Abstract
Background
One of the most novel and exciting findings in major depressive disorder research over the last decade is the discovery of the fast-acting and long-lasting antidepressant effects of ketamine. Indeed, the therapeutic effects of classic antidepressant such as SSRIs require a month or longer to be expressed, with about a third of MDD patients resistant to treatment. Clinical studies have shown that low dose of ketamine exhibits fast-acting relatively sustained antidepressant action even in treatment-resistant patients. However, the mechanisms of ketamine action at a systems level remain unclear.
Methods
Wistar-Kyoto rats were exposed to inescapable, uncontrollable footshocks. To evaluate learned helplessness behavior, we used an active avoidance task in a shuttle box equipped with an electrical grid floor. After helplessness assessment, we performed in vivo electrophysiological recordings first from ventral tegmental area dopaminergic (DA) neurons, and second from accumbens neurons responsive to fimbria stimulation. Ketamine was injected and tested on helpless behavior and electrophysiological recordings.
Results
We show that ketamine is able to restore the integrity of a network by acting on the DA system and restoring synaptic dysfunction observed in stress-induced depression. We show that part of the antidepressant effect of ketamine is via the DA system. Indeed injection of ketamine restores a decreased dopamine neuron population activity as well as restores synaptic plasticity (long-term potentiation) in the hippocampus-accumbens pathway, via in part, activation of D1 receptors.
Conclusions
This work provides for a unique systems perspective on the mechanisms of ketamine on a disrupted limbic system.
Keywords: ketamine, dopamine, learned helplessness, nucleus accumbens, ventral tegmental area, synaptic plasticity
Introduction
Major depressive (MDD) disorder is the most common mental disorder in the United States (1). A recent advance shows that a single low dose of ketamine, a functional N-methyl-D-aspartate indirect antagonist, relieves symptoms in treatment-resistant depression within hours and its effects can last for up to 10 days (2), and repeated injections induces sustained antidepressant action with mild side effects (3).
Cellular mechanisms of ketamine involve the rapid induction of synaptic proteins in the prefrontal cortex and the hippocampus of rats (4, 5). Ketamine rapidly reverses the stress-induced deficit in spine density (6) by activation of the mammalian target of rapamycin (mTor) signaling pathway (4, 6, 7). However, mechanisms at a systems level remain unclear.
We focused on two systems in the learned helplessness (LH) model of stress-induced depression: the dopaminergic (DA) reward system of the ventral tegmental area (VTA), in which dysfunctions (8) are thought to lead to the core MDD symptom of anhedonia, also found in the LH model (9). Secondly, the ventral subiculum of the hippocampus (vSub), involved in context-dependent regulation of behavior and stress integration (10), was examine due to its potential involvement in ruminative behavior, a condition associated with an abnormal focus on internal states (11, 12). Therefore, stress-induced disruptions of vSub-NAc contextual focus could drive an organism to a ruminative state (13). Mental rumination itself is not measurable in rats; however, LH can be maintained over time by processes that may be similar to those occurring in rumination (14). We thus propose to investigate the impact of LH on DA neuron activity and synaptic transmission in the vSub-NAc pathway, and how this system is influenced by ketamine administration.
Methods and Material
Animals
Adult male Wistar-Kyoto rats (300–350g, Charles River, USA) were used for their susceptibility to LH (15). Rats were housed singly on a reversed 12hr dark/light cycle (light on: 7.00 p.m.) with food and water ad libitum. All experiments were performed in accordance with the guidelines outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Behavioral and electrophysiological experiments are detailed in the Supplemental Methods
Learned Helplessness paradigm
In the LH paradigm (16, 17), inescapable stress occurred on day 1 in one chamber of a two-chamber shuttle box (Med Associates, VT, USA). Control animals (no-shock) were placed in the shocking chamber in parallel without shocking.
Helpless behavior and the effect of repeated injection of ketamine were assessed using an active avoidance task on days 2, 3 and 4. Failure was recorded if no crossing was made during the shock. The criterion of 40% failures to escape and 8 sec latency to escape (17) was used to discriminate between non-helpless and helpless rats.
A sub-anesthetic and sub-analgesic (18) dose of ketamine (dissolved in saline, 5mg/kg, i.p.) or saline (1ml/kg, i.p.) was injected 20 minutes or 2 hours before the beginning of the active avoidance task. Ketamine was injected either repeatedly (3 times, on days 3, 4 and 5) or acutely after testing for active avoidance on day 2. For acute injection, on day 3, one group of rats was tested for behavior and another was used for electrophysiological recordings.
Extracellular Recordings
Recordings were performed in chloral hydrate anesthetized rats (400 mg/kg, i.p.) 24 h after the last active avoidance task and as previously described (19–23).
VTA recordings
Microelectrodes were lowered through the VTA (A/P −5.5 to −5.9 mm, M/L +0.6 to +1.0 mm from bregma and −6.5 to −9.5 mm from dura). (24–26). Three parameters of activity were measured: (i) population activity (Supplemental figure S1) (ii) basal firing rate, and (iii) the proportion of action potentials occurring in bursts (24). Electrophysiological identification of dopaminergic neurons in the VTA is shown in supplemental figure S2. Ketamine (5mg/kg, i.p.) or saline (1ml/kg, i.p.) was injected 20 minutes, 2h or 24h before the beginning of the first track.
NAc recordings
Microelectrodes were lowered through the NAc (A/P +1.5 mm from bregma; M/L +1.1 mm from midline; D/V −5 to −7.5 mm from the dura). Single-pulse (intensity 1 mA; pulse-width 0.25 msec), and high-frequency stimulation (HFS; 50 Hz; 2s at suprathreshold) were applied to the fimbria (A/P −1.6 mm from bregma; M/L +1.3 mm from the midline; D/V −4.5 mm from the dura (20)). The D1 antagonist SCH23390 (0.5 μg/0.5 μl) or dPBS were infused locally into the NAc at a rate of 0.5 μL per minute via a 33 gauge cannula. Ketamine was injected i.p. (5mg/kg, in saline).
Histology
Recording electrode placement was verified via electrophoretic ejection of Chicago Sky Blue dye into the recording site and stimulation electrode placement was verified by delivering a 10 sec pulse at 200 μA. Rats were euthanized with a lethal dose of chloral hydrate (additional 400 mg/kg) and brains were removed following decapitation. The tissue was fixed in 8% paraformaldehyde for at least 48 h and then transferred to a 25% sucrose solution for cryoprotection. Once saturated, brains were frozen and sliced coronally at 60 μM thick using a cryostat (Leica Frigocut 2800) and mounted onto gelatin-chromalum-coated slides. Tissue was stained with combination of neutral red and cresyl violet.
Analysis
For behavior, results were expressed as mean number of failures (± SEM) and mean latency to escape (± SEM) recorded over 25 trials each day. Two-way ANOVA followed by a Dunnet’s t test was performed with treatment as the between-subject factor and session as the within-subject factor.
Electrophysiological data were analyzed using a one-way ANOVA (DA recordings) and a one-way ANOVA with repeated measures (NAc recordings) followed by the Holm–Sidak test, with time as the within-subject factor. When the normality test failed, a one-way ANOVA on Ranks was performed. Multiple comparisons were analyzed using a two-way ANOVA followed by the Holm–Sidak test, with treatment as the between-subject factor and time as the within-subject factor.
Results
Repeated injections of Ketamine restore escape behavior in helpless rats
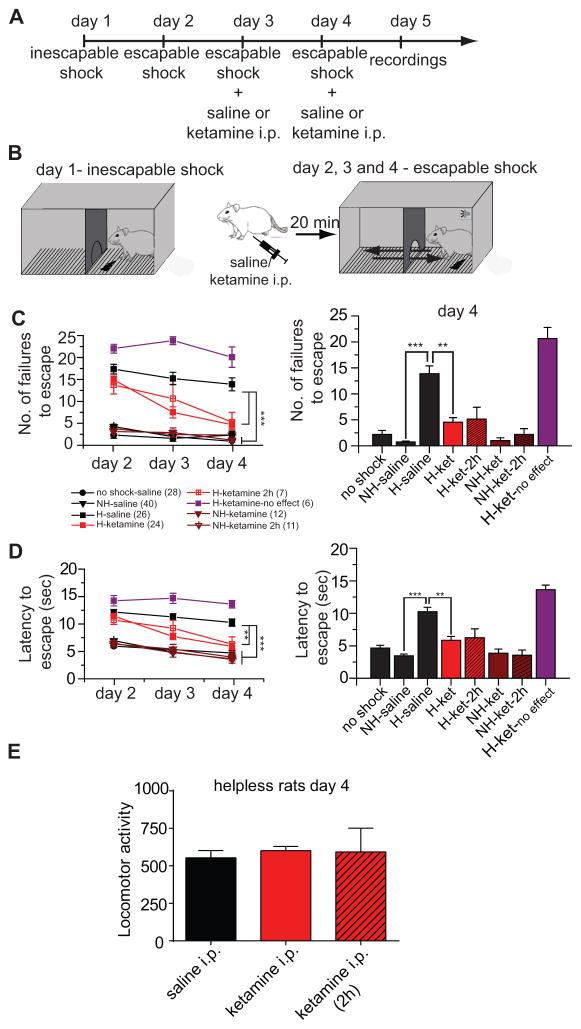
Rats received inescapable shocks on day 1 and were tested for escape behavior on 3 consecutive days before electrophysiological recordings (figure 1A and B). As previously reported (17, 29), inescapable shocks induce helpless behavior in 50% of the rats (non-helpless rats: 87 out of 172 rats or 50.6%; helpless rats: 85 out of 172 rats or 49.4 %). Between no-shock (n=28) and non-helpless rats (n=40), there was no difference in the number of failures to escape (F(1,27)=0.645, p=0.429) as well as the latency to escape (F(1,27)=0.042, p=0.840). Helpless rats (n=26) showed reduced escape and higher latency to escape compared to non-helpless rats (failures: F(1,25)= 115.265, p<0.001; latency: F(1,25)=71.954, p<0.001; figure 1C–D). Escape failure in helpless rats was reversed by prior administration of ketamine (20 minutes) (figure 1C–D left, red; summary figure 1C–D right). There was a significant difference between helpless rats treated with ketamine in comparison with saline injection (failures: F(1,23)=21.433, p<0.001 and latency: F(1,23)= 12.931, p<0.001) which was significant on days 3 and 4 (Holm-Sidak post-hoc). Ketamine (20 minutes), had no effect on the behavior of non-helpless rats in comparison to saline injection (failure: F(1,11)=0.048, p=0.504; latency: F(1,11)=0.291, p=0.6; figure C-D, brown, left). This is consistent with previous studies showing that low dose of ketamine does not interfere with active avoidance task performance (30, 31).
Figure 1. Learned helplessness is reversed by repeated injections of ketamine.
A. Experimental timeline B. Helplessness paradigm. C. Number of failures to escape across 3 consecutive days of escapable shocks session (left). Data for the escapable session on day 4 are summarized in bar graphs (right). Rats fall into two groups: those showing escape (triangles, non-helpless; circles, no-shock) and those failing to escape (squares, helpless). Ketamine (red) causes helpless rats to show escape behavior. D) Latency to escape across 3 consecutive days of escapable shocks session, showing results consistent with escape failures (left). Data for the escapable session on day 4 are summarized in bar graphs (right). Red, purple and brown represents data for ketamine. e) There was no difference in locomotor activity measured in both sides of the shuttle box during the escapable shock session on day 4 in helpless rats following saline or ketamine 20 minutes or 2 hours after(striped bar) the injection.
** p<0.01; ***p<0.001. Error bars represent SEM.
NH: non-helpless rats
H: helpless rats
In some cases, ketamine had no effect on escape behavior in helpless rats (6 out of 85 rats). There was no difference in latency or number of failures to escape before and 20 minutes after ketamine injection (latency: F(2,5)=1.928, p=0.196; failures: F(2,5)= 1.761, p=0.221; n=6).
Although the time course of drug action in rats is not directly comparable to that in humans, given clinical data showing that ketamine antidepressant effects are typically observed 2 hours after the injections (2), ketamine effects on escape behavior in helpless and non-helpless rats was tested 2 hours before the active avoidance tasks. For both groups, there was no difference in the number of failures to escape and the latency to escape in comparison to the 20-minute injection (helpless rats failures: F(1,6)=0.024, p=0.882; latency: F(1,6)=0.067; p=0.819; non-helpless failures: F(1,10)=0.261; p=0.621; latency: F(1,10)=0.016; p=0.902). This effect in helpless rats was significant on day 4, but not on day 3 (Holm-Sidak post-hoc).
Locomotor activity measured in helpless rats in the shuttle box on day 4 of the testing was not different between pre-injection of saline and pre-injection of ketamine (2h and 20 minutes) (F(1,54)= 0.379; p=0.686; figure 1E), ruling out ketamine-induced hyperactivity confounding measures of escape.
Ketamine restores dopaminergic population activity in the VTA of helpless rats
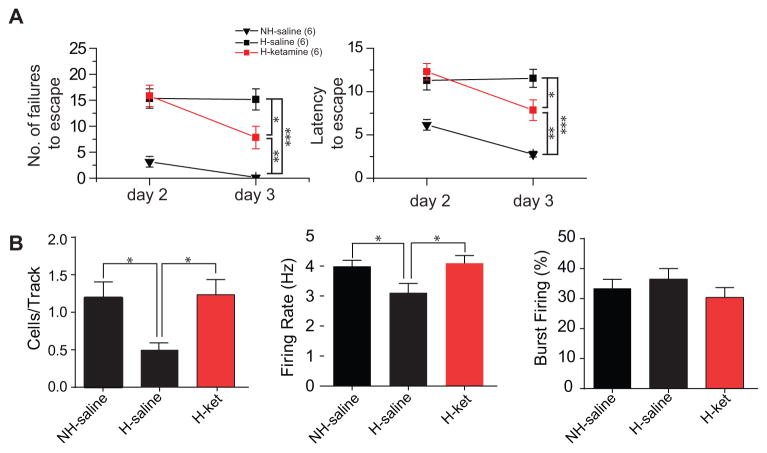
One of the core symptoms of MDD is anhedonia or the reduction of pleasure, which has been described in the LH model (9) and manifests as hyposensitivity to rewarding events; this likely involves dysfunction of the DA system (8, 32). In the current study, we examined whether DA activity was different in helpless and non-helpless animals (figure 2A–D). Non-helpless rats that received daily saline injections (n = 5 rats, 32 neurons) exhibited an average of 0.84 ± 0.05 spontaneously active DA neurons per electrode track that fired at an average rate of 4.1 ± 0.4 Hz and with 25.6± 4.4 % of the action potentials occurring in a burst discharge pattern, which is consistent with data obtained from home cage control rats (n=6 rats, 31 neurons; cells/track: F(1,10)=0.268; p=0.616; firing rate: F(1,61)=0.166; p=0.685; Burst firing: H=0.104; p=0.747, figure 2A-D) and no-shocks animals (n=4 rats, 23 neurons; cells/track: F(1,8)=0.444; p=0.524; firing rate: F(1,53)=1.662; p=0.203; Burst firing: H=0.671; p=0.413; figure 2A-C). Helpless rats (n=6 rats, 24 neurons) showed a significant decrease (approx. 50%) in DA neuron population activity compared to control (F(1,10)=6.713; p<0.05) and non-helpless rats (F(1,9)=13.455; p<0.01), (Figure 2A). No significant differences were observed in average firing rate (control versus helpless: F(1,52)=0.145; p=0.705; non-helpless versus helpless: F(1,53)=0.0397; p=0.843; figure 2B) or burst firing (control versus helpless, H=1.407; p=0.236; non-helpless versus helpless, H=0.700; p=0.403; figure 2C) across the population of neurons recorded. Injection of ketamine (20 minutes), compared to saline, restores the decreased population activity in helpless rats (F(1,9)= 14.33; p<0.01) with no change in firing rate (F(1,50)= 3.119; p=0.083) or burst firing (H=0.866; p=0.352). In non-helpless rats, ketamine (n=5 rats, 30 neurons) had no effect on the population activity (F(1,8)=0.756; p=0.410), firing rate (F(1,59)=3.424; p=0.07), or bursting activity (H= 3.553; p=0.06) compared to saline. However, in comparison to home cage control animals, ketamine injection increased firing rate and bursting for both helpless (firing rate: F(1,58)=4.091; p<0.05; bursting: H=6.663; p<0.05) and non-helpless rats (firing rate: F(1,59)=4.947; p<0.05; bursting: H=6.067; p<0.05). This is consistent with previous studies showing that non-competitive NMDA receptor antagonists increase DA firing in the VTA (33).
Figure 2. Helpless rats show selective decrease in dopamine neuron population activity that is reversed by repeated injections of ketamine.
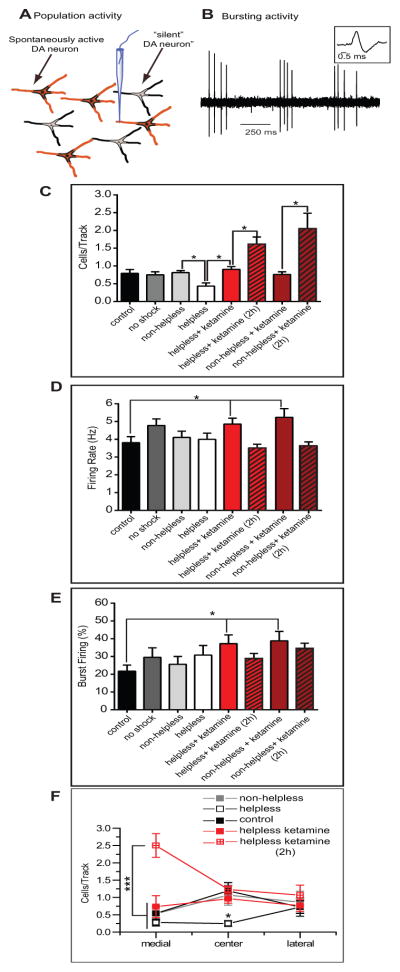
A) Number of spontaneously active DA neurons per electrode track. Only helpless rats showed a 50% decrease in number of active DA neurons (white bar) which was reversed by ketamine, 20 minutes post injection (red bar). Two hours after the injection, a significant increase in the population activity of non-helpless and helpless rats is observd (striped bar). In contrast, there was no change in firing rate (B) and burst firing (C) between helpless and non-helpless rats; ketamine administration 20 minutes prior recordings produced its typical increase in DA neuron rate and bursting in both groups, which is not seen 2 hours after the injection (striped bar). (D) Helpless rats show a decreased number of spontaneously active DA neurons located in the central (C) but not in the medial and lateral tracks in the VTA (white squares) compared to controls (black squares), non-helpless rats, and helpless rats treated with ketamine 20 minutes before recordings. Ketamine two hours post-injection induces an increase in population activity in the medial tracks. *p<0.05, ***p<0.001, error bars are ± SEM.
Red and brown bars represent data with injection of ketamine.
Ketamine injected 2 hours before the beginning of DA recordings induces a significant increase in population activity in helpless (n=5 rats, 65 neurons) and non-helpless rats (n=5 rats, 77 neurons), in comparison to ketamine injected 20 minutes prior (1.6 ± 0.2 cells per track, F(1,8)=10.945; p<0.05, n=5 in helpless rats vs 2.1 ± 0.4 in non-helpless rats, H=6.86; p<0.01, n=5; figure 2C). However, it had no effect on firing rate or burst firing in helpless and non-helpless rats compared to saline injection (non-helpless rats, firing rate: H=1.25; p=0.264; burst firing: H=2.374, p=0.123; helpless rats, firing rate: F(1, 86)=1.407; p=0.239; burst firing, H=0.003; p=0.958) (Figure 2B, C).
VTA DA neurons of helpless rats show a decreased population activity primarily in the central VTA (figure 2D) with no differences in medial and lateral tracks compared to control and non-helpless rats (medial: F(2,14)=0.744, p=0.493; central: H=9.417, p<0.001; lateral: F(2,14)=0.102, p=0.903). Injection of ketamine (20 minutes) induces an increase in the number of cells/track in the center tracks (F(1,9)=22.358, p<0.01, compared to saline) without affecting medial and lateral tracks (medial H=2.37, p=0.177; lateral H=0.035, p=0.931). In contrast, ketamine (2 hours) also increased DA cells/track in the medial track over that of ketamine administered 20 minutes prior (medial: F(1.8)= 14.295, p<0.001 vs lateral (F(1,8)= 1.161, p=0.31) and center (F(1,8)= 2.560, p=0.148).
Therefore, ketamine (20 minutes) in helpless rats restores escape behavior and DA activity in the VTA, mainly in the center tracks of the VTA. However, two hours after injection, ketamine also induces an overactivation of the medial tracks.
Ketamine has sustained action on escape behavior and activity in the VTA
Given that clinical studies show that ketamine exerts effects that are sustained for 24 hours (2), we tested the effects of a single injection of ketamine (after the end of the escapable session on day 2) on escape behavior (on day 3; Figure 3A). Helpless rats (n=6) showed reduced escape and higher latency to escape compared to non-helpless rats (n=6 rats) (failures: F(1,5)= 64.961, p<0.001; latency: F(1,5)=82.732, p<0.001; figure 3A) which was reduced by ketamine injected 24h before testing (failures: F(1,10)=6.027; p<0.05; latency: F(1,10)=5.438, p<0.05; figure 3A, red, n=6), but was still significantly different compared to non-helpless rats (failures: H=8.966, p<0.01; latency: H=8.308, p<0.01). In a separate group of rats, electrophysiological recordings were performed on identically treated rats on day 3. Non-helpless rats that received saline (n = 5 rats, 65 neurons) exhibited an average of 1.2 ± 0.2 spontaneously active DA neurons per electrode track that fired at an average rate of 3.8 ± 0.2 Hz and with 33.4 ± 3.0 % of the action potentials occurring in a burst discharge pattern. Helpless rats (n=5 rats, 24 neurons) showed a significant decrease in DA neuron population activity and in the firing rate compared to non-helpless rats (population activity, F(1,9)=10.115, p<0.05; firing rate: F(1,87)=5.176, p<0.05), which is restored by ketamine injected 24 hours before recording (population activity, F(1,8)=10.809, p<0.05; firing rate: F(1,69)=5.323; p<0.05). No difference in burst firing between non-helpless and helpless rats was observed after saline (H=2.413, p=0.12) or ketamine injection (H=3.195, p=0.072). The effect of ketamine injection on escape behavior and DA neuron recordings in non-helpless rats is shown in supplemental figure S3.
Figure 3. Acute injection of ketamine restores sustainably escape behavior and VTA DA activity.
A. Number of failures (left) and latency (right) to escape across 2 consecutive days of escapable shocks session. Injection of ketamine (red) after the first escapable session induces a significant decrease in the number of failures and the latency to escape (non-helpless rats: triangle; helpless rats: square). B. Number of spontaneously active DA neurons per electrode track (top). Helpless rats showed a 50% decrease in number of active DA neurons and a decrease in firing rate (middle) which was reversed by ketamine, 24h post injection (red bar). No change in the bursting activity (bottom) was observed between non-helpless, helpless rats, and helpless rats treated with ketamine. *p<0.05, **p<0.01, ***p<0.001, error bars are ± SEM; NH: non-helpless rats; H: helpless rats
Therefore, ketamine injection 24h prior recordings/behavioral task in helpless rats restores escape behavior in parallel with restoration of dopaminergic activity in the VTA.
Shell and core segments of the nucleus accumbens show opposite responses to HFS of the fimbria
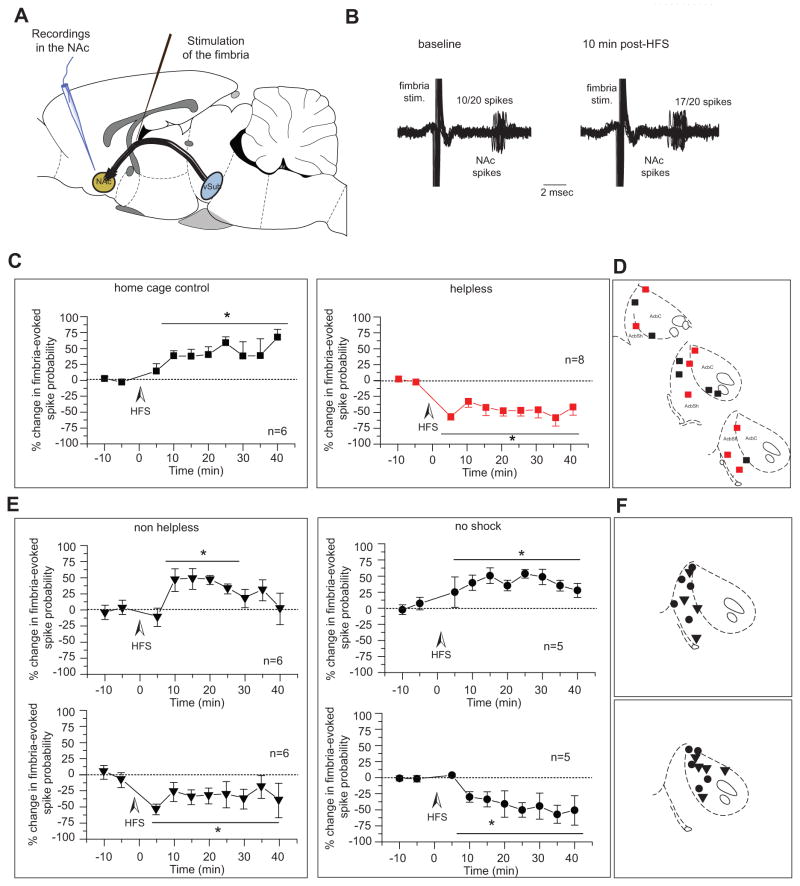
To examine potential changes in synaptic transmission in the vSub-NAc pathway in helpless rats, hippocampal-evoked activity was assessed in the NAc after high frequency stimulation (HFS) of the fimbria (figure 4A)(34). As previously described (19, 20), fimbria HFS induced long-term potentiation (LTP) in the vSub-NAc pathway of home cage control animals (figure 4B and 4C left) whereas long-term depression (LTD) was induced in helpless rats (figure 4C right). The baseline evoked spike probability in home cage control animals was 0.47 ± 0.05 and increased to 0.60 ± 0.08, 15 min post-HFS (n=6, F(5,31)=2.993; p<0.05) whereas in helpless animals baseline spike probability was 0.47 ± 0.04 and decreased to 0.29 ± 0.07, 15 min post-HFS (n=8; F(7,45)=8.095; p<0.001). Neurons were located either in the NAc-core or -shell (figure 4D).
Figure 4. Shell and core segments of the nucleus accumbens show different responses to HFS of fimbria in helpless vs non-helpless rats.
A) Schematic of recording and stimulating electrode placements. B) Extracellular recording trace showing a representative example of the increased fimbria-evoked spike probability recorded from a NAc neuron in a control animal, 10 min after high frequency stimulation. Twenty overlaid consecutive traces are shown with the numbers demonstrating the number of evoked spikes for 20 stimulations. c) HFS of the fimbria produced LTP in control rats (black squares) but produced LTD in helpless rats (red squares). D) Recording electrode placements in the NAc of home cage control (black squares) and helpless rats (red squares) animals shown as coronal sections of the rat brain, taken from Paxinos and Watson (53). E) HFS of the fimbria produced LTP in the accumbens shell in non-helpless and no-shock rats (top) but produced LTD in the accumbens core (bottom). Plots show mean percent change (± SEM) in fimbria-evoked spike probability, normalized to the baseline. F) Recording electrode placements in the NAc of non-helpless (triangles) and helpless rats (circles) animals, shown as coronal sections of the rat brain, taken from Paxinos and Watson (53).
*p<0.05; ***p<0.001; arrow indicates the time of stimulation
vSub: ventral subiculum of the hippocampus; NAc: nucleus accumbens; HFS: high-frequency stimulation
In non-helpless rats, HFS to the fimbria induced 2 types of responses: an LTP (baseline spike probability: 0.46 ± 0.02, post-HFS: 0.58 ± 0.05, F(5,35)=4.477, p<0.05, n=6; Figure 4E top left) or an LTD (baseline spike probability: 0.49 ± 0.03, post-HFS: 0.32 ± 0.03, F(5,28)=2.925, p<0.05, n=6; Figure 4E bottom left). Interestingly, the majority of the electrode placements for LTP were located in the NAc-shell and the majority of the LTD placements were located in the NAc-core (figure 4F). No-shock rats have the same responses, with neurons located in the NAc-shell showing a significant increase of the spike probability (baseline spike probability: 0.53 ± 0.03, post-HFS: 0.66 ± 0.03; F(4,28)=4.432, p<0.01 n=5, Figure 4E top right) and neurons in the core a significant decrease (baseline spike probability: 0.59 ± 0.07, post-HFS: 0.41 ± 0.06, F(4,14)= 4.828, p<0.05; n=5; Figure 4E bottom right).
Therefore, in the LH model, LTD in the vSub-shell NAc but not in the vSub-core NAc appears to be a marker of helplessness.
Ketamine restores long-term potentiation in the hippocampus-accumbens pathway of helpless rats
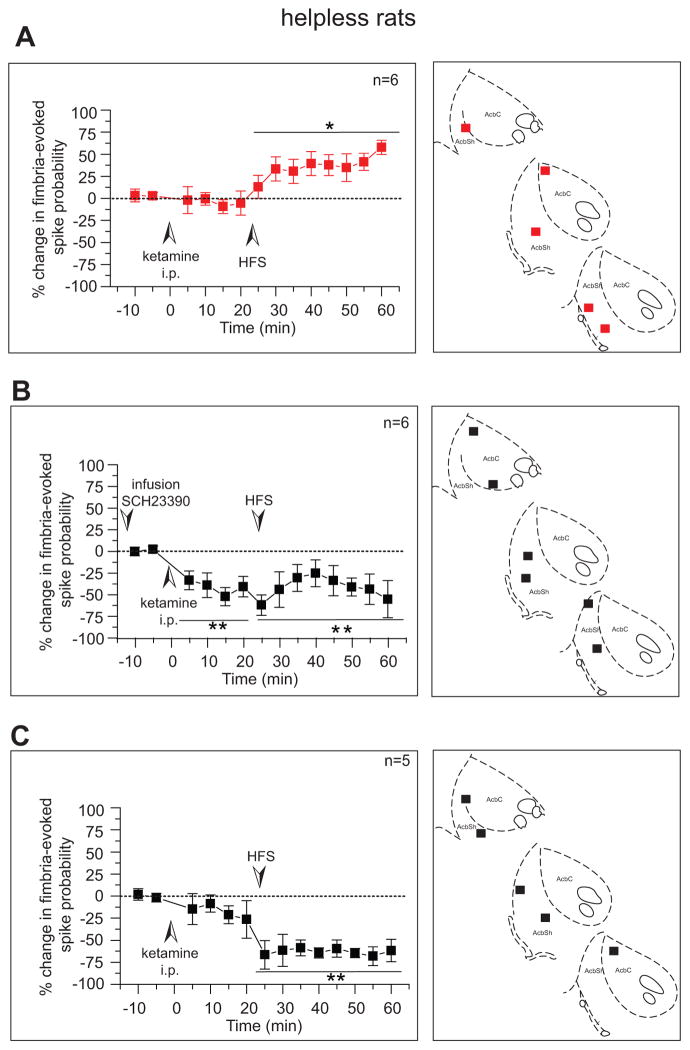
When ketamine was injected 20 minutes before HFS, LTP of the vSub-NAc pathway was restored (n=6; figure 5A left). This effect was not due to the injection since pre-injection of saline had no effect on the HFS-induced LTD (n=4, data not shown). Neurons were located either in the NAc-core or -shell (figure 5A right). Therefore, besides restoring escape behavior and dopaminergic activity in the VTA, ketamine restores synaptic plasticity in the hippocampus-accumbens pathway.
Figure 5. Ketamine restores long-term potentiation in the hippocampus-accumbens pathway of helpless rats that depends on D1 receptors.
Left a) injection of ketamine (i.p. 5mg/kg) in helpless animals restored fimbria HFS-induced LTP in the accumbens shell following high frequency stimulation of the fimbria and b) infusion of the D1 antagonist SCH23390 in the NAc (0.5 μg/0.5 μl) prevented ketamine (i.p. 5mg/kg) from restoring LTP in the fimbria-NAc pathway. c) Ketamine did not affect HFS-induced LTD in rats that did not show behavioral improvement. Plots show mean percent change (±SEM) in fimbria-evoked spike probability, normalized to baseline. Right. Recording electrode sites shown as coronal sections of the rat brain taken from Paxinos and Watson (53).
*p<0.05, **p<0.01.
HFS: high-frequency stimulation
The HFS-induced LTP in control rats is, at least in part, dopamine-dependent (35). Thus, in a separate group of helpless rats (n=6), the D1 antagonist SCH23390 was infused into the NAc 15 min before recording baseline activity. In these neurons, ketamine induced a decrease of fimbria-evoked spiking activity and after HFS of the fimbria, an LTD was observed (F(5,27)=3.769; p<0.01, n=6). This effect was not due to infusion in the NAc since pre-infusion of the vehicle dPBS had no effect on the HFS-induced LTP previously described (n=3, data not shown). Therefore, ketamine restores synaptic plasticity in the NAc via activation of D1 receptors.
As previously mentioned, ketamine had no effect on escape behavior in a subset of helpless rats. In these neurons, ketamine before HFS did not restore LTP of the vSub-NAc pathway and induced an LTD (F(4,25)=3.385; p<0.01). Therefore, ketamine restores escape behavior likely via reinstatement of adequate response to fimbria HFS (synaptic plasticity) in the NAc.
Discussion
In this study we examined the effect of repeated and acute ketamine injections in a behavioral model of depression. We find that ketamine reverses helpless behavior, restores normal DA neuron population activity and restores LTP in the vSub-NAc pathway. Our findings indicate that a normal LTP in the NAc-shell correlates with a failure to induce helplessness. Moreover, this study shows that the effect of ketamine on synaptic plasticity is at least in part due to activation of D1 receptors in the NAc. Therefore, our results suggest that the antidepressant action of ketamine acts via the DA system, at least in part, and the NAc-shell.
Several models of depression, such as the chronic mild stress model (32) and the LH model (9) induce anhedonia in rats, thought to involve disruptions of the DA system (8, 32). In particular, inhibition of VTA DA neurons can acutely induce multiple depression-like behaviors, and activation of VTA DA neurons can rescue stress-induced depression-like phenotype (32). In the present study, helpless rats exhibited a decrease in the population activity specifically in the central tracks of the VTA, with no change in the firing rate or the bursting activity of single DA neurons. This is consistent with our study showing that chronic stress selectively decreases DA neuron population activity in the medial and central tracks of the VTA (36). Therefore, in helpless animals a decrease in the number of spontaneously active DA neurons is consistent with a decrease in VTA activity inducing stress-induced depressive-like behavior (32).
In the present study, repeated injections of ketamine restored escape behavior in helpless rats independent of the timing of the injection (20 minutes or 2 hours), confirming the antidepressant effect of this agent at low doses. We also showed that in these rats, 20 minutes after the injection, the DA population activity in the VTA is restored to what is observed in control animals. Two hours after the injection, ketamine not only restored the population activity in helpless rats, but induced a significant increase both in helpless and non-helpless rats, which is consistent with that observed in rodent models of psychosis (37), and might correspond to the dissociative and psychotic symptoms described in patients ½ h after i.v. infusion of ketamine (2). These restorative actions of ketamine were maintained 24 hours post-injection with respect to both decreased escape failures and restoration of VTA DA neuron activity, albeit the escape behavior remained below control levels. This is consistent with recent studies highlighting the beneficial effect of repeated dose of ketamine, in comparison to acute injection, in sustainably treating major-depressive disorder in patients (3).
One of the challenges in treating depression is not only to restore sensitivity to positive rewarding events but also to diminish negative mood state. In depressed patients, ruminative self-focus is associated with hyperactivity of limbic regions such as the medial prefrontal cortex, the amygdala and the hippocampus (38). In our study we show that non-helpless rats display normal synaptic plasticity in the vSub-shell pathway, but disruption of the vSub-core pathway. However, helpless rats show disruption of plasticity in both the vSub-shell and -core pathways. ll groups of rats received footshocks during escape behavior testing; therefore this footshock stress appears to correspond to vSub-core LTD across groups. Indeed, stress can enhance synaptic strength (39) and elevate DA levels (40) in the NAc-shell, but not the core (39, 40). In contrast, helpless rats differ from non-helpless and no-shock rats by an LTD in the vSub-shell pathway. Thus, in no-shock and non-helpless rats, LTP in the vSub-shell pathway produced by footshock-induced stress may protect them from helpless behavior. Ketamine injected before HFS restored normal plasticity in the vSub-shell and -core of helpless rats. Although it is not clear why an NMDA antagonist like ketamine would restore LTP, ketamine induces an increase in glutamate and aspartate release by a presynaptic action (41). This increase may then activate glutamatergic neurotransmission at non-NMDA receptors, including AMPA and kainate receptors. Ketamine also increases the release of DA (42) in the striatum, which is a potent modulator of synaptic plasticity (43). Therefore, ketamine could act to increase LTP via an indirect activation of AMPA and DA D1 receptors, since blockade of D1 receptors prevents ketamine actions in helpless rats. Consistent with previous studies (35) ketamine or SCH23390 injected alone have no effect on the basal fimbria-evoked spiking activity in the NAc. However, with pre-infusion of SCH23390, ketamine had an inhibitory effect, consistent with studies showing that D1-NMDA interactions in the NAc affect the excitability of NAc neurons (44).
In contrast, when ketamine has no effect on escape deficit in helpless rats, it does not restore normal synaptic plasticity in the vSub-NAc pathway, suggesting that normal plasticity in the vSub-NAc pathway is necessary for escape behavior. This is also consistent with recent studies (31, 45) showing that ketamine rapidly increases m-Tor-dependent synaptogenesis in the PFC (4, 6) and the hippocampus (5), and increases hippocampal BDNF and mTOR levels during the forced swim test in rats (46). Moreover, the rapid and sustained antidepressant-like effect of ketamine in the LH model also involves stimulation of AMPA receptors (47). Since LTD involves internalization of AMPA receptors (48), it is feasible that, in response to HFS, ketamine reverses the altered synaptic plasticity of helpless rats via increased glutamate and DA release and activation of AMPA receptors. In the social defeat stress model, a decrease in AMPA function in the NAc is proposed to mediate resilience (49). However, in the present study the effect of ketamine was examined after LTP induction, which by itself induces changes in AMPA function (50). Moreover, ketamine has been injected i.p. which we expect will have an effect on structures others than the NAc such as the hippocampus, the prefrontal cortex (4, 5) or the basolateral amygdala (51), which play an important role in depressive-like behavior (52) and have a critical influence on synaptic plasticity in the NAc.
Taken together, the present data provide strong evidence that both repeated and acute injections of ketamine may be effective in reversing depression symptoms by restoring an abnormally attenuated dopaminergic population activity, which we predict will reverse hyposensitivity to rewarding events. Ketamine may also be effective in treating ruminative behavior by restoring normal information processing in the vSub-NAc shell pathway via activation of D1 receptors in the NAc. These two processes may be related, in that the decrease in vSub-NAc drive could contribute to the decrease in DA neuron activity, since activation of this circuit increases DA neuron population activity (23, 37). Such information is a significant step towards the understanding of the mechanisms of ketamine at a systems level for the treatment of depression and may point to a direction for more effective treatments.
Supplementary Material
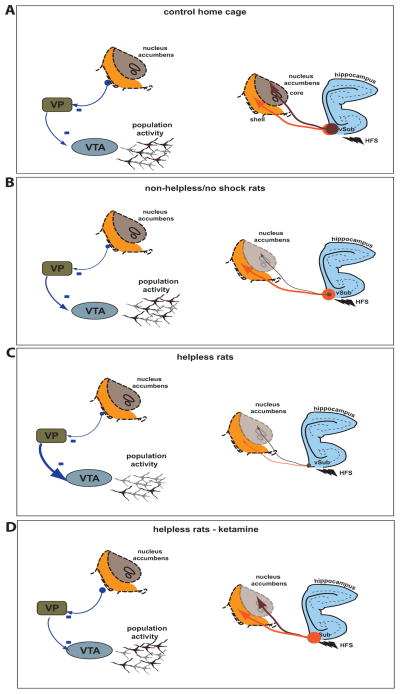
Figure 6. Summarizing schematic.
Schematic summarizing the population activity in the VTA (left) and effect of HFS on the vSub-NAc shell and core (right). (a) In home cage control rats, HFS of the vSub produces LTP in the NAc core and shell (right), (b) following inescapable shock, rats that did not show helplessness showed HFS-induced LTD in the vSub-NAc core projection, with the vSub-shell projection showing normal HFS-induced LTP (right) and a DA neuron population activity comparable to control rats. (c) In contrast, in helpless rats, the vSub-shell and -core pathways shows LTD in response to HFS (right), which corresponds to a decrease in DA neuron population activity (left). (d) Following injection of ketamine, both DA neuron activity (left) and vSub-NAc LTP (right) is restored in both core and shell regions in helpless rats.
Thin arrow, LTD, thick arrow, LTP.
VP: ventral pallidum; VTA: ventral tegmental area; vSub : ventral subiculum of the hippocampus; HFS: High frequency stimulation
Acknowledgments
We thank Niki MacMurdo, Hannah Dollish and Nicole Jakobowski for technical assistance and Kathryn Gill and Jared Moreines for comments. This work was funded by a Young Investigator Award from NARSAD - The Brain and Behavior Research Foundation (PB), the United States Public Health Service Grant MH57440 and MH191180 and a gift from Lundbeck (A.A.G.)
Footnotes
Financial disclosure
Dr. Belujon reports no biomedical financial interests or potential conflicts of interest. Dr. Grace received funds from Johnson and Johnson, Lundbeck, Pfizer, GlaxoSmithKline, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, and Asubio.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 3.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchis-Segura C, Spanagel R, Henn FA, Vollmayr B. Reduced sensitivity to sucrose in rats bred for helplessness: a study using the matching law. Behav Pharmacol. 2005;16:267–270. doi: 10.1097/01.fbp.0000171772.61669.6f. [DOI] [PubMed] [Google Scholar]
- 10.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- 12.Bagby RM, Rector NA, Segal ZV, Joffe RT, Levitt AJ, Kennedy SH, et al. Rumination and distraction in major depression: assessing response to pharmacological treatment. J Affect Disord. 1999;55:225–229. doi: 10.1016/s0165-0327(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 13.Papageorgiou C, AW Positive beliefs about depressive rumination: development and preliminary validation of a self-report scale. Behavior Therapy. 2001;32:13–26. [Google Scholar]
- 14.Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol Psychiatry. 2001;49:763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- 15.Wieland S, Boren JL, Consroe PF, Martin A. Stock differences in the susceptibility of rats to learned helplessness training. Life Sci. 1986;39:937–944. doi: 10.1016/0024-3205(86)90376-0. [DOI] [PubMed] [Google Scholar]
- 16.Valentine G, Dow A, Banasr M, Pittman B, Duman R. Differential effects of chronic antidepressant treatment on shuttle box escape deficits induced by uncontrollable stress. Psychopharmacology (Berl) 2008;200:585–596. doi: 10.1007/s00213-008-1239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kissin I, Bright CA, Bradley EL., Jr The effect of ketamine on opioid-induced acute tolerance: can it explain reduction of opioid consumption with ketamine-opioid analgesic combinations? Anesthesia and analgesia. 2000;91:1483–1488. doi: 10.1097/00000539-200012000-00035. [DOI] [PubMed] [Google Scholar]
- 19.Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. J Neurosci. 2008;28:9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belujon P, Patton MH, Grace AA. Role of the Prefrontal Cortex in Altered Hippocampal-Accumbens Synaptic Plasticity in a Developmental Animal Model of Schizophrenia. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel alpha5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- 24.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 25.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–430. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci. 1998;18:5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petty F, Kramer GL, Wu J. Serotonergic modulation of learned helplessness. Ann N Y Acad Sci. 1997;821:538–541. doi: 10.1111/j.1749-6632.1997.tb48324.x. [DOI] [PubMed] [Google Scholar]
- 30.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of Striatal DeltaFosB Overexpression and Ketamine on Social Defeat Stress-Induced Anhedonia in Mice. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 32.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.French ED, Ceci A. Non-competitive N-methyl-D-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons. Neurosci Lett. 1990;119:159–162. doi: 10.1016/0304-3940(90)90823-r. [DOI] [PubMed] [Google Scholar]
- 34.Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 35.Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenti O, Gill KM, Grace AA. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci. 2012;35:1312–1321. doi: 10.1111/j.1460-9568.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Moghaddam B. Regulation of glutamate efflux by excitatory amino acid receptors: evidence for tonic inhibitory and phasic excitatory regulation. J Pharmacol Exp Ther. 1995;274:1209–1215. [PubMed] [Google Scholar]
- 42.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 44.Huppe-Gourgues F, O’Donnell P. D(1)-NMDA receptor interactions in the rat nucleus accumbens change during adolescence. Synapse. 2012;66:584–591. doi: 10.1002/syn.21544. [DOI] [PubMed] [Google Scholar]
- 45.Dwyer JM, Duman RS. Activation of Mammalian Target of Rapamycin and Synaptogenesis: Role in the Actions of Rapid-Acting Antidepressants. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 48.Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 49.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kessal K, Chessel A, Spennato G, Garcia R. Ketamine and amphetamine both enhance synaptic transmission in the amygdala-nucleus accumbens pathway but with different time-courses. Synapse. 2005;57:61–65. doi: 10.1002/syn.20154. [DOI] [PubMed] [Google Scholar]
- 52.Chang CH, Grace AA. Amygdala-Ventral Pallidum Pathway Decreases Dopamine Activity After Chronic Mild Stress in Rats. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The Rat Brain in Stereotatic Coordinates. Academic Press; San Diego: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.