Abstract
Background
Allopurinol is the most commonly used urate-lowering therapy, with rare but potentially fatal adverse effects. However, its impact on overall mortality remains largely unknown. In this study, we evaluated the impact of allopurinol initiation on the risk of mortality among individuals with hyperuricaemia and among those with gout in the general population.
Methods
We conducted an incident user cohort study with propensity score matching using a UK general population database. The study population included individuals aged ≥40 years who had a record of hyperuricaemia (serum urate level >357 µmol/L for women and >416 µmol/L for men) between January 2000 and May 2010. To closely account for potential confounders of allopurinol use and risk of death, we constructed propensity score matched cohorts of allopurinol initiators and comparators (non-initiators) within 6-month cohort accrual blocks.
Results
Of 5927 allopurinol initiators and 5927 matched comparators, 654 and 718, respectively, died during the follow-up (mean=2.9 years). The baseline characteristics were well balanced in the two groups, including the prevalence of gout in each group (84%). Allopurinol initiation was associated with a lower risk of all-cause mortality (matched HR 0.89 (95% CI 0.80 to 0.99)). When we limited the analysis to those with gout, the corresponding HR was 0.81 (95% CI 0.70 to 0.92).
Conclusions
In this general population study, allopurinol initiation was associated with a modestly reduced risk of death in patients with hyperuricaemia and patients with gout. The overall benefit of allopurinol on survival may outweigh the impact of rare serious adverse effects.
INTRODUCTION
Hyperuricaemia and gout have been shown to be associated with an elevated risk of premature death.1,2 Allopurinol, the most commonly used uratelowering medication (up to 95% of treated cases), might also have cardiovascular and renal benefits;3–6 however, its use is not free of adverse effects. A rare but potentially fatal adverse reaction (ie, allopurinol hypersensitivity syndrome) that affects approximately 1 in 260–1540 allopurinol users, usually during the 1st year of use,7–9 has led to reluctance among some physicians to prescribe allopurinol, even when clinically indicated. If the impact of these severe side effects is substantial, it may shorten the overall survival of patients who were started on allopurinol.
To date, data on the survival impact of allopurinol in patients with hyperuricaemia or gout are scarce. One study based on a US Veterans Affairs (VA) population found that allopurinol initiation was associated with a 23% lower risk of death among individuals with hyperuricaemia.10 It is unknown whether these findings based on 99% male veteran allopurinol users10 are replicable among patients with gout or in more generalisable populations. To address these issues, we evaluated the impact of allopurinol initiation on the risk of death among individuals with hyperuricaemia and among patients with gout in a general population context.
METHODS
Study population
We used The Health Improvement Network (THIN) database, which contains computerised medical records entered by general practitioners (GPs) in the UK. The current THIN dataset contains data from 479 practices with a total of 9.1 million patients. The computerised information includes demographics, details from GP visits, diagnoses from specialist referrals and hospital admissions, results of laboratory tests, and additional health information recorded systematically, including height, weight, body mass index (BMI), smoking and alcohol use. THIN uses the Read classification, a hierarchical clinical terminology system routinely used in the UK to code symptoms and medical diagnoses. Prescriptions issued by the GP are directly generated from the computer, and are coded in THIN according to Multilex classification, a standard drug terminology library used throughout the UK that includes information on drug formulation and strength.
Our study population included individuals aged ≥40 years who had a record of hyperuricaemia (serum uric acid (SUA) level >357 µmol/L (6 mg/dL) for women and >416 µmol/L (7 mg/dL) for men) between January 2000 and May 2010. Study cohort members were required to have ≥2 years of enrolment with the general practice before entering the study cohort to allow for exposure and covariate assessment. Individuals were excluded if they had an estimated glomerular filtration rate of <30 mL/min (estimated according to the Modification of Diet in Renal Disease Equation), a history of dialysis, renal or organ transplantation, malignancy or previous allopurinol use.10
The propensity score matched cohorts stratified by time blocks
Confounding by indication can be a major concern in pharmacoepidemiological studies such as ours. Specifically, the baseline characteristics of allopurinol initiators and non-initiators may systematically differ, causing a lack of comparability between the two groups. Therefore, we conducted an incident allopurinol user cohort study with propensity score matching. Furthermore, in order to closely account for potential secular trends in allopurinol use in relation to various confounders at different times,11 matched cohorts were constructed within 6-month blocks of calendar time (21 blocks from January 2000 to May 2010). Within each cohort accrual block, allopurinol initiators were defined as patients who started to use allopurinol during that 6-month period. Propensity scores (predicted probability of allopurinol initiation) were estimated using logistic regression, separately for each cohort accrual block with stepwise model selection at level of significance α=0.05. For each allopurinol initiator, we identified a propensity score matched subject who did not initiate allopurinol during the accrual block. Matched non-initiators were ineligible for selection in subsequent accrual blocks (greedy matching algorithm).11 The first allopurinol prescription date was assigned as the index date for allopurinol initiators, and a random date within the 6-month block was assigned as the index date for non-initiators.
The variables included in the propensity score estimation consisted of demographics, BMI, comorbidities, medication use, laboratory measurements and healthcare utilisation, as assessed over 2 years prior to the index date. Specifically, demographics included age at index date and sex. Comorbidities included hypertension, cardiovascular disease, diabetes, gout and Charlson comorbidity index. The Charlson comorbidity index is a composite index of diagnoses that includes myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, peptic ulcer disease, liver disease, diabetes with complications, renal disease, cancer and AIDS/HIV.12 Medications included statins, fibrates, ACE inhibitors, angiotensin II receptor blockers, β blockers, calcium channel blockers, aspirin, non-steroidal anti-inflammatory drugs, loop diuretics, hydrochlorothiazide, losartan and insulin. Laboratory values included SUA, cholesterol, albumin and glomerular filtration rate, calculated from serum creatine using the simplified Modification of Diet in Renal Disease study equation.13–15 The number of primary care visits was used as a measure of healthcare utilisation.
Statistical analysis
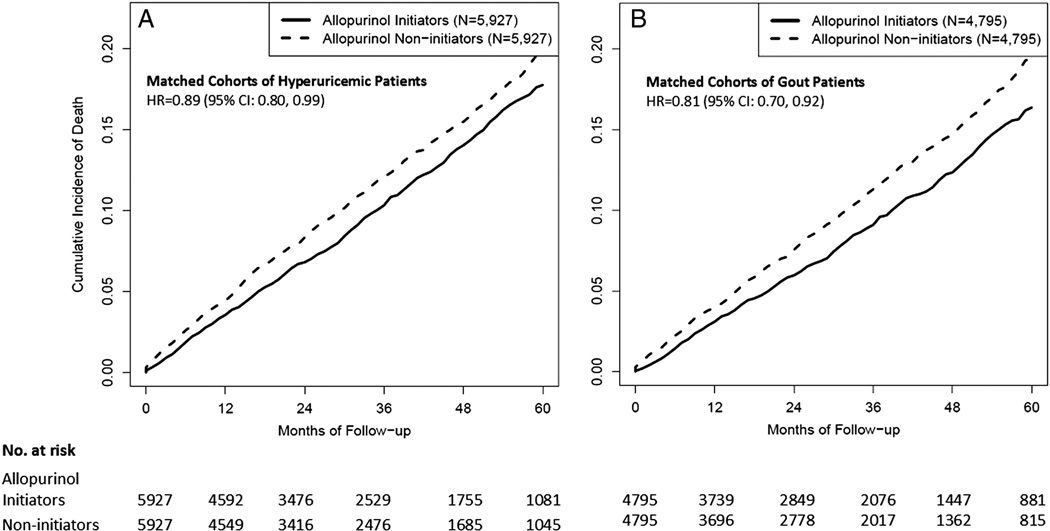
The outcome of interest was all-cause mortality as defined by the death date recorded in THIN. Allopurinol initiators and matched non-initiators began accruing risk time from the index date until death, discontinuity of enrolment or the end of study period, whichever came first. In the final matched cohort, each individual was identified as either an allopurinol initiator or non-initiator, and retained that exposure status throughout follow-up. This approach maintains the comparability of these two exposure groups in terms of the baseline characteristics and provides conservative estimates, similar to an intent-to-treat approach used in clinical trials (figure 1).
Figure 1.
Time to death for the propensity score matched cohorts among (A) patients with hyperuricaemia and (B) patients with gout.
Cox proportional hazard models were used to estimate the effect of allopurinol initiation on all-cause mortality, stratified by 6-month cohort accrual blocks. Survival plots were generated as estimates of cumulative mortality to identify time-trends in the occurrence of death. In order to address potential residual imbalance between the two comparison groups, multivariate analysis was also performed by further adjusting for all confounders included in propensity score estimation. We repeated the same analyses limited to individuals with gout in our hyperuricaemic cohort. We explored a potential interaction of allopurinol initiation and sex by testing the significance of the interaction term. To address the potential for allopurinol initiators to discontinue the drug over time, we performed sensitivity analyses with follow-up time truncated at 1 year, 2 years and 3 years for all subjects. For all HRs, we calculated 95% CIs. All p values were two-sided. All statistical analyses were performed using SAS V.9.2 (SAS Institute, Cary, North Carolina, USA).
RESULTS
There were an average of 289 allopurinol initiators and 5812 allopurinol non-initiators among the subjects who were eligible for propensity score estimation and cohort inclusion in each 6-month accrual block. When a non-initiator was randomly selected for each initiator (without propensity score matching) within each 6-month cohort accrual block, allopurinol initiators had a higher prevalence of comorbidities and use of cardiovascular and other medications at baseline (n=6587 in each group; table 1). As expected, the SUA levels were higher among allopurinol initiators and prevalence of gout was almost three times that of non-initiators (85.4% vs 30.0%, table 1). During the follow-up of these unmatched cohorts, 780 individuals died in the allopurinol initiator group and 586 in the non-initiator group (HR 1.39, 95% CI 1.25 to 1.55, see online web figure 1).
Table 1.
Baseline characteristics in the unmatched cohorts and propensity score matched cohorts among patients with hyperuricaemia
| Unmatched* cohorts | Propensity score matched cohorts | |||
|---|---|---|---|---|
| Baseline characteristics | Allopurinol initiators (N=6587) |
Non-initiators (N=6587) |
Allopurinol initiators (N=5927) |
Non-initiators (N=5927) |
| Demographics | ||||
| Age, years | 67.5 | 66.8 | 67.4 | 67.6 |
| Male, % | 70 | 59 | 69 | 72 |
| BMI, kg/m2 | 30.2 | 29.6 | 30.1 | 30.0 |
| Measures of comorbidity | ||||
| Charlson index, mean | 1.0 | 0.8 | 0.9 | 0.9 |
| Hypertension, % | 67.7 | 67.6 | 67.0 | 69.5 |
| Stroke, % | 10.9 | 9.4 | 10.5 | 11.9 |
| Myocardial infarction, % | 1.9 | 1.6 | 1.8 | 1.9 |
| Diabetes, % | 14.8 | 14.4 | 14.1 | 14.5 |
| Gout, % | 85.4 | 30.0 | 83.7 | 84.4 |
| Primary care visits, N | 12.4 | 11.0 | 12.3 | 11.8 |
| Medications | ||||
| Statin, % | 57.8 | 50.9 | 57.0 | 54.6 |
| Fibrate, % | 2.7 | 2.5 | 2.6 | 2.3 |
| ACE inhibitors, % | 52.0 | 45.6 | 50.9 | 50.1 |
| ARBs, % | 15.8 | 12.6 | 15.2 | 14.6 |
| β-blockers, % | 44.8 | 38.1 | 43.9 | 44.0 |
| Calcium channel blockers, % | 35.5 | 35.7 | 35.8 | 36.5 |
| Aspirin, % | 42.8 | 38.2 | 41.8 | 42.2 |
| NSAIDs, % | 73.9 | 49.9 | 73.0 | 75.4 |
| Loop diuretics, % | 36.3 | 20.2 | 34.0 | 31.1 |
| Hydrochlorothiazide, % | 36.1 | 40.3 | 36.7 | 37.3 |
| Losartan, % | 4.0 | 4.3 | 4.0 | 4.5 |
| Insulin, % | 4.4 | 3.3 | 4.0 | 3.8 |
| Laboratory measurements (baseline) | ||||
| Serum uric acid, µmol/L | 531 | 442 | 522 | 519 |
| Albumin, mmol/L | 41.9 | 42.2 | 41.9 | 42.1 |
| GFR, mL/min per 1.73 m2 | 59.26 | 64.68 | 60.01 | 61.00 |
| Cholesterol, mmol/L | 4.93 | 5.06 | 4.95 | 4.95 |
A non-initiator was randomly selected for each initiator (without propensity score matching) within each 6-month cohort accrual block.
ARB, angiotensin receptor blocker; BMI, body mass index; GFR, glomerular filtration rate; NSAID, non-steroidal anti-inflammatory drug.
In contrast, after propensity score matching (n=5927 in each group), the baseline characteristics were well balanced in the two comparison groups (table 1). In both groups, 70% were male, the mean age was 67 years. Notably, 84% of each group had gout. There were 654 deaths in the allopurinol initiator group and 718 deaths in the non-initiator group during the follow-up (mean=2.9 years), resulting in incidence rates of 1.5 and 2.1 per 1000 person-years, respectively, and a HR of 0.89 (95% CI 0.80 to 0.99; table 2) associated with allopurinol initiation. Further adjustment for other potential confounders did not change the estimate materially (HR 0.86 (95% CI 0.77 to 0.96)). The effect of allopurinol initiation did not differ across sex (p for interaction=0.5). The HRs in the analyses with follow-up truncated at 1 year, 2 years and 3 years were 0.77 (95% CI 0.63 to 0.94), 0.79 (95% CI 0.68 to 0.93) and 0.82 (95% CI 0.72 to 0.94), respectively (table 2).
Table 2.
HR for mortality associated with initiation of allopurinol in the propensity score matched cohorts
| Allopurinol initiators |
Non-initiators | HR (95% CI) | |
| Deaths (N) | |||
| Hyperuricaemic cohorts | (N=5927) | (N=5927) | |
| Total follow-up | 654 | 718 | 0.89 (0.80 to 0.99) |
| 1 year follow-up | 183 | 233 | 0.77 (0.63 to0.94) |
| 2-year follow-up | 325 | 395 | 0.79 (0.68 to 0.93) |
| 3year follow-up | 439 | 521 | 0.82 (0.72 to 0.94) |
| Gout cohorts | (N=4795) | (N=4795) | |
| Total follow-up | 483 | 556 | 0.81 (0.70 to 0.92) |
| 1 year follow-up | 127 | 169 | 0.75 (0.59 to 0.94) |
| 2-year follow-up | 229 | 288 | 0.76 (0.63 to 0.91) |
| 3 year follow-up | 312 | 388 | 0.77 (0.66 to 0.97) |
When we repeated the matched analysis of patients with hyperuricaemia with gout (n=4795 in each group), the HR of allopurinol initiation for mortality was 0.81 (95% CI 0.70 to 0.92). The corresponding HRs in the analyses with follow-up truncated at 1 year, 2 years and 3 years were 0.75, 0.76 and 0.77, respectively (table 2).
DISCUSSION
In this large-scale cohort study of a general population, we found that allopurinol initiation was associated with an 11% lower risk of all-cause mortality compared with non-initiators in patients with hyperuricaemia, and a 19% lower risk of mortality in patients with gout. These risk reductions were apparent from the 1st year and throughout the subsequent years of follow-up. These associations were independent of age, sex, BMI, relevant comorbidities, healthcare utilisation, use of cardiovascular medications and SUA levels.
These results are consistent with the aforementioned VA cohort study finding that allopurinol use was associated with a 23% lower risk of all-cause death in individuals with hyperuricaemia.10 In the VA study, gout was present in 83% of allopurinol initiators and 20% of controls, and allopurinol users also had higher levels of comorbidities, similar to our study findings. However, that VA study did not provide any gout-specific data. Similarly, a nested case-control study of elderly patients (≥66 years) with gout and congestive heart failure showed that allopurinol use was associated with a 26% lower risk of all-cause mortality.16 Nevertheless, these previous findings are consistent with our general population data in patients with hyperuricaemia as well as in patients with gout, suggesting that allopurinol could contribute to reducing the 9–28% increased risk of premature death observed in patients with gout.1,2
It remains unclear if the survival benefit observed in allopurinol initiators is due to its urate-lowering effect, reduction in oxidative stress from xanthine oxidoreductase (XOR) inhibition or other mechanisms.17–21 Allopurinol or oxypurinol was shown to improve endothelial function in patients with hypertension, type II diabetes and dyslipidaemia, in smokers, in patients with hyperuricaemia with elevated cardiovascular risk, and in patients with established coronary artery disease, compared with controls.2,21–23 However, the levels of urate reduction from allopurinol and improved endothelial function have not been consistently correlated, and uricosuric agents such as probenecid and benzbromarone did not show similar benefits to endothelial function.20,24 Furthermore, effects of XOR inhibition on accumulation of upstream precursors (eg, inosine and adenosine) may contribute to beneficial effects of XOR inhibition in models of vascular disease. Nevertheless, given allopurinol’s low-cost and extensive history of use, it has been favoured for use in clinical trials of potential cardiovascular risk reduction.25
Several strengths of our study deserve comment. This is a large-scale study in a general population, and it addressed the limited generalisability of the previous study based on a veteran population and the lack of data specifically on patients with gout. In a pharmacoepidemiological study such as ours, confounding by indication may bias results. Indeed, in the unmatched analysis, allopurinol initiator and non-initiator cohorts lacked comparability in many regards; allopurinol initiators had a greater comorbidity burden (gout, hypertension, diabetes, previous stroke or myocardial infarction, and overall Charlson comorbidity index) and had higher use of medications (non-steroidal anti-inflammatory drugs, aspirin, lipid-lowering drugs and antihypertensives). Thus, allopurinol initiators would be expected to have a greater risk of mortality than non-initiators, as was the finding in the comparison of the unmatched cohorts. However, after propensity score matching to balance potential confounders between compared cohorts, our study found the opposite, suggesting a protective effect of allopurinol. Our incident user cohort study design with propensity score matching addressed imbalances in numerous baseline characteristics so that the observed protective effect of allopurinol on all-cause mortality is unlikely to be the result of confounding by baseline characteristics. Moreover, matching within 6-month blocks flexibly accounts for changes in the relative importance of confounding variables at different times. The benefits of matching must be weighed against the problem of loss of generalisability that is likely a result from incomplete matching; however, in this study, more than 90% of the allopurinol initiators were matched, making generalisability less of a concern.
Potential limitations of our study also deserve comment. Our study focused on the impact of allopurinol on all-cause mortality in the general population and could not address its potential impact on cause-specific death, because the latter data tend to be incomplete within THIN data. Nevertheless, accurate knowledge on the overall mortality impact is critically important in its own right. Although we speculate that the potential survival benefit with allopurinol use is due to reduction of excess cardiovascular or renal outcomes,3–6 this hypothesis calls for future studies examining specific causes of death. Finally, our study was observational; thus, we cannot rule out the possibility of residual or unknown confounding.
In conclusion, allopurinol initiation was associated with a modestly reduced risk of death in patients with hyperuricaemia and patients with gout. The overall survival benefit of allopurinol may outweigh the impact of rare, potentially serious adverse effects at a population level.
Acknowledgments
Funding In part from grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (USA) (P60AR047785, R01AR056291 and R01AR065944), the VA Boston Fellowship in Advanced Geriatrics (MD), and the Arthritis Foundation Clinical to Research Transition Award (MD).
Ethical approval This study was approved by THIN Scientific Review Committee (Reference number 12-005), and judged exempt from review by the Boston University Medical Campus Institutional Review Board (Protocol H-31311).
Footnotes
Contributors All listed authors have made substantial contributions to the study conception/design, or the acquisition, analysis, or interpretation of data for the work. All authors have drafted or critically revised the manuscript, and approved the version to be published. MD and HKC are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests HKC has served on advisory boards for Takeda Pharmaceuticals and Astra-Zeneca Pharmaceuticals, and has received investigator initiated research grants from Takeda Pharmaceuticals and Savient Pharmaceuticals; no other relationships or activities that could appear to have influenced the submitted work. The other authors declare no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Data Sharing Statement No additional data available.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116:894–900. doi: 10.1161/CIRCULATIONAHA.107.703389. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan E, Svendsen K, Neaton JD, et al. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104–1110. doi: 10.1001/archinte.168.10.1104. [DOI] [PubMed] [Google Scholar]
- 3.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimaldi-Bensouda L, Alperovitch A, Aubrun E, et al. Impact of allopurinol on risk of myocardial infarction. [Published Online First 6 Jan 2014];Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2012-202972. [DOI] [PubMed] [Google Scholar]
- 6.Siu YP, Leung KT, Tong MK, et al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Kim SC, Newcomb C, Margolis D, et al. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: a population-based cohort study. Arthritis Care Res (Hoboken) 2013;65:578–584. doi: 10.1002/acr.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: A proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529–2536. doi: 10.1002/art.34488. [DOI] [PubMed] [Google Scholar]
- 9.Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. 1986;29:82–87. doi: 10.1002/art.1780290111. [DOI] [PubMed] [Google Scholar]
- 10.Luk AJ, Levin GP, Moore EE, et al. Allopurinol and Mortality in Hyperuricemic Patients. Rheumatology. 2009;48:804–806. doi: 10.1093/rheumatology/kep069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14:465–476. doi: 10.1002/pds.1062. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CY, Vittinghoff E, Lin F, et al. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004;141:95–101. doi: 10.7326/0003-4819-141-2-200407200-00007. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Greene T, Kusek JW, et al. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 16.Thanassoulis G, Brophy JM, Richard H, et al. Gout, allopurinol use, heart failure outcomes. Arch Intern Med. 2010;170:1358–1364. doi: 10.1001/archinternmed.2010.198. [DOI] [PubMed] [Google Scholar]
- 17.Butler R, Morris AD, Belch JJ, et al. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 18.Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 19.Farquharson CA, Butler R, Hill A, et al. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 20.George J, Carr E, Davies J, et al. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 21.Guthikonda S, Sinkey C, Barenz T, et al. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 22.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, et al. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 23.Mercuro G, Vitale C, Cerquetani E, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004;94:932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Ogino K, Kato M, Furuse Y, et al. Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circ Heart Fail. 2010;3:73–81. doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 25.Dawson J, Quinn T, Walters M. Uric acid reduction: a new paradigm in the management of cardiovascular risk? Curr Med Chem. 2007;14:1879–1886. doi: 10.2174/092986707781058797. [DOI] [PubMed] [Google Scholar]