Abstract
The present study was designed to determine whether treatment with erythropoietin (EPO) could protect cerebral microvasculature against the pathological consequences of endothelial nitric oxide synthase (eNOS) uncoupling. Wild-type and GTP cyclohydrolase I (GTPCH-I)-deficient hph1 mice were administered EPO (1000 U/kg/day, sc, 3 days). Cerebral microvessels of hph1 mice demonstrated reduced BH4 bioavailability, increased production of superoxide anions and impaired endothelial nitric oxide (NO) signaling. Treatment of hph1 mice with EPO attenuated the levels of 7,8-dihydrobiopterin (7,8-BH2), the oxidized product of BH4, and significantly increased the ratio of BH4 to 7,8-BH2. Moreover, EPO decreased levels of superoxide anions and increased NO bioavailability in cerebral microvessels of hph1 mice. Attenuated oxidation of BH4 and inhibition of eNOS uncoupling were explained by the increased expression of antioxidant proteins, manganese superoxide dismutase and catalase. The protective effects of EPO observed in cerebral microvessels of hph1 mice were also observed in GTPCH-I siRNA-treated human brain microvascular endothelial cells exposed to EPO (1 U/ml or 10 U/ml; 3 days). Our results suggest that EPO might protect the neurovascular unit against oxidative stress by restoring bioavailability of BH4 and endothelial NO in the cerebral microvascular endothelium.
Keywords: GTP cyclohydrolase I, superoxide, nitric oxide, brain, cerebral microvessels, hph1 mice
Introduction
Plethora of preclinical studies indicated that recombinant erythropoietin (EPO), a pleiotropic cytokine, is neuroprotective (Siren et al. 2001, Erbayraktar et al. 2003, Brines et al. 2004, Brines & Cerami 2005, Grasso et al. 2007, Buemi et al. 2009, Ghezzi et al. 2010, Zhang et al. 2012). Furthermore, studies from our laboratory indicated that vascular protective effects of EPO could also be extended to large cerebral arteries (Santhanam et al. 2005, Santhanam & Katusic 2006, Santhanam et al. 2006, d'Uscio & Katusic 2008, d'Uscio et al. 2010). While existing evidence supporting neuroprotective and vascular protective effects of EPO makes it an appealing candidate for evaluation of protection against cerebrovascular disorders, the effects of EPO on cerebral microvessels, the vascular component in the neurovascular unit mediating trophic support and regulating blood flow in brain, has not been investigated. While oxidative stress and concomitant loss of endothelial NO has been recognized as one of the most common initiating events in the pathogenesis of cerebrovascular disorders (Chrissobolis & Faraci 2008, Chrissobolis et al. 2011, Faraci 2011), the ability of EPO to protect cerebral microvasculature against oxidative stress has not been demonstrated.
Our previous studies have shown that EPO may increase endothelial biosynthesis of tetrahydrobiopterin (BH4, an essential co-factor for eNOS activation) as well as stimulate antioxidant protein expression in the peripheral vasculature (d'Uscio et al. 2003, d'Uscio & Katusic 2008, d'Uscio et al. 2010). However, the effects of EPO on the bioavailability of BH4 in cerebral microcirculation remain unknown. In a recent study, we used the GTP cyclohydrolase I (GTPCH-I, rate limiting enzyme in BH4 biosynthesis)-deficient hph1 mice and demonstrated that loss of BH4 resulted in uncoupling of eNOS and elevated superoxide anion production in cerebral microvessels (Santhanam et al. 2012b). Furthermore, we have demonstrated that oxidative stress in the cerebral microvasculature of BH4-deficient hph1 mice is amenable to pharmacological interventions (Santhanam et al. 2012a, Santhanam et al. 2012b). The present study was designed to determine whether treatment with EPO could protect cerebral microvasculature against the pathological consequences of eNOS uncoupling in the cerebral microvessels of hph1 mice. To specifically determine the therapeutic significance of EPO in relation to the neurovascular unit, we sought to determine whether the protective effects of EPO observed in cerebral microvessels of hph1 mice could also be observed in BH4-deficient brain microvascular endothelial cells.
Methods
Mice
Twelve- to sixteen-week-old male hph-1 mutant mice and wild-type (C57BL/6J) littermates were used for the present study. Breeder pairs of hph1 mice were provided to us by Dr. Keith M. Channon (University of Oxford, Oxford, UK). Breeding and genotyping of the mice were performed as reported earlier (Khoo et al. 2004, d'Uscio et al. 2011). Mice were either treated with saline or recombinant human EPO (1000 U/kg body weight per day, 3 days, subcutaneous; Amgen). This dose and treatment regimen of EPO has been demonstrated in our prior studies to exert vascular protection without affecting hematopoiesis (d'Uscio et al. 2007, d'Uscio & Katusic 2008). Following treatment, mice were euthanized by intraperitoneal injections of pentobarbital (250 mg/kg bw). All experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee of Mayo Clinic and were in compliance with NIH Guide for Care and Use of Laboratory Animals.
Isolation of cerebral microvessels
Following euthanasia, brains were removed and placed in cold (4° C) Krebs-Ringer bicarbonate solution (in mmol/l: NaCl 118.6; KCl 4.7; CaCl2 2.5; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25.1; glucose 10.1; EDTA 0.026) and large cerebral arteries (basilar, anterior cerebral, posterior cerebral, middle cerebral arteries) were discarded. Cerebral microvessels were subsequently isolated using the experimental procedures reported earlier (Ospina et al. 2002, Park et al. 2004). Briefly, brains were minced in a Dounce homogenizer and the homogenates were suspended in 15% Dextran solution (in PBS). The supernatant was discarded, and cerebral microvessels were retained on a 40 µm nylon filter (Austin et al. 2010, Santhanam et al. 2012a, Santhanam et al. 2012b). The microvessels thus obtained have been extensively characterized by previous studies, including ours, and are a heterogeneous mixture of small arteries, arterioles, small veins, venules and capillaries (Ospina et al. 2002, Park et al. 2004, Santhanam et al. 2012b).
Brain microvascular endothelial cell culture
Human brain microvascular endothelial cells (BMECs) were purchased from Applied Cell Biology Research Institute (Kirkland, WA). BMECs were grown in endothelial growth medium 2 (EGM2; Lonza, Basel, Switzerland), as described earlier (Austin et al. 2010). BMECs between passages 7 and 8 were exposed to recombinant human EPO (0, 1 U/ml, 10 U/ml, EGM2; Epogen, Amgen) for 3 days. During the course of incubation, EGM2 and EPO were changed daily.
siRNA Transfection
BMECs, at 30–50% confluency, were transfected with 30 nM of GTPCH-I siRNA (sc60675; Santa Cruz Biotech) or Control siRNA (sc37007, Santa Cruz Biotech) in the presence of Lipofectamine 2000 (Invitrogen) in serum free endothelial basal medium (EBM-2; Lonza) according to manufacturer’s instructions. Fresh EGM-2 was added 8 hours after transfection. Forty eight hours after transfection, cells were exposed to recombinant human EPO (0, 1 U/ml or 10 U/ml) for 3 days. This concentration of EPO in cell culture media was chosen from our previous studies wherein we have shown EPO to exert protection of vascular endothelium (d'Uscio & Katusic 2008).
Measurement of biopterin levels
Cerebral microvessels were minced in extraction buffer [Composition: 50 mmol/l Tris (pH 7.4), 1 mmol/L dithiothreitol, 1 mmol/L EDTA]. The homogenates were then centrifuged at 10,000 g (8 min at 4°C) and supernatants were used for biopterin measurements. In a separate set of experiments, BMECs exposed to EPO were harvested and sonicated in extraction buffer, and centrifuged at 8,000 g (5 min at 4 °C). Levels of tetrahydrobiopterin (BH4) and its oxidized product, 7,8-dihydrobiopterin (7,8-BH2) were determined by reverse phase HPLC, as reported previously (Santhanam et al. 2012a, Santhanam et al. 2012b).
Detection of intracellular superoxide anions
Microvessels as well as BMECs were incubated in Krebs-HEPES buffer containing 50 µmol/l of dihydroethidium (Molecular Probes) at 37°C for 15 minutes and intracellular superoxide anion levels were quantified using a HPLC-based fluorescence detection of the oxidation of dihydroethidium to 2-hydroxyethidium, as described earlier. In some experiments, microvessels were incubated with L-NAME (30 µmol/L) for 30 minutes prior to addition of dihydroethidium (Santhanam et al. 2012a, Santhanam et al. 2012b).
Western blot analysis
Cerebral microvessels and BMECs were lysed in lysis buffer containing [50 mmol/L NaCl, 50 mmol/L NaF, 50 mmol/L sodium pyrophosphate, 5 mmol/L EDTA, 5 mmol/L EGTA, 0.1 mmol/L Na3VO4, 1% Triton X-100, 10 mmol/L HEPES, pH 7.4, and protease inhibitor cocktail (Sigma)]. The experimental methods for protein expression studies are published elsewhere (Santhanam et al. 2012a, Santhanam et al. 2012b). Monoclonal antibodies against eNOS (1:500), iNOS (1:500) [BD Transduction], catalase (1:250), β-actin (1:5000) [Sigma] and polyclonal antibodies (1:1000 dilution) against CuZn superoxide dismutase (CuZnSOD), manganese superoxide dismutase (MnSOD) [Assay Designs] were used. Protein expression bands were detected by enhanced chemiluminescence (Super Signal West Pico Chemiluminescence, Thermo Scientific, IL) and densitometric analysis was performed by UN-SCAN-IT software (Version 6.1, Silk Scientific Corporation, UT).
Determination of NO production
Nitric oxide production in the cerebral microvessels and media supernatants of BMECs were measured as total nitrite and nitrate (NOx = NO3 + NO2) using a commercially available fluorimetric nitrite/nitrate assay kit according to manufacturer’s instructions (Cayman Chemical Co., Ann Arbor, MI) (Santhanam et al. 2012a, Santhanam et al. 2012b).
Determination of cGMP levels
Basal levels of cGMP were determined in cerebral microvascular lysates by enzyme immunoassay according to manufacturer’s instructions (Cell Bio Labs, Inc., San Diego, CA) (Santhanam et al. 2012a, Santhanam et al. 2012b).
Statistical analysis
Data are presented as mean ± SEM. For in vivo studies, ‘n’ represents the number of mice used, and for in vitro studies ‘n’ represents the number of independent experiments performed in each group. Un-paired students t-test was used to determine statistical difference between two groups, and multiple comparisons were performed by one-way ANOVA followed by Bonferroni’s post-hoc test. A value of P<0.05 was considered statistically significant.
Results
EPO increased bioavailability of BH4 in cerebral microvessels of hph1 mice
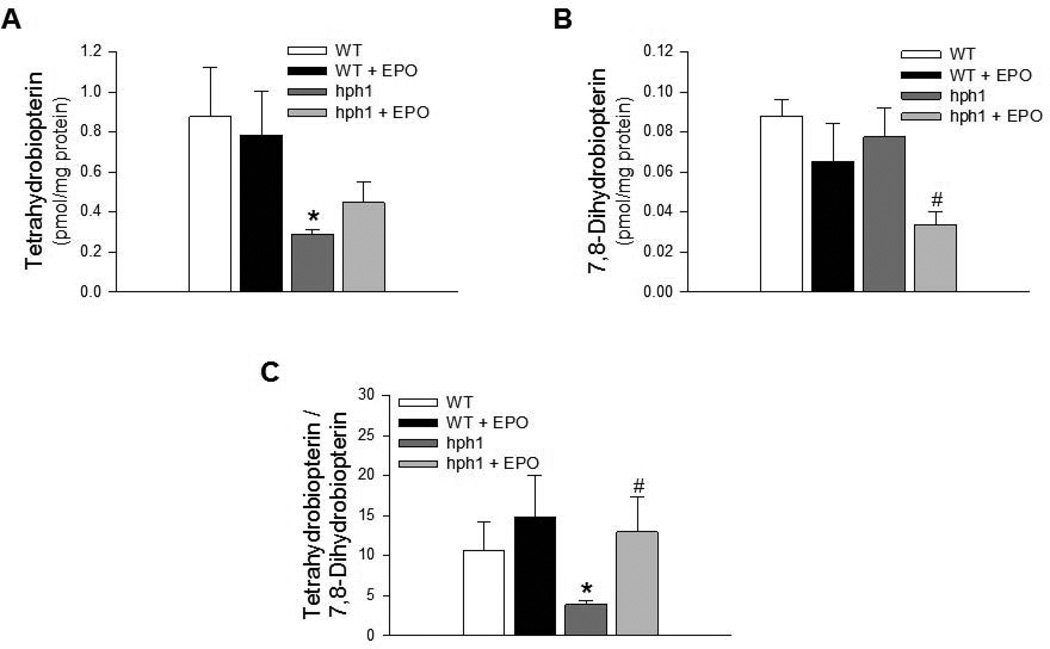
Cerebral microvessels of hph1 mice demonstrated significant impairment in bioavailability of BH4, as indicated by the significantly reduced ratio of BH4 to 7,8-BH2, consistent with our previous study (Figure 1) (Santhanam et al. 2012b). Treatment of wild-type mice with EPO did not affect BH4 levels, 7,8-BH2 levels or the ratio of BH4 to 7,8-BH2 (Figure 1A – 1C). In contrast, EPO prevented oxidative inactivation of BH4 as indicated by the significant reduction in the levels of 7,8-BH2, oxidized product of BH4 (Figure 1B). Furthermore, ratio of BH4 to 7,8-BH2, indicative of net BH4 bioavailable for eNOS activation, was significantly improved in cerebral microvessels of EPO-treated hph1 mice as compared to hph1 mice (Figure 1C).
Figure 1.
Treatment with EPO increased BH4 bioavailability. A) BH4 levels in cerebral microvessels of hph1 mice were significantly reduced (* P<0.05 vs. WT, n =6). EPO treatment did not affect BH4 levels in cerebral microvessels of wild-type mice and hph1 mice. B) EPO inhibited oxidation of BH4, as indicated by the significantly reduced levels of 7,8-dihydrobiopterin in cerebral microvessels of hph1 mice treated with EPO (# P<0.05 vs. hph1, n=6). C) The ratio of BH4 to 7,8-BH2, indicative of BH4 bioavailability, was significantly decreased in cerebral microvessels of hph1 mice (* P<0.05 vs. WT, n=6). Treatment with EPO significantly increased the ratio of BH4 to 7,8-BH2 in cerebral microvessels of hph1 mice (# P<0.05 vs. hph1, n=6).
EPO inhibited eNOS uncoupling-induced superoxide anion production
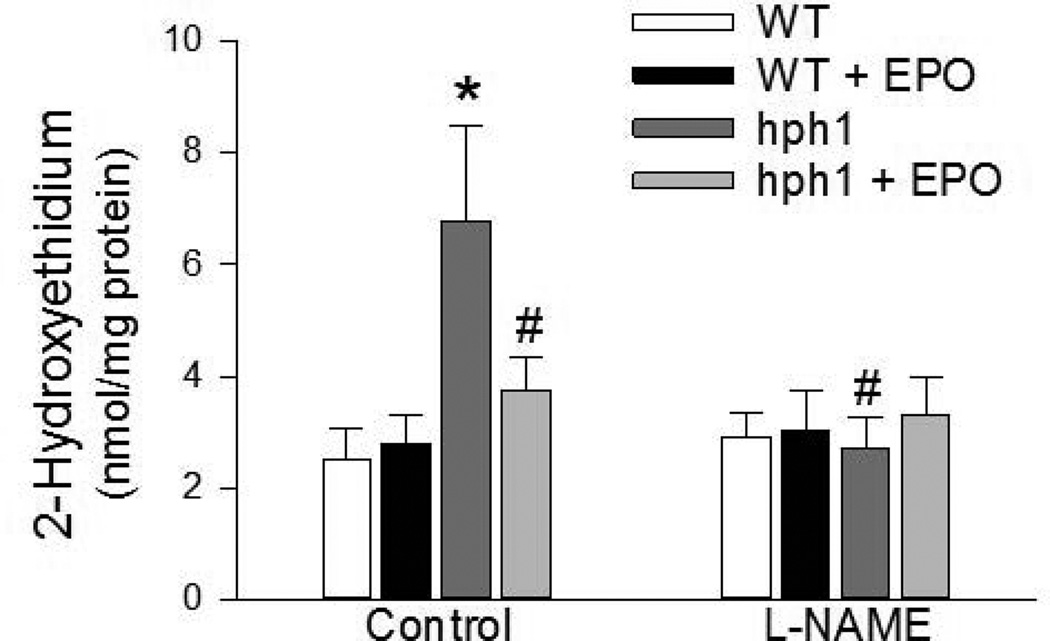
Levels of 2-hydroxyethidium, product derived from selective oxidation of dihydroethidium by superoxide anions, were significantly increased in cerebral microvessels of hph1 mice (Figure 2). Furthermore, sensitivity of the increased production of superoxide anions in cerebral microvessels of hph1 mice to NOS inhibitor, L-NAME, confirmed that eNOS is the principal source of superoxide anions (Figure 2). While EPO treatment did not affect superoxide anion production in cerebral microvessels of wild-type mice, EPO abolished the increased production of superoxide anions in cerebral microvessels of hph1 mice. Notably, L-NAME did not affect superoxide anion production in cerebral microvessels of EPO-treated hph1 mice (Figure 2).
Figure 2.
Treatment with EPO inhibited superoxide anion production. Cerebral microvessels of hph1 mice demonstrated increased production of superoxide anions (* P<0.05 vs. WT, n=8), and the increased superoxide anion production was sensitive to L-NAME (30 µM, # P<0.05 vs. hph1, n=4).Treatment with EPO significantly attenuated superoxide anion production in cerebral microvessels of hph1 mice. Incubation with L-NAME (30 µM) did not affect superoxide anion production in cerebral microvessels of hph1 mice treated with EPO.
EPO stimulated expression of antioxidant proteins
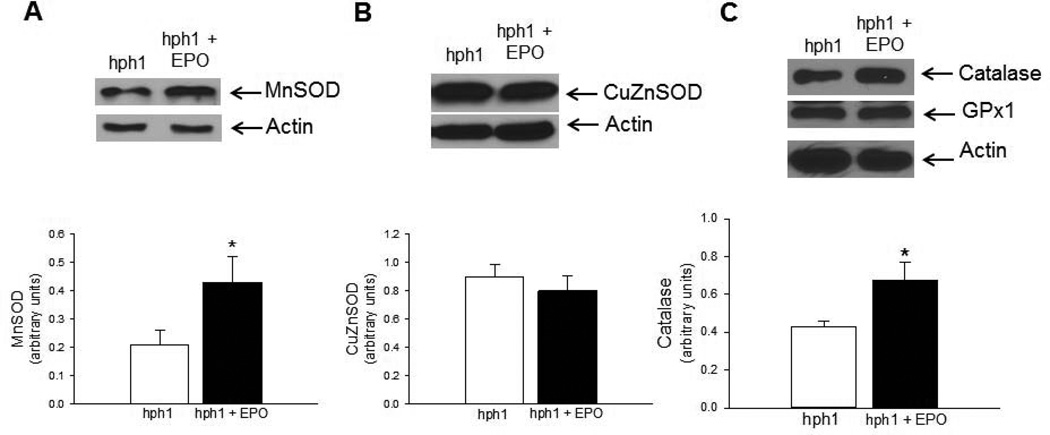
To determine the mechanism underlying attenuated oxidation of BH4 EPO in cerebral microvessels of hph1 mice treated with EPO, we studied expression of antioxidant proteins. We observed that EPO selectively increased expression of MnSOD (Figure 3A, P<0.05), while expression of CuZnSOD remained unchanged in cerebral microvessels of hph1 mice (Figure 3B). Furthermore, expression of catalase, a H2O2 scavenging enzyme, was also significantly increased by EPO in cerebral microvessels of hph1 mice, while expression of GPx1, another enzyme that could scavenge and inactivate H2O2, remained unchanged (Figure 3C).
Figure 3.
EPO stimulated expression of antioxidant proteins in cerebral microvessels of hph1 mice. Representative Western blots and densitometric analysis indicated that treatment with EPO significantly increased expression of antioxidant protein MnSOD (A, * P<0.05, n=5) in cerebral microvessels of hph1 mice, while expression of CuZnSOD remained unchanged (B). Treatment with EPO also increased expression of catalase in cerebral microvessels of hph1 mice (C), while expression of GPx1 remained unchanged (* P<0.05, n=5).
Effect of EPO on endothelial NO in vivo
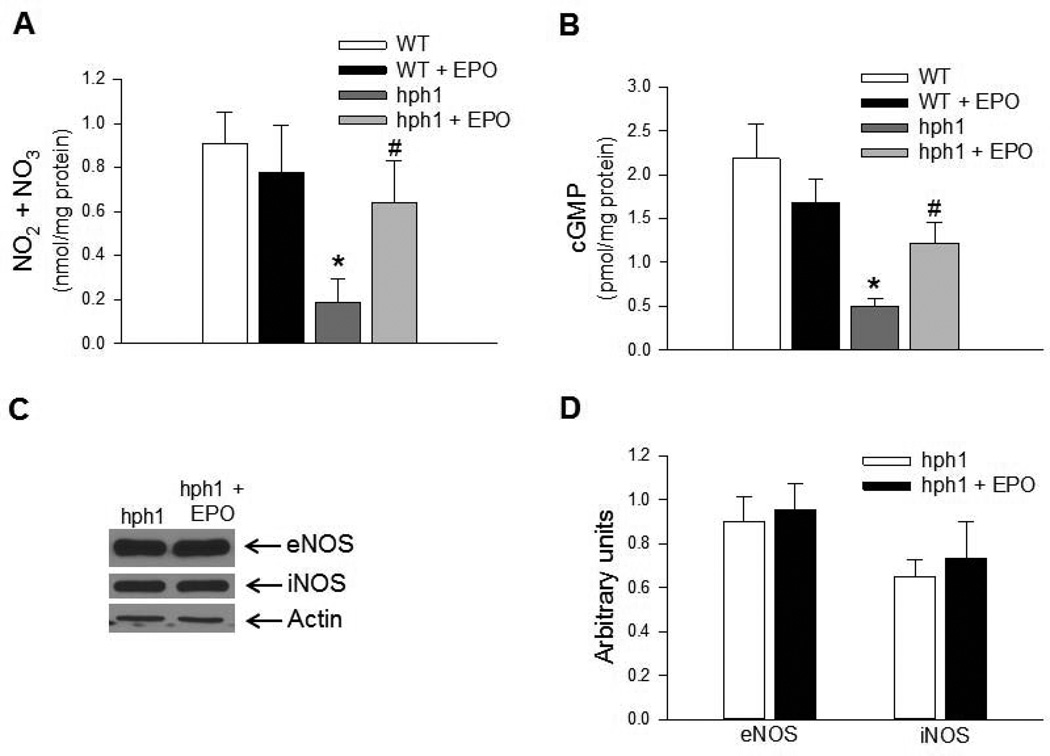
Treatment of hph1 mice with EPO increased levels of total nitrite/nitrate in cerebral microvessels in vivo (Figure 4A). Moreover, reduction of basal cGMP levels caused by BH4 deficiency was abolished by EPO treatment in cerebral microvessels of hph1 mice (Figure 4B). Taken together, these results indicate that EPO treatment increased bioavailability of endothelial NO in cerebral microvessels of hph1 mice. Importantly, protein expressions of both eNOS and iNOS remained unaffected (Figures 4 C&D), indicating that the increased bioavailability of endothelial NO by EPO could be most likely mediated by increased bioavailability of BH4 and inhibition of eNOS uncoupling.
Figure 4.
Treatment with EPO increased bioavailability of endothelial NO in cerebral microvessels of hph1 mice. A) Levels of total nitrite/nitrate were significantly reduced in cerebral microvessels of hph1 mice (* P<0.05 vs. WT, n=6). While EPO treatment did not affect total nitrite/nitrate levels in cerebral microvessels of wild-type mice, levels of NOx in cerebral microvessels of hph1 mice were significantly increased (# P<0.05 vs. hph1, n=6). B) Basal levels of cGMP were significantly attenuated in cerebral microvessels of hph1 mice (* P<0.05 vs. WT, n=8–9). Treatment with EPO significantly increased levels of cGMP in cerebral microvessels of hph1 mice (# P<0.05 vs. hph1, n=8), while the levels of cGMP in wild-type mice remained unchanged following EPO treatment. C) Representative Western blots demonstrating that EPO treatment did not affect expressions of eNOS or iNOS in cerebral microvessels of hph1 mice. D) Bar diagram representing the densitometric analysis of eNOS and iNOS expressions in cerebral microvessels of hph1 mice treated with EPO.
Effects of EPO on cultured BMECs
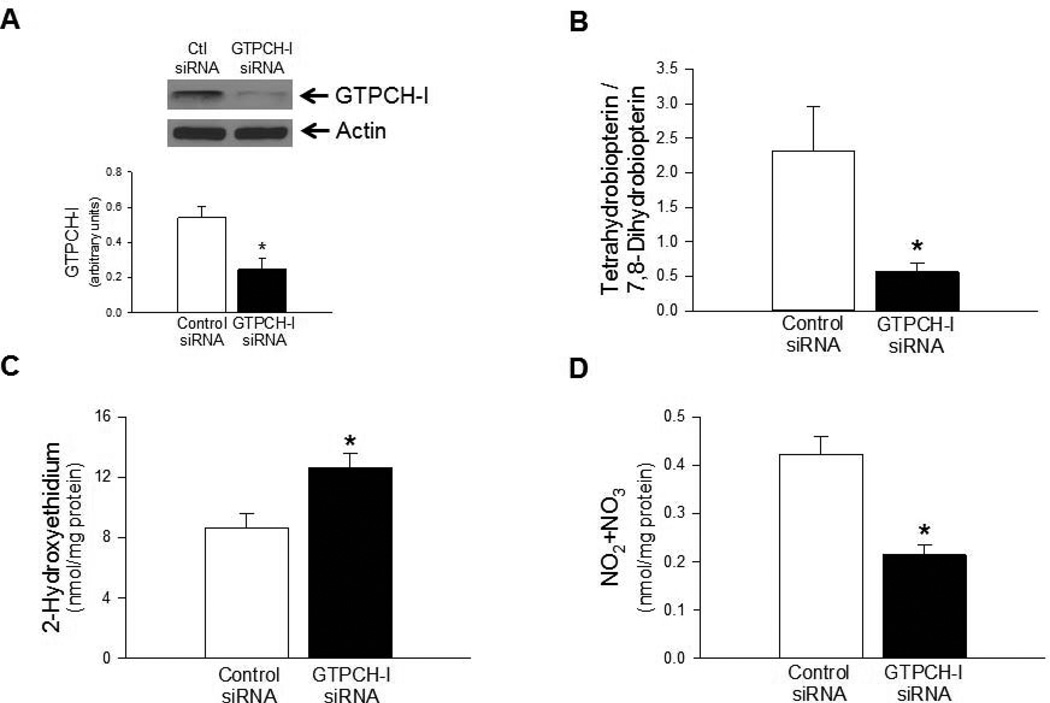
To demonstrate that the effects of EPO to stimulate antioxidant protein expression and increase bioavailability of BH4 could be explained by direct action of EPO on cerebral microvascular endothelium, we subsequently performed studies in cultured human BMECs. To mimic BH4 deficiency, we treated BMECs with siRNA against GTPCH-I, rate limiting enzyme in BH4 biosynthesis. As expected, treatment of BMECs with GTPCH-I siRNA resulted in significant attenuation of GTPCH-I expression in BMECs (Figure 5A) and significant impairment of BH4 to 7,8-BH2 ratio (Figure 5B). Consistent with the results obtained from cerebral microvessels of hph1 mice, deficiency of BH4 in BMECs treated with GTPCH-I siRNA was associated with significantly increased production of superoxide anions (Figure 5C). While GTPCH-I siRNA treatment did not affect eNOS expression in BMECs (data not shown), levels of total nitrite/nitrate was significantly reduced in BH4-deficient BMECs (Figure 5D).
Figure 5.
Treatment of BMECs with GTPCH-I siRNA suppressed bioavailability of BH4 and endothelial NO. A) Exposure of BMECs to GTPCH-I siRNA resulted in significantly attenuated expression of GTPCH-I (* P<0.05 vs. Control siRNA-treated BMECs, n=4). (B) Ratio of BH4 to 7,8-BH2 was significantly reduced in BMECs treated with GTPCH-I siRNA (*P<0.05, n=5). Reduced bioavailability of BH4 was associated with increased production of superoxide anions (C, *P<0.05, n=7) and reduced bioavailability of NO (D, * P<0.05, n=5).
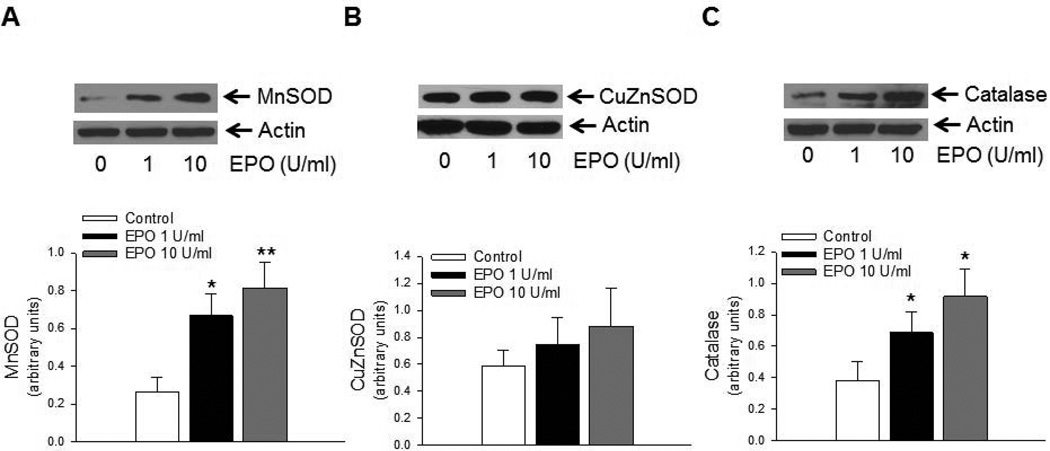
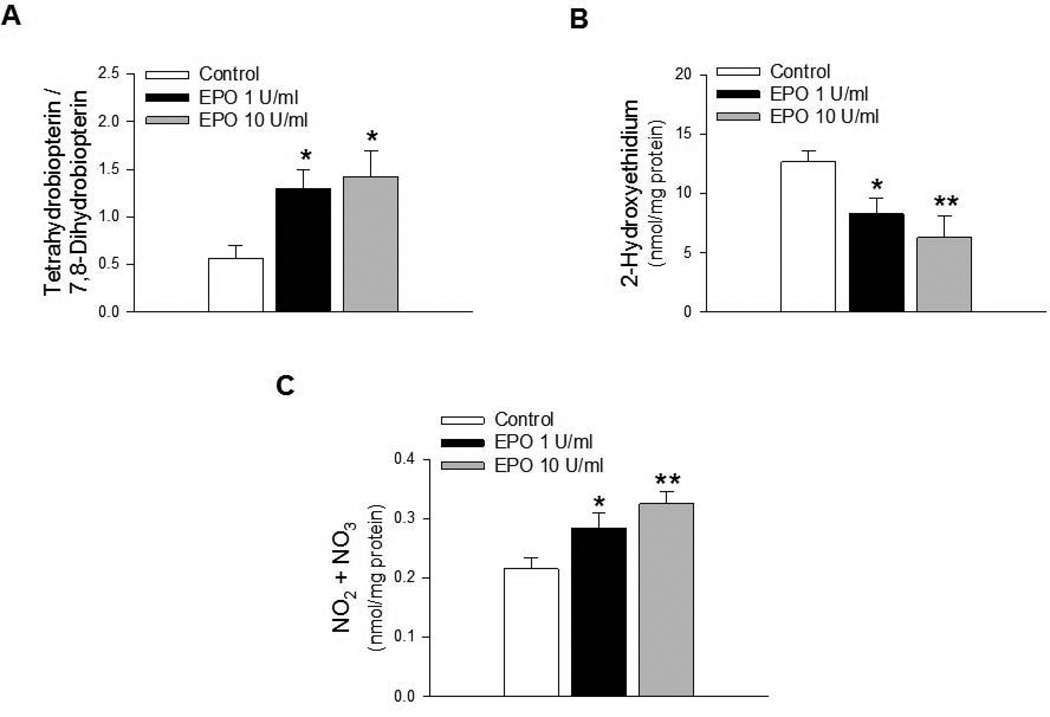
Exposure of GTPCH-I siRNA treated BMECs to EPO also resulted in increased expression of MnSOD (Figure 6A), while expression of CuZnSOD remained unchanged (Figures 6B). Furthermore, EPO also increased expression of catalase in GTPCH-I siRNA treated BMECs (Figure 6C), while expression of GPx1 remained unchanged (data not shown). EPO also increased the ratio of BH4 to 7,8-BH2 in GTPCH-I siRNA treated BMECs (Figure 7A). Furthermore, increased expression of antioxidant proteins and BH4 bioavailability were reflected in attenuation of superoxide anion production (Figure 7B) and increased levels of total nitrite/nitrate in GTPCH-I treated BMECs exposed to EPO (Figure 7C).
Figure 6.
EPO stimulated antioxidant protein expression in brain microvascular endothelial cells. Representative Western blots and densitometric analysis from GTPCH-I siRNA treated BMECs exposed to EPO demonstrated significant increase in expression of MnSOD (A), while expression of CuZnSOD (B) remained unchanged (*P<0.05, ** P<0.01, n=6). Furthermore, EPO treatment also increased expression of catalase (C, * P<0.05, n=6).
Figure 7.
EPO increased bioavailability of endothelial NO in brain microvascular endothelial cells. Bioavailability of BH4 was significantly increased in GTPCH-I siRNA treated BMECs exposed to EPO (A, * P<0.05, n=6). EPO also attenuated superoxide anion production in GTPCH- I siRNA treated BMECs (B, * P<0.05, ** P<0.01, n=8). Levels of total nitrite was significantly increased in GTPCH-I treated BMECs exposed to EPO (C, * P<0.05, ** P<0.01, n=6).
Discussion
Our study presents several novel findings. First, treatment with EPO prevented oxidative inactivation of BH4 and restored bioavailability of BH4 in cerebral microvessels of GTPCH-I-deficient hph1 mice. Second, EPO reversed eNOS uncoupling-induced superoxide anion production. Third, EPO treatment selectively increased expression of MnSOD and catalase. Fourth, during BH4 deficiency, treatment with EPO significantly improved endothelial NO bioavailability. Fifth, the ability of EPO to restore BH4 bioavailability and attenuate superoxide anion production in cerebral microvessels of hph1 mice was also observed in GTPCH-I siRNA-treated BMECs exposed to EPO. Taken together, our study demonstrated that EPO stimulated expression of antioxidant proteins, inhibited oxidation of BH4, restored BH4 bioavailability for eNOS activation and increased endothelial NO/cGMP signaling in BH4-deficient cerebral microvasculature.
To the best of our knowledge, this is the first study to define the beneficial effects of EPO responsible for prevention of pathological consequences of eNOS uncoupling in cerebral circulation. To evaluate the protective effects of EPO in cerebral microvasculature, we employed the GTPCH-I-deficient hph1 mice. Our prior studies have identified that genetic deficiency of GTPCH-I resulted in reduced bioavailability of BH4 for eNOS activation, increased production of eNOS-derived superoxide anions and impaired endothelial NO/cGMP signaling in cerebral microvasculature (Santhanam et al. 2012b). Since endothelium contributes to ~ 80% of the total BH4 in the vascular wall (d'Uscio & Katusic 2008), we also performed studies to determine the effects of EPO in brain microvascular endothelial cells. To mimic BH4 deficiency in vitro, we employed GTPCH-I siRNA and demonstrated reduced bioavailability of BH4 for eNOS activation and augmented superoxide anion production. We would like to underscore that the effects of EPO observed in intact cerebral microvessels could also be observed in EPO-treated brain microvascular endothelial cells cultured in vitro.
Treatment of BH4-deficient brain microvessels with EPO reduced the levels of 7,8-BH2, the oxidized derivative of BH4 that competitively binds and inactivates eNOS (Vasquez-Vivar et al. 2002, d'Uscio et al. 2003). Absence of a change in BH4 levels per se reflected lack of an effect on enzymatic activity of GTPCH-I, the rate-limiting enzyme in BH4 biosynthesis, by EPO. The most likely explanation for increased BH4 bioavailability by EPO in cerebral microvessels of hph1 mice was provided by attenuated oxidative inactivation of BH4 to 7,8-BH2. Hence, we sought to determine whether EPO could stimulate expression of antioxidant enzyme(s) in cerebral microvessels of hph1 mice. Indeed, we identified that EPO selectively up-regulated protein expression of MnSOD in cerebral microvasculature of hph1 mice as well as in BMECs. To date, up-regulation of antioxidant proteins have been observed in vascular and neuronal tissues in response to neurotrophins and growth factors, including EPO (Kong et al. 1993, Digicaylioglu & Lipton 2001, Kops et al. 2002, Park & Rho 2002, Rojo et al. 2004, d'Uscio et al. 2010). In line with these results, the present study presents compelling evidence to suggest that EPO protects cerebral microcirculation against pathological consequences of eNOS uncoupling by stimulating expression of antioxidant proteins. The ability of EPO to stimulate expression of MnSOD and catalase could be explained by either of the two mechanisms. First, EPO had been shown to up-regulate antioxidant proteins by activation of NFκB pathway (Digicaylioglu & Lipton 2001). Second, cytoprotective effects of EPO have also been shown to be mediated by activation of NF-E2 related factor 2 (Nrf2), a transcription factor that regulates expression of many antioxidant enzymes, including heme oxygenase 1, superoxide dismutases, glutathione S-transferase, quinine oxidoreductase (Itoh et al. 1997, Zhang et al. 2010, Genc et al. 2010, Jin et al. 2011, Jin et al. 2014). The contribution of each of these pathways to EPO-induced stimulation of expressions of MnSOD and catalase in cerebral microvessels remains to be determined.
Andresen and coworkers have suggested that preserving MnSOD expression may help preserve NO bioavailability during oxidative stress (Andresen et al. 2004). Consistent with our observations, studies on MnSOD-deficient mice (Faraci et al. 2006) have demonstrated that NO-dependent relaxations to acetylcholine were impaired in cerebral arterioles of MnSOD+/− mice. Notably, the superoxide-mediated endothelial dysfunction was not observed in large cerebral arteries, demonstrating that MnSOD is functionally important for regulation of endothelial function in cerebral microvessels. Superoxide anions and other reactive oxygen species have been shown to induce permeabilization of mitochondrial membrane (Lemasters et al. 1998, Schild & Reiser 2005). With excessive oxidative stress, as seen in cerebral microvessels of hph1 mice, endogenous MnSOD could be overwhelmed and mitochondria also may become an important source of superoxide (Widlansky & Gutterman 2011). Results obtained from our study demonstrated that treatment of hph1 mice with EPO increased expression of MnSOD in cerebral microvessels. This EPO-induced increase in MnSOD expression might be sufficient to abrogate mitochondria-centered superoxide anion production and, in turn, protect NO from inactivation by superoxide anion. Indeed, the ability of EPO to stimulate expression of antioxidants and increase BH4 bioavailability resulted in increased availability of endothelial NO, as reflected in elevation of NO2/NO3 levels and significantly increased cGMP. Besides regulating cerebral blood flow, optimal availability of endothelial NO and activation of cGMP in cerebral microcirculation could also affect function of neuronal and microglial cells in the neurovascular unit (Faraci 2011, Katusic & Austin 2013). In this regard, obtained results suggest that EPO could protect the neurovascular unit against the pathological consequences of oxidative stress.
In summary, results from the present study provide the first evidence to suggest that EPO restores biological activity of NO in cerebral microvasculature injured by uncoupling of eNOS. Furthermore, obtained results suggest that increasing expression of antioxidant enzymes in cerebral microvascular endothelium by EPO is an important mechanism responsible for beneficial effects of EPO in cerebral circulation.
Acknowledgments
This study was funded in part by National Institutes of Health Grants (HL 91867, HL111062 to ZSK), Roche Foundation for Anemia Research and the Mayo Foundation. We would like to thank Ms. Leslie Smith for her technical assistance.
Footnotes
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am J Physiol Heart Circ Physiol. 2004;287:H1141–H1148. doi: 10.1152/ajpheart.01215.2003. [DOI] [PubMed] [Google Scholar]
- Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res. 2010;107:1498–1502. doi: 10.1161/CIRCRESAHA.110.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buemi M, Lacquaniti A, Bolignano D, Cernaro V, Campo S, Grasso G, Buemi A, Donato V, Sturiale A. Down with the erythropoietin. Long live the erythropoietin! Curr Drug Targets. 2009;10:1028–1032. doi: 10.2174/138945009789577981. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrissobolis S, Miller AA, Drummond GR, Kemp-Harper BK, Sobey CG. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci (Landmark Ed) 2011;16:1733–1745. doi: 10.2741/3816. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Katusic ZS. Erythropoietin increases endothelial biosynthesis of tetrahydrobiopterin by activation of protein kinase B alpha/Akt1. Hypertension. 2008;52:93–99. doi: 10.1161/HYPERTENSIONAHA.108.114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Smith LA, Katusic ZS. Erythropoietin increases expression and function of vascular copper- and zinc-containing superoxide dismutase. Hypertension. 2010;55:998–1004. doi: 10.1161/HYPERTENSIONAHA.110.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Uscio LV, Smith LA, Katusic ZS. Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice. Am J Physiol Heart Circ Physiol. 2011;301:H2227–H2234. doi: 10.1152/ajpheart.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Uscio LV, Smith LA, Santhanam AV, Richardson D, Nath KA, Katusic ZS. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension. 2007;49:1142–1148. doi: 10.1161/HYPERTENSIONAHA.106.085704. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Erbayraktar S, Yilmaz O, Gokmen N, Brines M. Erythropoietin is a multifunctional tissue-protective cytokine. Curr Hematol Rep. 2003;2:465–470. [PubMed] [Google Scholar]
- Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol. 2011;300:H1566–H1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol. 2006;100(1985):2089–2093. doi: 10.1152/japplphysiol.00939.2005. [DOI] [PubMed] [Google Scholar]
- Genc K, Egrilmez MY, Genc S. Erythropoietin induces nuclear translocation of Nrf2 and heme oxygenase-1 expression in SH-SY5Y cells. Cell Biochem Funct. 2010;28:197–201. doi: 10.1002/cbf.1639. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Bernaudin M, Bianchi R, et al. Erythropoietin: not just about erythropoiesis. Lancet. 2010;375:2142. doi: 10.1016/S0140-6736(10)60992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Meli F, Passalacqua M, Fodale V, Buemi M, Giambartino F, Iacopino DG, Tomasello F. The role of erythropoietin in neuroprotection: therapeutic perspectives. Drug News Perspect. 2007;20:315–320. doi: 10.1358/dnp.2007.20.5.1120219. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jin W, Ming X, Hou X, et al. Protective effects of erythropoietin in traumatic spinal cord injury by inducing the Nrf2 signaling pathway activation. J Trauma Acute Care Surg. 2014;76:1228–1234. doi: 10.1097/TA.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Jin W, Wu J, Wang H, Kong J, Ni H, Liang W. Erythropoietin administration modulates pulmonary Nrf2 signaling pathway after traumatic brain injury in mice. J Trauma. 2011;71:680–686. doi: 10.1097/TA.0b013e3181f6b984. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Austin SA. Endothelial nitric oxide: protector of a healthy mind. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo JP, Nicoli T, Alp NJ, Fullerton J, Flint J, Channon KM. Congenic mapping and genotyping of the tetrahydrobiopterin-deficient hph-1 mouse. Mol Genet Metab. 2004;82:251–254. doi: 10.1016/j.ymgme.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Kong XJ, Lee SL, Lanzillo JJ, Fanburg BL. Cu,Zn superoxide dismutase in vascular cells: changes during cell cycling and exposure to hyperoxia. Am J Physiol. 1993;264:L365–L375. doi: 10.1152/ajplung.1993.264.4.L365. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Nieminen AL, Qian T, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Krause DN, Duckles SP. 17beta-estradiol increases rat cerebrovascular prostacyclin synthesis by elevating cyclooxygenase-1 and prostacyclin synthase. Stroke. 2002;33:600–605. doi: 10.1161/hs0202.102732. [DOI] [PubMed] [Google Scholar]
- Park EY, Rho HM. The transcriptional activation of the human copper/zinc superoxide dismutase gene by 2,3,7,8-tetrachlorodibenzo-p-dioxin through two different regulator sites, the antioxidant responsive element and xenobiotic responsive element. Mol Cell Biochem. 2002;240:47–55. doi: 10.1023/a:1020600509965. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C. Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24:334–342. doi: 10.1097/01.WCB.0000105800.49957.1E. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Salinas M, Martin D, Perona R, Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci. 2004;24:7324–7334. doi: 10.1523/JNEUROSCI.2111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam AV, d'Uscio LV, He T, Katusic ZS. PPARdelta agonist GW501516 prevents uncoupling of endothelial nitric oxide synthase in cerebral microvessels of hph-1 mice. Brain Res. 2012a;1483:89–95. doi: 10.1016/j.brainres.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam AV, d'Uscio LV, Smith LA, Katusic ZS. Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice. J Neurochem. 2012b;122:1211–1218. doi: 10.1111/j.1471-4159.2012.07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam AV, Katusic ZS. Erythropoietin and cerebral vascular protection: role of nitric oxide. Acta Pharmacol Sin. 2006;27:1389–1394. doi: 10.1111/j.1745-7254.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- Santhanam AV, Smith LA, Akiyama M, Rosales AG, Bailey KR, Katusic ZS. Role of endothelial NO synthase phosphorylation in cerebrovascular protective effect of recombinant erythropoietin during subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2005;36:2731–2737. doi: 10.1161/01.STR.0000190021.85035.5b. [DOI] [PubMed] [Google Scholar]
- Santhanam AV, Smith LA, Nath KA, Katusic ZS. In vivo stimulatory effect of erythropoietin on endothelial n itric oxide synthase in cerebral arteries. Am J Physiol Heart Circ Physiol. 2006;291:H781–H786. doi: 10.1152/ajpheart.00045.2006. [DOI] [PubMed] [Google Scholar]
- Schild L, Reiser G. Oxidative stress is involved in the permeabilization of the inner membrane of brain mitochondria exposed to hypoxia/reoxygenation and low micromolar Ca2+ Febs J. 2005;272:3593–3601. doi: 10.1111/j.1742-4658.2005.04781.x. [DOI] [PubMed] [Google Scholar]
- Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal. 2011;15:1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol Sci. 2012;33:415–422. doi: 10.1016/j.tips.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 2010;244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]