan M, et al. mTOR complex 2 regulates proper turnover of insulin receptor substrate-1 via the ubiquitin ligase subunit Fbw8. Mol Cell. 2012;48:875–87. doi: 10.1016/j.molcel.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebi H, Costa C, Faber AC, Nishtala M, Kotani H, Juric D, et al. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proc Natl Acad Sci U S A. 2013;110:21124–9. doi: 10.1073/pnas.1314124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borisov N, Aksamitiene E, Kiyatkin A, Legewie S, Berkhout J, Maiwald T, et al. Systems-level interactions between insulin-EGF networks amplify mitogenic signaling. Mol Syst Biol. 2009;5:256. doi: 10.1038/msb.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieterle AM, Bohler P, Keppeler H, Alers S, Berleth N, Driesen S, et al. PDK1 controls upstream PI3K expression and PIP3 generation. Oncogene. 2013 doi: 10.1038/onc.2013.266. Epub 2013 Jul 29. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–8. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li QL, Gu FM, Wang Z, Jiang JH, Yao LQ, Tan CJ, et al. Activation of PI3K/AKT and MAPK Pathway through a PDGFRβ-Dependent Feedback Loop Is Involved in Rapamycin Resistance in Hepatocellular Carcinoma. PLoS ONE. 2012;7:e33379. doi: 10.1371/journal.pone.0033379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith J, Rozengurt E. Different Patterns of Akt and ERK Feedback Activation in Response to Rapamycin, Active-Site mTOR Inhibitors and Metformin in Pancreatic Cancer Cells. PLoS ONE. 2013;8:e57289. doi: 10.1371/journal.pone.0057289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benz o[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem. 2010;53:7146–7155. doi: 10.1021/jm101144f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–98. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 30.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR Kinase Inhibition Causes Feedback-Dependent Biphasic Regulation of AKT Signaling. Cancer Discov. 2011;1:248–59. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoang B, Benavides A, Shi Y, Yang Y, Frost P, Gera J, et al. The PP242 Mammalian Target of Rapamycin (mTOR) Inhibitor Activates Extracellular Signal-regulated Kinase (ERK) in Multiple Myeloma Cells via a Target of Rapamycin Complex 1 (TORC1)/ Eukaryotic Translation Initiation Factor 4E (eIF-4E)/RAF Pathway and Activation Is a Mechanism of Resistance. J Biol Chem. 2012;287:21796–805. doi: 10.1074/jbc.M111.304626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 33.Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Santos ED, et al. Antitumor Efficacy of PKI-587, a Highly Potent Dual PI3K/mTOR Kinase Inhibitor. Clin Cancer Res. 2011;17:3193–203. doi: 10.1158/1078-0432.CCR-10-1694. [DOI] [PubMed] [Google Scholar]
- 34.Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, Lesnick JD, et al. GDC-0980 Is a Novel Class I PI3K/mTOR Kinase Inhibitor with Robust Activity in Cancer Models Driven by the PI3K Pathway. Mol Cancer Ther. 2011;10:2426–36. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 35.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–22. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, et al. Phosphoproteomic Analysis Identifies Grb10 as an mTORC1 Substrate That Negatively Regulates Insulin Signaling. Science. 2011;332:1322–26. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desbuquois B, Carré N, Burnol AF. Regulation of insulin and type 1 insulin-like growth factor signaling and action by the Grb10/14 and SH2B1/B2 adaptor proteins. FEBS Journal. 2013;280:794–816. doi: 10.1111/febs.12080. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, et al. Peripheral Disruption of the Grb10 Gene Enhances Insulin Signaling and Sensitivity In Vivo. Mol Cell Biol. 2007;27:6497–505. doi: 10.1128/MCB.00679-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Q, Szebenyi DME. Structural Basis for the Interaction between the Growth Factor-binding Protein GRB10 and the E3 Ubiquitin Ligase NEDD4. J Biol Chem. 2010;285:42130–9. doi: 10.1074/jbc.M110.143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santiskulvong C, Rozengurt E. Protein kinase Calpha mediates feedback inhibition of EGF receptor transactivation induced by G(q)-coupled receptor agonists. Cell Signal. 2007;19:1348–57. doi: 10.1016/j.cellsig.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. Embo J. 2008;27:1919–31. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. Embo J. 2008;27:1932–43. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 44.Er EE, Mendoza MC, Mackey AM, Rameh LE, Blenis J. AKT Facilitates EGFR Trafficking and Degradation by Phosphorylating and Activating PIKfyve. Sci. Signal. 2013;6:ra45. doi: 10.1126/scisignal.2004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Muller U, et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012;22:796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 46.Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, et al. Basal Subtype and MAPK/ERK Kinase (MEK)-Phosphoinositide 3-Kinase Feedback Signaling Determine Susceptibility of Breast Cancer Cells to MEK Inhibition. Cancer Res. 2009;69:565–72. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turke AB, Song Y, Costa C, Cook R, Arteaga CL, Asara JM, et al. MEK Inhibition Leads to PI3K/AKT Activation by Relieving a Negative Feedback on ERBB Receptors. Cancer Res. 2012;72:3228–37. doi: 10.1158/0008-5472.CAN-11-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 49.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-Mediated Reactivation of MAPK Signaling Contributes to Insensitivity of BRAF-Mutant Colorectal Cancers to RAF Inhibition with Vemurafenib. Cancer Discov. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lahn MM, Sundell KL. The role of protein kinase C-alpha (PKC-alpha) in melanoma. Melanoma Res. 2004;14:85–9. doi: 10.1097/00008390-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Dupasquier S, Abdel-Samad R, Glazer RI, Bastide P, Jay P, Joubert D, et al. A new mechanism of SOX9 action to regulate PKC-alpha expression in the intestine epithelium. J Cell Sci. 2009;122:2191–6. doi: 10.1242/jcs.036483. [DOI] [PubMed] [Google Scholar]
- 52.Smadja-Lamere N, Shum M, Deleris P, Roux PP, Abe JI, Marette A. Insulin Activates RSK (p90 Ribosomal S6 Kinase) to Trigger a New Negative Feedback Loop That Regulates Insulin Signaling for Glucose Metabolism. J Biol Chem. 2013;288:31165–76. doi: 10.1074/jbc.M113.474148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zmajkovicova K, Jesenberger V, Catalanotti F, Baumgartner C, Reyes G, Baccarini M. MEK1 is required for PTEN membrane recruitment, AKT regulation, and the maintenance of peripheral tolerance. Mol Cell. 2013;50:43–55. doi: 10.1016/j.molcel.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109:2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serra V, Scaltriti M, Prudkin L, Eichhorn PJA, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21:227–39. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cen B, Mahajan S, Wang W, Kraft AS. Elevation of Receptor Tyrosine Kinases by Small Molecule AKT Inhibitors in Prostate Cancer Is Mediated by Pim-1. Cancer Res. 2013;73:3402–11. doi: 10.1158/0008-5472.CAN-12-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Y, Serra V, Prudkin L, Scaltriti M, Murli S, Rodriguez O, et al. Evaluation and Clinical Analyses of Downstream Targets of the Akt Inhibitor GDC-0068. Clin Cancer Res. 2013;19:6976–86. doi: 10.1158/1078-0432.CCR-13-0978. [DOI] [PubMed] [Google Scholar]
- 60.Lin A, Piao Hl, Zhuang L, Sarbassov DD, Ma L, Gan B. FoxO transcription factors promote AKT Ser473 phosphorylation and renal tumor growth in response to pharmacological inhibition of the PI3K-AKT pathway. Cancer Res. 2014;74:1682–93. doi: 10.1158/0008-5472.CAN-13-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gala K, Chandarlapaty S. Molecular Pathways: HER3 Targeted Therapy. Clin Cancer Res. 2014;20:1410–6. doi: 10.1158/1078-0432.CCR-13-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 63.Sahra IB, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 64.Kalender A, Selvaraj A, Kim SY, Gulati P, Brulé S, Viollet B, et al. Metformin, Independent of AMPK, Inhibits mTORC1 in a Rag GTPase-Dependent Manner. Cell Metabol. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–92. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Yue W, Yang CS, DiPaola RS, Tan XL. Repurposing of Metformin and Aspirin by Targeting AMPK-mTOR and Inflammation for Pancreatic Cancer Prevention and Treatment. Cancer Prev Res. 2014;7:388–97. doi: 10.1158/1940-6207.CAPR-13-0337. [DOI] [PubMed] [Google Scholar]
- 67.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between Insulin/Insulin-like Growth Factor-1 Receptors and G Protein-Coupled Receptor Signaling Systems: A Novel Target for the Antidiabetic Drug Metformin in Pancreatic Cancer. Clin Cancer Res. 2010;16:2505–11. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinnett-Smith J, Kisfalvi K, Kui R, Rozengurt E. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun. 2013;430:352–57. doi: 10.1016/j.bbrc.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 70.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tzatsos A, Tsichlis PN. Energy depletion inhibits phosphatidylinositol 3-kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J Biol Chem. 2007;282:18069–82. doi: 10.1074/jbc.M610101200. [DOI] [PubMed] [Google Scholar]
- 72.Ning J, Clemmons DR. AMP-Activated Protein Kinase Inhibits IGF-I Signaling and Protein Synthesis in Vascular Smooth Muscle Cells via Stimulation of Insulin Receptor Substrate 1 S794 and Tuberous Sclerosis 2 S1345 Phosphorylation. Mol Endocrinol. 2010;24:1218–29. doi: 10.1210/me.2009-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen CH, Kiyan V, Zhylkibayev AA, Kazyken D, Bulgakova O, Page KE, et al. Autoregulation of the Mechanistic Target of Rapamycin (mTOR) Complex 2 Integrity Is Controlled by an ATP-dependent Mechanism. J Biol Chem. 2013;288:27019–30. doi: 10.1074/jbc.M113.498055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y, Lee SX, Ou Y, et al. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol Cell. 2013;52:161–72. doi: 10.1016/j.molcel.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rozengurt E. Early signals in the mitogenic response. Science. 1986;234:161–6. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]