Abstract
T2A-1 is a newly developed transgenic rice that expresses a synthesized cry2Aa gene driven by the maize ubiquitin promoter. T2A-1 exhibits high resistance against lepidopteran pests of rice. The brown planthopper, Nilapavarta lugens (Stål), is a main nontarget sap-sucking insect pest of rice, and Cyrtorhinus lividipennis (Reuter) is the major predator of the eggs and young nymphs of planthoppers. As C. lividipennis may expose to the Cry2Aa protein via N. lugens, it is therefore essential to assess the potential effects of transgenic cry2Aa rice on this predator. In the present study, three experiments were conducted to evaluate the ecological risk of transgenic cry2Aa rice to C. lividipennis: (1) a direct feeding experiment in which C. lividipennis was fed an artificial diet containing Cry2Aa at the dose of 10-time higher than that it may encounter in the realistic field condition; (2) a tritrophic experiment in which the Cry2Aa protein was delivered to C. lividipennis indirectly through prey eggs or nymphs; (3) a realistic field experiment in which the population dynamics of C. lividipennis were investigated using vacuum-suction. Both direct exposure to elevated doses of the Cry2Aa protein and prey-mediated exposure to realistic doses of the protein did not result in significant detrimental effects on the development, survival, female ratio and body weight of C. lividipennis. No significant differences in population density and population dynamics were observed between C. lividipennis in transgenic cry2Aa and nontransgenic rice fields. It may be concluded that transgenic cry2Aa rice had no detrimental effects on C. lividipennis. This study represents the first report of an assessment continuum for the effects of transgenic cry2Aa rice on C. lividipennis.
Introduction
Rice, Oryza sativa L., is the staple food of more than three billion people of Asia [1]. More than 200 species of insect pests infest rice during its growing season [2], and this causes 15% to 25% yield losses of rice [3], [4]. Among these insects, lepidopteran species such as stem borers and leaffolders are particularly serious chronic pests and cause large annual yield losses [5], [6]. Traditional management of lepidopteran pests is mainly dependent on the spraying of pesticides. However, excessive or continual applications of pesticide not only cause environmental contamination and the resurgence of herbivores but also reduce populations of the natural enemies of these herbivores [7], [8]. Researchers have therefore been encouraged to seek more effective and environmentally friendly methods to control lepidopteran pests.
The Bacillus thuringiensis (Bt) insecticidal δ-endotoxin has been used as a biological insecticide for more than 50 years, and it is now possible to introduce different Bt genes into rice. Transgenic rice plants expressing Bt proteins have been shown to be effective against many lepidopteran insect pests [9] and have led to great reductions in the use of insecticides [10], [11]. A series of rice lines that express various Bt genes (e.g., cry1Ab, cry1Ab/1Ac, cry1C and cry2A) have been developed and can effectively suppress the infestation of target lepidopteran insect pests [12]–[14]. Despite these successes, concerns have been raised regarding the potential impacts of transgenic Bt rice on nontarget herbivores and their natural enemies through tritrophic transmission. It is therefore necessary to conduct an environmental risk assessment of any novel transgenic Bt rice prior to its commercialization, and the environmental risks of each rice line must be evaluated on a case-by-case basis.
The brown planthopper, Nilapavarta lugens (Stål) (Hemiptera: Delphacidae), is the main nontarget sap-sucking insect pest of rice and causes significant annual reductions in the rice yield [15], [16]. The evaluation of nontarget effects of transgenic rice on the natural enemies of N. lugens is an important part of the environmental risk assessment, and should be conducted before the commercialization of any novel Bt rice. Several previous reports have examined the effects of transgenic Bt rice on the predators of N. lugens. Although the Cry1Ab protein can be transferred from transgenic rice plants to predators via N. lugens, no adverse effects have been found on any of the fitness parameters (survival, developmental time, weight and fecundity) of numerous predators [Cyrtorhinus lividipennis (Reuter) (Hemiptera: Miridae), Pardosa pseudoannulata (Bösenberg et Strand) (Araneae: Lycosidae), Pirata subpiraticus (Bösenberg et Strand) (Araneae: Lycosidae), Propylea japonica (Thunberg) (Coleoptera: Coccinellidae) and Ummeliata insecticeps (Bösenberg et Strand) (Araneae: Linyphiidae)] that preyed on N. lugens reared on Bt-transformed rice lines in the laboratory [17]–[22]. Additionally, the results from field experiments have also shown that the population densities and population dynamics of U. insecticeps, C. lividipennis and P. pseudoannulata are not significantly different between transgenic cry1Ab rice and non-Bt rice fields [21]–[23]. All of these results have indicated that the predators of N. lugens are not affected by the Cry1Ab protein. T2A-1 is a relatively newly developed transgenic rice line expressing cry2Aa driven by the maize ubiquitin promoter, and it exhibits high resistance against lepidopteran pests of rice [12]. However, the potential effects of this rice line on the predators of N. lugens have received little attention. T2A-1 did not cause direct detrimental effects on the larvae of Chrysoperla sinica (Tjeder) (Neuroptera: Chrysopidae), a general predator of N. lugens [24]; that study represents the only existing report about the effects of Cry2Aa on the predators of N. lugens.
Cyrtorhinus lividipennis (Reuter) (Hemiptera: Miridae) is a major predator of the eggs and young nymphs of planthoppers, and it is a primary factor regulating the population density of N. lugens in rice fields [7], [25], [26]. Thus, C. lividipennis may expose to the Bt protein via N. lugens. The potential effects of transgenic Bt rice on C. lividipennis should be evaluated. Transgenic cry1Ab rice had no negative effects on the life-table parameters, population density and population dynamics of C. lividipennis [18], [23]. However, no such study of transgenic cry2Aa rice has yet been conducted with C. lividipennis. In the current study, we conducted comprehensive experiments in the laboratory and in rice fields to examine the effects of T2A-1 on C. lividipennis. The effects of T2A-1 via prey on the life-table parameters and the functional response of C. lividipennis to N. lugens, and the direct toxicity of the Cry2Aa protein to C. lividipennis were evaluated in the laboratory. The effects of T2A-1 on population density and population dynamics were investigated through a 3-year experiment in rice fields. ELISA was used to determine whether the Cry2Aa protein could be transferred to C. lividipennis via N. lugens.
Materials and Methods
Ethics Statement
All necessary permits were obtained for the described field studies. Permission of small-field test of the transgenic line (T2A-1) at the suburbs of Xiaogan City and the suburbs of Suizhou City during the 2011-2013 was issued by Ministry of Agriculture of the People's Republic of China. Contact: Hao Chen; Phone: +86 27 87280516.
Plant materials
The transgenic Bt rice line (T2A-1) and the nontransgenic parental indica rice line Minghui 63 were selected for the experiments. T2A-1 expresses a synthesized cry2Aa gene driven by the maize ubiquitin promoter. T2A-1 is homozygous and exhibits high resistance against lepidopteran pests of rice [12]. Minghui 63 is an elite indica restorer strain, and served as nontransgenic control. Both rice lines were gifted by National Key Laboratory of Crop Genetic Improvement, Wuhan, China.
The two rice lines used for the laboratory experiments were cultured in different plastic tanks (25 cm length×20 cm width×3 cm height) in Yoshida culture solution [27]. Fifteen-day-old rice seedlings (approximately 15 cm in height) were used in the experiments. All plants were maintained at 26°C±2°C, and the relative humidity was approximately 80%.
Insects for the laboratory experiments
The original adults of N. lugens were collected from paddy fields in Wuhan, Hubei Province, China. Prior to the tritrophic bioassay, independent colonies of N. lugens were established on T2A-1 and Minghui 63 and maintained for more than ten generations. The C. lividipennis individuals were collected from paddy fields in Xiaogan and reared with eggs of N. lugens infested on Minghui 63. The colony was maintained for more than six generations before its use in the present study. The insects were cultured at 28±1°C, RH 70±5% and with a light-dark cycle of 14 h:10 h. Both susceptible strains of Plodia interpunctella (Hubner) (Lepidoptera: Pyralidae) and Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae) were used to confirm the bioactivity of the Cry protein.
Prey egg–mediated effects of transgenic cry2Aa rice on the life-table parameters of C. lividipennis
Two reproductive females of N. lugens were placed into a glass tube (3 cm diameter×25 cm length) containing six or seven 15-day-old rice seedlings (Minghui 63 and T2A-1) and allowed to lay eggs for 2 days. The N. lugens adults were then removed, and the eggs of N. lugens on the rice seedlings were used as the prey of C. lividipennis. Newly hatched nymphs of C. lividipennis (<24 h) were placed individually in glass tubes. All tubes were sealed with nylon mesh. During the period from the first to the third instar of C. lividipennis, the rice seedlings were refreshed every 2 days, and during the period from the fourth instar to adulthood, the rice seedlings were refreshed every day. Yoshida culture solution [27] was used to keep the rice seedlings fresh. The survival and molting of the C. lividipennis nymphs were recorded every day. After the C. lividipennis adults emerged, the sex and body weight of these adults were recorded. Thirty-two nymphs of C. lividipennis were tested for each rice line.
Prey nymph–mediated effects of transgenic cry2Aa rice on the life-table parameters of C. lividipennis
Newly molted second-instar nymphs (<24 h) of C. lividipennis were placed individually in glass tubes (2 cm diameter×12 cm length) covered with tampons. The bottom of each tube was filled with a piece of wetted sponge to maintain humidity. Newly hatched nymphs of N. lugens (24–48 h after hatching), reared on either Minghui 63 or T2A-1 rice plants, were employed as the prey of C. lividipennis. During the period from the second to the third instar of C. lividipennis, 10 nymphs of N. lugens were provided daily, and during the period from the fourth instar to adulthood, 20 nymphs were provided daily. The survival and molting of the C. lividipennis nymphs were monitored on a daily basis. After the adults of C. lividipennis emerged, the sex and body weight of these adults were recorded. Eighty nymphs of C. lividipennis were tested for each rice line.
Cry2Aa contents in rice plants, N. lugens and C. lividipennis
The sheaths of 15-day-old rice seedlings, neonates of N. lugens fed on T2A-1 or Minghui 63 for 2 days, N. lugens eggs laid by N. lugens adults fed on T2A-1 or Minghui 63, and third- or fourth-instar nymphs of C. lividipennis that preyed on eggs or nymphs of N. lugens fed on T2A-1 or Minghui 63 were collected. Four or five samples were collected for each treatment. Before the assay, the insect samples were washed four times with PBST (PBS/0.55% Tween-20) to remove any Cry protein from their outer surfaces. The Cry protein contents were determined using AP005 ENVIRONLOGIX kits (ENVIRONLOGIX, USA). The kits were used according to the manufacturer's instructions.
Exposure of C. lividipennis to Cry2Aa at high dose
Lyophilized Cry2Aa protein was purchased from the Biochemistry Department Laboratory, School of Medicine, Case Western Reserve University, USA. The Cry2Aa protoxin was expressed by Escherichia coli, then the protoxin inclusion bodies were solubilized and trypsinized, subsequently the toxins were purified and lyophilized. The purity of toxins is about 95–98%, and the molecular size of activated toxin is 65 kDa. Potassium arsenate (PA, KH2AsO4), which has been previously reported as toxic to insects [24], [28], was used as a toxic model compound and was purchased from Sigma-Aldrich (St. Louis, MO).
The results of the preliminary experiments showed that the chemically defined diet for N. lugens as described by Fu et al. [29] was sufficient for sustaining the growth and development of C. lividipennis from the second instar to adulthood (The survival of C. lividipennis from the first instar to adulthood was low). The artificial diet for N. lugens was therefore used as the medium to deliver the Cry2Aa protein to the gut of C. lividipennis. Second-instar nymphs of C. lividipennis were reared on one of three different diets: i) an artificial diet (negative control); ii) an artificial diet containing 300 µg/ml of Cry2Aa (a level over ten times higher than that to which C. lividipennis would realistically be exposed in rice fields); iii) an artificial diet containing 40 µg/ml of PA (positive control). The diets were refreshed daily. The molting and survival of C. lividipennis were observed daily. When the adults emerged, their genders and body weights were recorded. Thirty-six nymphs of C. lividipennis were evaluated for each treatment.
To ensure the stability of the Cry2Aa protein in the artificial diets before and after 24 h of feeding exposure, the Cry2Aa proteins were extracted from the artificial diets, and the concentrations of Cry2Aa protein in the diet were determined using AP005 ENVIRONLOGIX kits.
To examine the bioactivity of this batch of Cry2Aa on lepidopteran insects, Cry2Aa was mixed with an artificial diet for P. interpunctella. Each bioassay included five concentrations for Cry2Aa (2, 10, 20, 30, 40 µg/g) plus a control. Forty newly hatched P. interpunctella larvae javascript:void(0); were introduced into the artificial diets of each concentration and five replicates were tested. Mortality of the larvae was determined after 1 week. The LC50 (concentration resulting in 50% P. interpunctella larval mortality) of this batch of Cry2A protein was measured.
To examine the bioactivity of Cry2Aa protein in the artificial diets before and after 24 h of feeding exposure, the artificial diets containing Cry2Aa were appropriately diluted and sprinkled on the leaves of corn. After 2 h of air-drying, 15 Bt-susceptible second-instar larvae of C. medinalis were distributed onto the leaves for each treatment. Four replicates were tested in each treatment. Mortality of the insects were recorded 48 h later.
Effects of transgenic cry2Aa rice on the functional response of female C. lividipennis
C. lividipennis individuals were reared on T2A-1 or Minghui 63 rice plants infested with N. lugens eggs for one generation. Female adults of C. lividipennis (2 days after eclosion) were starved for 24 h, and each female was then transferred to one glass tube (2 cm diameter×12 cm length) containing three or four 15-day-old rice seedlings infested with first-instar nymphs of N. lugens. The experimental densities of N. lugens were 10, 20, 30, 40 and 50 nymphs per tube. The number of N. lugens consumed by C. lividipennis was recorded after 24 h. The experiment was repeated five times for each density.
Field experiment design
The experiments were conducted during the growing seasons of 2011–2013 at the two different sites, where field trials of Bt rice were permitted, in Hubei Province, China. The first site located in the suburbs of Xiaogan City, and the second site located in the suburbs of Suizhou City. The layout of the plots in the field followed a completely randomized block design with four replications for each rice line. Each experimental plot was 150 m2 (10 m×15 m) and was surrounded by a 1 m wide unplanted border. The entire experimental field was bordered by five rows of the nontransgenic control plants. The Bt and non-Bt control rice lines were sown in early May and transplanted 1 month after sowing. The seedlings were manually transplanted with one seedling per pot, 13.3 cm between plants within a row, and 29.9 cm between rows. The agronomic practices used for growing the rice, including fertilization and irrigation, were consistent with those followed by local farmers, except that no insecticides were applied during the whole growing season.
Field sampling with a vacuum-suction machine
Sampling of C. lividipennis was conducted as described by Xu et al. [30]. Arthropods at both field sites were collected using a vacuum-suction machine, constructed basing on a description by Carino et al. [31] and supplemented by a square sampling box (50 cm length×50 cm width×120 cm height) with a metal frame enclosed by Mylar film. Samples were collected every 10–15 days, starting 1 month after transplantation and continuing until the rice was ripe (as measured by grain maturity and harvest). On each sampling date, a square sampling box was placed at random along the diagonal line of each test plot at each site, with five subsamples per plot. The sample locations in each plot were marked with bamboo stakes to avoid resampling at the same location. Arthropods inside the frame enclosure were collected using the vacuum-suction machine for 5 min at each sampling location and were preserved in 75% ethanol. All samples were taken back to the laboratory and identified to the species level.
Data analysis
ELISA data and body weights were compared using the Student's t-tests. The Chi-square test was used for the parameters of preimaginal survival and female ratio. Nymphal developmental time was analyzed using Mann–Whitney U-tests, as the data did not fulfill the assumptions required for parametric analyses (normal distribution of residues and homogeneity of error variances). Survival response to the artificial diets containing Cry2Aa was analyzed using the Kaplan-Meier procedure, and the log-rank test was used in the purified toxin experiment.
In the field experiment, the population density and population dynamics determined by vacuum-suction were used to evaluate the impacts of the transgenic Bt rice on C. lividipennis populations in the fields. The population density of C. lividipennis was represented by seasonal means as captured by vacuum-suction. The population dynamics of the predators were measured by the means at each sampling date. Population density and population dynamics were analyzed using the Student's t-test.
The data from the functional response experiment were fitted to Holling's “Type II” disc equation, which estimates Na as follows: Na = aTN/(1 + aThN), where Na is the number of prey attacked, N is prey density, and T is the duration of the experiment (T = 1 day in the present study). The parameters a (instantaneous search rate) and Th (time required to handle a prey item) were calculated via least-squares nonlinear regression based on the Gauss-Newton method.
The percentage data were arcsine–square root transformed, and all count data were square root (x+1) or log 10 (x+1) transformed before being subjected to data analysis. The untransformed means are presented in the results. All statistical analyses were performed using the software package SPSS (version 16.0 for Windows, 2007).
Results
Prey-mediated effects of transgenic cry2Aa rice on the life-table parameters of C. lividipennis
No significant differences were observed between the developmental time, preimaginal survival, female ratio and fresh body weight of C. lividipennis adults reared with eggs or nymphs of N. lugens fed on Bt and non-Bt rice (P>0.05) (Tables 1, 2).
Table 1. Prey-mediated effects of Cry2Aa on life-table parameters of Cyrtorhinus lividipennis preying eggs of Nilapavarta lugens reared with T2A-1 or Minghui 63 rice plants.
| Parameters | T2A-1 | Minghui 63 | Statistics |
| 1st instar developmental time (days ± SE)a | 2.3±0.09 (32) | 2.5±0.11 (32) | U = 415, P = 0.117 |
| 2nd instar developmental time (days ± SE)a | 1.7±0.09 (30) | 1.6±0.10 (30) | U = 400.5, P = 0.387 |
| 3rd instar developmental time (days ± SE)a | 1.5±0.09 (29) | 1.5±0.10 (30) | U = 435, P = 1.000 |
| 4th instar developmental time (days ± SE)a | 1.8±0.09 (29) | 1.8±0.07 (30) | U = 400, P = 0.473 |
| 5th instar developmental time (days ± SE)a | 2.7±0.09 (29) | 2.9±0.14 (29) | U = 364, P = 0.399 |
| Whole nymphal stage developmental time (days ± SE)a | 10.0±0.13 (28) | 10.3±0.13 (29) | U = 313, P = 0.080 |
| Preimaginal survival (%)b | 87.5 | 90.6 | χ 2 = 0.002, P = 0.967 |
| Female ratio (%)b | 42.9 | 55.2 | χ 2 = 0.864, P = 0.352 |
| Male weight (mg ± SE)c | 0.55±0.03 | 0.49±0.03 | t = −1.492, P = 0.179 |
| Female weight (mg ± SE)c | 0.93±0.02 | 0.92±0.04 | t = −0.193, P = 0.851 |
The experiment started with 32 nymphs per treatment. (n), number of individuals at each development stage.
Mann–Whitney U-test.
Chi-square test.
Student's t-test.
Table 2. Prey-mediated effects of Cry2Aa on life-table parameters of Cyrtorhinus lividipennis preying nymphs of Nilapavarta lugens reared with T2A-1 or Minghui 63 rice plants.
| Parameters | T2A-1 | Minghui 63 | Statistics |
| 2nd instar developmental time (days ± SE)a | 1.9±0.08 (76) | 1.8±0.06 (77) | U = 2.538E3*, P = 0.102 |
| 3rd instar developmental time (days ± SE)a | 1.8±0.07 (63) | 2.0±0.08 (70) | U = 1.875E3*, P = 0.087 |
| 4th instar developmental time (days ± SE)a | 2.4±0.09 (50) | 2.4±0.13 (48) | U = 1.180E3*, P = 0.873 |
| 5th instar developmental time (days ± SE)a | 2.8±0.17 (29) | 2.7±0.15 (27) | U = 367.5, P = 0.654 |
| 2nd instar-adult developmental time (days ± SE)a | 8.8±0.21 (28) | 9.2±0.20 (26) | U = 292, P = 0.190 |
| Preimaginal survival (%)b | 35.0 | 32.5 | χ 2 = 0.112, P = 0.738 |
| Female ratio (%)b | 42.9 | 38.5 | χ 2 = 0.108, P = 0.743 |
| Male weight (mg ± SE)c | 0.33±0.05 | 0.30±0.01 | t = −0.420, P = 0.696 |
| Female weight (mg ± SE)c | 0.46±0.02 | 0.43±0.09 | t = −0.322, P = 0.764 |
The experiment started with 80 nymphs per treatment. (n), number of individuals at each development stage.
*E3 = 10−3.
Mann–Whitney U-test.
Chi-square test.
Student's t-test.
The Cry2Aa content in T2A-1 rice sheaths was 13.9±1.2 µg/g. The content of Cry2Aa in nymphs of N. lugens fed on T2A-1 was 5.3±0.8 ng/g, only 0.04% of that in the rice sheaths. The content of Cry2Aa in the eggs of N. lugens fed on T2A-1 was undetectable (Table 3).
Table 3. Contents of Cry2Aa protein in rice sheath tissue, Nilapavarta lugens and Cyrtorhinus lividipennis.
| Treatments | T2A-1 | Minghui 63 |
| Sheath of rice plants | 13.9±1.2 µg/g | Not detectable |
| Eggs of N.lugens | Not detectable | Not detectable |
| Nymphs of N. lugens | 5.3±0.8 ng/g | Not detectable |
| C. lividipennis provided with N. lugens eggs with rice plants | 200.2±33.2 ng/g | Not detectable |
| C. lividipennis provided with N. lugens nymphs with rice plants | 48.5±13.0 ng/g | Not detectable |
| C. lividipennis provided with N. lugens eggs without rice plants | Not detectable | Not detectable |
| C. lividipennis provided with N. lugens nymphs without rice plants | Not detectable | Not detectable |
| C. lividipennis provided with rice plants | 17.1±8.6 ng/g | Not detectable |
Data are represented as mean ± SE.
When N. lugens nymphs and T2A-1 seedlings were provided simultaneously to C. lividipennis, Cry2Aa was detectable, and the concentration of Cry2Aa in C. lividipennis was 48.5±13.0 ng/g; this value was much lower than that in the rice sheaths (13.9±1.2 µg/g) but higher than that in the nymphs of N. lugens (5.3±0.8 ng/g). However, when the N. lugens nymphs fed on T2A-1 were removed from T2A-1 and the nymphs alone were provided to C. lividipennis, no Cry2Aa was detected in the predator. When N. lugens eggs and T2A-1 seedlings were provided to C. lividipennis simultaneously, Cry2Aa was detectable, and the concentration of Cry2Aa in C. lividipennis was 200.2±33.2 ng/g. However, when the N. lugens eggs were removed from the T2A-1 seedlings and the eggs alone were provided to C. lividipennis, Cry2Aa was not detectable in the predator. As was expected, no Cry2Aa protein was detected in the Minghui 63 rice plants (Table 3).
Purified Cry2Aa protein bioassay
Before and after exposure to C. lividipennis for 24 h, the concentrations of Cry2Aa in the artificial diets were 98.4±3.2 µg/g and 91.1±3.7 µg/g, respectively. After 24 h of exposure, the concentration of Cry2Aa in the diets decreased by 7.42%, and no statistical differences were detected between the values before and after exposure (Student's t-test, P = 0.185). Therefore, it could be concluded that the Cry2Aa protein in the artificial diets was stable.
The LC50 of this batch of Cry2Aa for P. interpunctella larvae was 14.9 µg/g fresh weight. Before and after exposure to C. lividipennis for 24 h, the artificial diets containing 300 µg/ml Cry2Aa resulted in 90% and 80% mortality of C. medinalis larvae, respectively; these mortalities were significantly higher than those that occurred in larvae fed with the pure artificial diet (13%). These results indicated that the Cry2Aa protein in the artificial diets for C. lividipennis was bioactive.
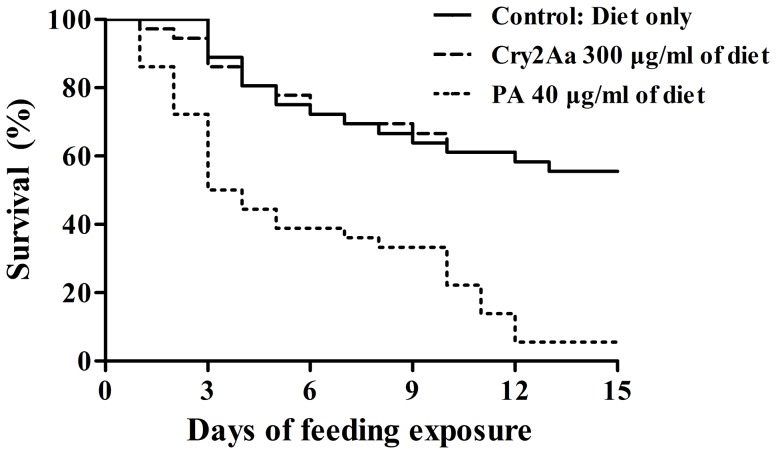
Survival of the C. lividipennis fed with the artificial diet containing 40 µg/ml PA was significantly decreased compared to that of the C. lividipennis fed with the pure artificial diet (P<0.001) (Fig. 1). This result indicated that the test system employed in the present study could detect the dietary effects of insecticidal compounds. High-dosage exposure to the Cry2Aa protein had no adverse effects on the survival response of C. lividipennis in comparison to the pure artificial diet (negative control) (Fig. 1). Similarly, the developmental time from the second instar to adulthood, preimaginal survival and body weight of C. lividipennis were unaffected by the Cry2Aa protein in comparison to the negative control (Table 4).
Figure 1. Survival of Cyrtorhinus lividipennis fed pure artificial diet or diet containing different insecticidal compounds.
300 µg Cry2Aa and 40 µg PA per ml were incorporated into artificial diets. Pure diet served as a negative control (N = 36).
Table 4. Effects of purified Cry2Aa incorporating into artificial diet on life-table parameters of Cyrtorhinus lividipennis.
| Parameters | Treatments | ||
| Control | 300 µg/ml Cry2Aa | 40 µg/ml PA | |
| 2nd instar developmental time (days ± SE)a | 2.1±0.15 (35) | 2.3±0.13 (35) | 2.4±0.21 (23) |
| 3rd instar developmental time (days ± SE)a | 2.5±0.10 (28) | 2.6±0.13(29) | 4.2±0.36 (13)** |
| 4th instar developmental time (days ± SE)a | 3.1±0.17 (21) | 3.1±0.24 (22) | 7.0±0.58 (3)** |
| 5th instar developmental time (days ± SE)a | 4.2±0.18 (20) | 4.1±0.20 (20) | — |
| 2nd instar-adult developmental time (days ± SE)a | 11.8±0.40 (20) | 11.7±0.40 (20) | — |
| Preimaginal survival (%)b | 55.6 | 55.6 | 0.0 |
| Male weight (mg ± SE)c | 0.41±0.02 | 0.40±0.02 | — |
| Female weight (mg ± SE)c | 0.58±0.02 | 0.54±0.02 | — |
Nymphs of C. lividipennis were fed with an artificial diet containing 300 µg/ml Cry2Aa or 40 µg/ml PA (positive control). Pure diet served as a negative control (N = 36). The experiment lasted until adult eclosed. Statistical comparisons were made separately for each of the insecticidal compounds comparing with the control. Asterisks denote significant differences: P<0.01.
Mann–Whitney U-test with Bonferroni correction (adjusted α = 0.025).
Chi-square test with Bonferroni correction (adjusted α = 0.025).
Student's t-test.
Effects of Cry2Aa on the functional response of C. lividipennis
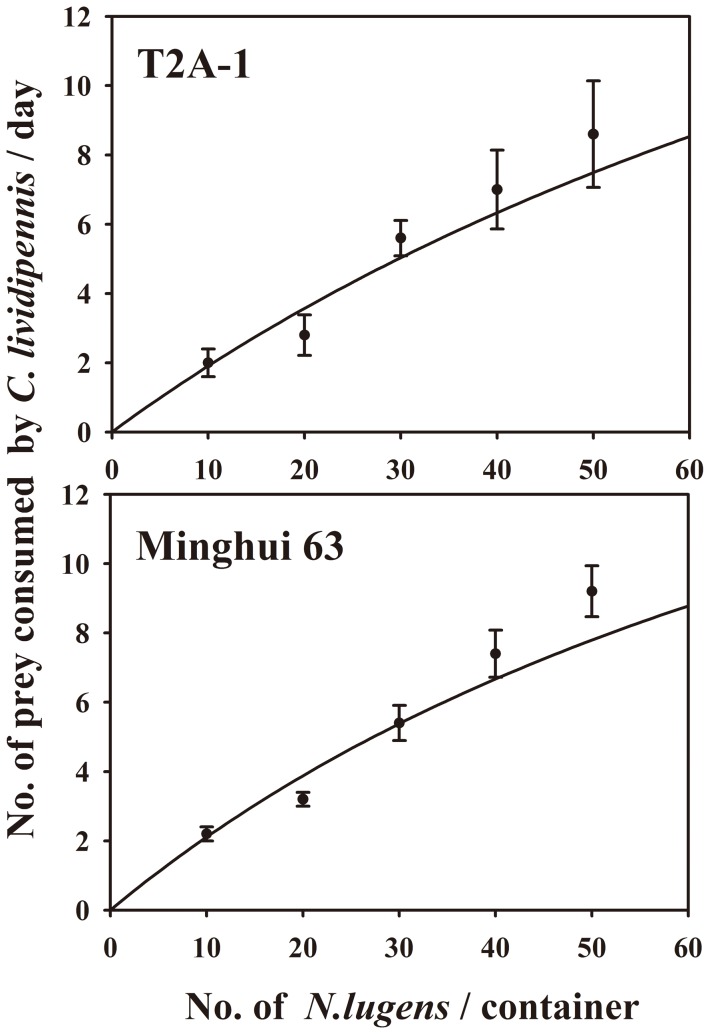
The results indicated that the functional response of C. lividipennis to N. lugens on both rice lines was typically Type II as described by Holling [32], [33] (Fig. 2). The instantaneous search rate and handling time were not significantly affected by rice line (P>0.05) (Table 5).
Figure 2. Functional response of Cyrtorhinus lividipennis collected from T2A-1 and Minghui 63.
Table 5. Parameters of Type II functional response of Cyrtorhinus lividipennis to Nilapavarta lugens nymph fed on Bt or non-Bt rice.
| Rice materials | a | Th | R2 (%) |
| T2A-1 | 0.251±0.028 | 0.036±0.008 | 95.83 |
| Minghui 63 | 0.231±0.025 | 0.037±0.007 | 95.20 |
a: instantaneous search rate (day−1). Th: time required to handle a prey (day). Data are represented as mean ± SE.
There was no significant difference between T2A-1 and Minghui 63, based on Student's t-test (P<0.05).
Effects of transgenic cry2Aa rice on the population density and dynamics of C. lividipennis
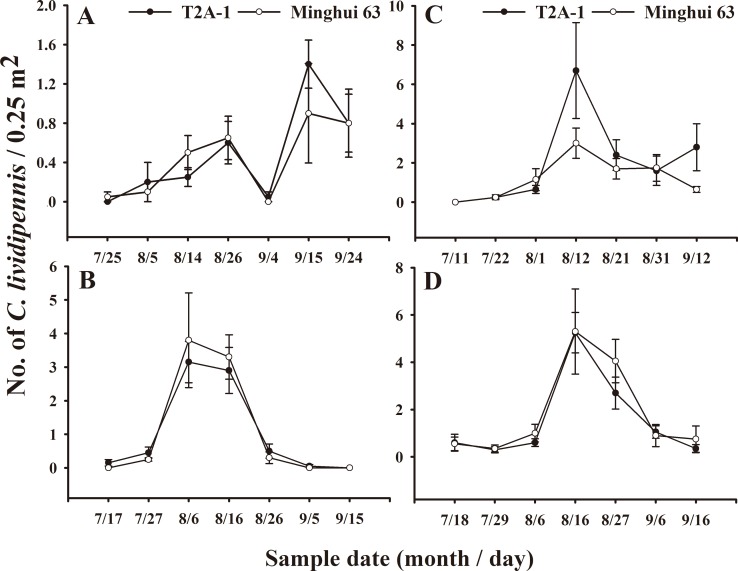
The population density of C. lividipennis is shown in Table 6. Compared with that on the nontransgenic Minghui 63, the population density of C. lividipennis was not significantly influenced by transgenic cry2Aa rice at any site or in any year (Student's t-test, P>0.05) (Table 6). The population dynamics (means of each sampling date) of C. lividipennis are shown in Fig. 3. No significant differences in the population dynamics of C. lividipennis were observed between Minghui 63 and transgenic cry2Aa rice fields at any sampling date, at any site or in any year (Student's t-test, all P>0.05). Repeated measures ANOVA analysis showed that the population dynamics were unaffected by rice line (P>0.05).
Table 6. Population densities (no. of per 0.25 m2) of Cyrtorhinus lividipennis collected by vacuum-suction.
| Rice materials | Xiaogan | Suizhou | ||
| 2011 | 2012 | 2013 | 2012 | |
| T2A-1 | 0.47±0.10 | 1.03±0.09 | 2.06±0.68 | 1.55±0.06 |
| Minghui 63 | 0.43±0.14 | 1.09±0.20 | 1.21±0.23 | 1.84±0.29 |
N = 4 at both sites in 2011, 2012, and 2013. Data are represented as mean ± SE. There was no significant difference between T2A-1 and Minghui 63 field, based on Student's t-test.
Figure 3. Population dynamics of Cyrtorhinus lividipennis collected by vacuum-suction.
Data are represented as mean ± SE. (A). Xiaogan, 2011; (B). Xiaogan, 2012; (C). Xiaogan, 2013; (D) Suizhou, 2012. There was no significant difference between Bt rice and control plots at the same sampling time, based on Student's t-test (N = 4). Repeated measures ANOVA: (A). F1,6 = 0.059, P = 0.816; (B). F1,6 = 0.058, P = 0.777; (C). F1,6 = 1.366, P = 0.287; (D). F1,6 = 0.964, P = 0.364.
Discussion
The ecological risk assessment of an insect-resistant transgenic crop for a nontarget arthropod (NTA) should be conducted within a tiered scheme: (i) effects on the NTA at elevated doses in a replicated controlled system; (ii) effects on the NTA at realistic doses in a replicated controlled system; (iii) effects on the population of the NTA at realistic doses in a realistic agricultural system [34]. According to these criteria, three experiments were conducted to evaluate the ecological risk of transgenic cry2Aa rice to C. lividipennis, a primary predator of N. lugens that is the main NTA of transgenic Bt rice in the present study: (1) a direct feeding experiment, in which C. lividipennis was fed an artificial diet containing Cry2Aa at the dose of 10-time higher than that it may encounter in the realistic field condition; (2) a tritrophic experiment, in which the Cry2Aa protein was delivered to C. lividipennis indirectly through prey eggs or nymphs; (3) a realistic field experiment, in which the population dynamics of C. lividipennis were investigated by vacuum-suction. Direct exposure to elevated doses of the Cry2Aa protein and prey-mediated exposure to realistic doses of the Cry2Aa protein did not result in significant detrimental effects on the developmental time, preimaginal survival, female ratio and body weight of C. lividipennis. No significant differences in population density and population dynamics were observed between C. lividipennis populations in transgenic cry2Aa and nontransgenic rice fields. It could be concluded that transgenic cry2Aa rice had no adverse effects on C. lividipennis. This study represents the first report of an assessment continuum for the effects of transgenic cry2Aa rice on C. lividipennis.
Planthoppers are the main nontarget herbivores in transgenic Bt rice fields. Determining whether the Bt protein may be transmitted to predators via planthoppers is important for the ecological risk assessment of transgenic Bt rice. Several reports have examined Bt protein transmission to predators via predation on planthoppers infesting transgenic Bt rice, but their results were inconclusive. Cry1Ab could be transferred to P. subpiraticus by predation on N. lugens fed on transgenic cry1Ab rice, and the content of Cry1Ab in P. subpiraticus was significantly higher than that in N. lugens fed on transgenic cry1Ab rice [19]. Cry1Ab could also be transferred to U. insecticeps via predation on N. lugens fed on transgenic cry1Ab rice, and the concentration of Cry1Ab in U. insecticeps was significantly lower than that in N. lugens [21]. However, no Cry2Aa was detected in a study of the larvae of a general predator (C. sinica) of planthoppers, in which C. sinica was provided with Laodelphax striatellus fed on transgenic cry2Aa rice [24].
In the present study, when N. lugens nymphs and T2A-1 seedlings were provided simultaneously to C. lividipennis, Cry2Aa was detected in the predator. However, when the N. lugens nymphs fed on T2A-1 were removed from the rice and provided alone to C. lividipennis, no Cry2Aa was detected in the predator. Similarly, when N. lugens eggs and T2A-1 seedlings were provided to C. lividipennis simultaneously, Cry2Aa was detected in the predator. However, when the N. lugens eggs were removed from the T2A-1 seedlings and provided alone to C. lividipennis, no Cry2Aa was detected in the predator. Therefore, it may be inferred that Cry2Aa was not transmitted to C. lividipennis via predation on the eggs and nymphs of N. lugens; the protein may instead be transferred by the piercing-sucking foraging behavior of C. lividipennis on rice. This hypothesis was verified through the results of our supplementary experiments: when C. lividipennis nymphs were provided with T2A-1 seedlings alone for 1 day, Cry2Aa was detected in the predator, and the concentration of Cry2Aa in C. lividipennis was 17.1±8.6 ng/g (Table 3). Therefore, C. lividipennis may serve as a good indicator species in ecological risk assessments of transgenic Bt tice.
In the ecological risk assessment of an arthropod-resistant genetically engineered crop, researchers normally use “Tier-1 assays” as the initial step to determine the toxicity of the insecticidal compounds expressed by the transgenic crop on NTAs. In Tier-1 tests, insecticidal compounds are added to artificial diets for the tested NTAs, and the tested organisms are directly exposed to doses of the insecticidal compounds several times higher than those realistically present in the field. Tier-1 tests increase the likelihood that a hazard will be detected if the hazard exists, and therefore provide confidence that minimal risk is present if no adverse effect is detected [34], [35]. Three important factors must be considered in Tier-1 assays: (i) the methods for the delivery of the insecticidal proteins to the test organisms; (ii) the need for and selection of the compounds used as positive controls; and (iii) the methods for monitoring the concentration, stability and bioactivity of the insecticidal proteins during the assay [35]. In the present study, a dietary exposure experiment was conducted in which purified Cry2Aa protein was directly fed to C. lividipennis nymphs through its incorporation into a previously described artificial diet for N. lugens [29]. This artificial diet was sufficient for sustaining the growth and development of C. lividipennis from the second instar to adulthood. Before and after exposure to C. lividipennis for 24 h, the Cry2Aa protein in the artificial diets was stable and bioactive. The oral poison PA was used as a positive control to validate our dietary exposure assay. The survival of the C. lividipennis fed with the artificial diet containing 40 µg/ml PA significantly decreased compared to that of the C. lividipennis fed with the pure artificial diet (P<0.001) (Fig. 1). This result confirmed that the test system employed in the current study was able to detect the dietary effects of insecticidal compounds. No detrimental effects on the life-table parameters of C. lividipennis were observed when the insects were provided with an artificial diet containing Cry2Aa at a concentration that was nearly ten times higher than that measured in the rice sheaths. This study represents the first report of the use of a Tier-1 system to evaluate the potential effects of Cry2Aa on C. lividipennis.
The survival rate of C. lividipennis preying the nymphs of N. lugens was 32–35%, which was much lower than that of C. lividipennis preying eggs of N. lugens (87–91%) in the tritrophic experiment. So did body weight of adults of C. lividipennis. Similar results have been reported by Chua and Mikil and Chen et al., and they concluded that N. lugens eggs was an essential food type for C. lividipennis, and N. lugens nymphs was not an ideal food for the predator [36], [37]. The different nutrient composition of prey may cause biological parameters difference of predator. Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) is a polyphagous species, the complete development of this predator can be accomplished using the aphid Acyrthosiphon pisum (Harris) (Homoptera: Aphididae) or Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae) eggs as substitution prey. Biochemical analyses indicated that amino acids and lipids of E. kuehniella eggs were richer than A. pisum adults, but, on the contrary, the glycogen of aphids was richer than E. kuehniella eggs. Some biological parameters such as larval mortality, adult weight, and fecundity, were modified according to the food eaten [38]. Whether the different survival of C. lividipennis in the present study is caused by different nutrient composition of prey needs to be further explored.
Conclusions
In summary, this comprehensive study, involving a Tier-1 examination system, a tritrophic bioassay, functional response experiments in the laboratory and population dynamics determinations in the field, provides the most complete information to date on the impacts of Bt rice expressing Cry2Aa on C. lividipennis, a major predator in rice ecosystems. These results indicate that C. lividipennis is not sensitive to the Cry2Aa protein and that Bt rice (T2A-1) poses a negligible risk to this nontarget organism.
Acknowledgments
We thank Prof. Yongjun Lin (National Key Laboratory of Crop Genetic Improvement at Huazhong Agricultural University) for providing the transgenic rice seeds.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the National Genetically Modified Organisms Breeding Major Project: Technology of Environmental Risk Assessment on Transgenic Rice (2014ZX08011-001). HH received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zeigler RS, Barclay A (2008) The relevance of rice. Rice 1: 3–10. [Google Scholar]
- 2.Cheng JA, He J (1996) Rice insect pests. China A gricultural Press, Beijing.
- 3. Kiritani K (1979) Pest management in rice. Annu Rev Entomol 24: 279–312. [Google Scholar]
- 4.Oerke EC, Dehne HW, Schönbeck F, Weber A (1994) Crop production and crop protection: estimated losses in major food and cash crops. Amsterdam: Elsevier.
- 5.Pathak MD, Khan ZR (1994) Insect Pests of Rice. Los Baños, Laguna, Philippines: International Rice Research Institute.
- 6. Sheng CF, Wang HT, Gao LD, Xuan JW (2003) The occurrence status, damage cost estimate and control strategies of stem borers in China. Plant Prot 29: 37–39 (in Chinese with English summary) [Google Scholar]
- 7. Lou YG, Zhang GR, Zhang WQ, Hu Y, Zhang J (2013) Biological control of rice insect pests in China. Biol Control 67: 8–20. [Google Scholar]
- 8. Matteson PC (2000) Insect pest management in tropical Asian irrigated rice. Annu Rev Entomol 45: 549–574. [DOI] [PubMed] [Google Scholar]
- 9. Tu J, Zhang G, Datta K, Xu C, He Y, et al. (2000) Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis-endotoxin. Nat Biotechnol 18: 1101–1104. [DOI] [PubMed] [Google Scholar]
- 10. Huang JK, Hu RF, Rozelle S, Pray C (2005) Insect-resistant GM rice in farmers' fields: assessing productivity and health effects in China. Science 308: 688–690. [DOI] [PubMed] [Google Scholar]
- 11. Shelton AM, Zhao JZ, Roush RT (2002) Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Ann Rev Entomol 47: 845–881. [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Tang W, Xu CG, Li XH, Lin YJ, et al. (2005) Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor Appl Genet 111: 1330–1337. [DOI] [PubMed] [Google Scholar]
- 13. Cheng X, Sardana R, Kaplan H, Altosaar I (1998) Agrobacterium-transformed rice plants expressing synthetic cry1A(b) and cry1A(c) genes are highly toxic to yellow stem borer and striped stem borer. Proc Natl Acad Sci U S A 95: 2767–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang W, Chen H, Xu CG, Li XH, Lin YJ, et al. (2006) Development of insect-resistant transgenic indica rice with a synthetic cry1C* gene. Mol Breeding 18: 1–10. [Google Scholar]
- 15.Heinrichs EA (1979) Control of leafhopper and planthopper vectors of rice viruses. In: Moramorosch K, Arris KF, Editors. Leafhopper vectors and planthopper disease agents. New York: Academic Press. pp. 529–558.
- 16.Sogawa K, Liu GJ, Shen JH (2003) A review on the hyper susceptibility of Chinese hybrid rice to insect pests. Chinese J Rice Sci 17, 23–30. (in Chinese with English summary).
- 17. Bai YY, Jiang MX, Cheng JA (2005) Effects of transgenic cry1Ab rice pollen on fitness of Propylea japonica (Thunberg). J Pest Sci 78: 123–128. [Google Scholar]
- 18. Bernal CC, Aguda RM, Cohen MB (2002) Effect of rice lines transformed with Bacillus thuringiensis toxin genes on the brown planthopper and its predator Cyrtorhinus lividipennis . Entomol Exp Appl 102: 21–28. [Google Scholar]
- 19. Chen M, Ye GY, Lu XM, Hu C, Peng YF, et al. (2005) Biotransfer and bioaccumulation of Cry1Ab insecticidal protein in rice plant-brown planthopper-wolf spider food chain. Acta Entomol Sinica 48: 208–213 (in Chinese with English summary) [Google Scholar]
- 20. Jiang YH, Fu Q, Cheng JA, Zhu ZR, Jiang MX, et al. (2004) Dynamics of Cry1Ab protein from transgenic Bt rice in herbivores and their predators. Acta Entomol Sinica 47: 454–460 (in Chinese with English summary) [Google Scholar]
- 21. Tian JC, Liu ZC, Chen M, Chen Y, Chen XX, et al. (2010) Laboratory and field assessments of prey-mediated effects of transgenic Bt rice on Ummeliata insecticeps (Araneida: Linyphiidae). Environ Entomol 39: 1369–1377. [DOI] [PubMed] [Google Scholar]
- 22. Tian JC, Chen Y, Li ZL, Li K, Chen M, et al. (2012) Transgenic Cry1Ab rice does not impact ecological fitness and predation of a generalist spider. PLoS ONE 7: e35164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen M, Liu ZC, Ye GY, Shen ZC, Hu C, et al. (2007) Impacts of transgenic cry1Ab rice on non-target planthoppers and their main predator Cyrtorhinus lividipennis (Hemiptera: Miridae)-A case study of the compatibility of Bt rice with biological control. Biol Control 42: 242–250. [Google Scholar]
- 24. Li YH, Wang YY, Romeis J, Liu QS, Lin KJ, et al. (2013) Bt rice expressing Cry2Aa does not cause direct detrimental effects on larvae of Chrysoperla sinica . Ecotoxicology 22: 1413–1421. [DOI] [PubMed] [Google Scholar]
- 25. Chen JM, Cheng JA, He JH (1992) Review on Cyrtorrhinus livdipennis Reuter. Entomol Knowledg 25: 370–373. [Google Scholar]
- 26. Sigsgaard L (2007) Early season natural control of the brown planthopper, Nilaparvata lugens: the contribution and interaction of two spider species and a predatory bug. Bull Entomol Res 97: 533–544. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physical studies of rice, third editon. Los Baños, Laguna, Philippines: International Rice Research Institute. pp. 61–65.
- 28. Duan JJ, Head G, McKee MJ, Nickson TE, Martin JW, et al. (2002) Evaluation of dietary effects of transgenic corn pollen expressing Cry3Bb1 protein on a non-target ladybird beetle, Coleomegilla maculata . Entomol Exp Appl 104: 271–280. [Google Scholar]
- 29. Fu Q, Zhang ZT, Hu C, Lai FX, Sun ZX (2001) A chemically defined diet enables continuous rearing of the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Appl Entomol Zool 36: 111–116. [Google Scholar]
- 30. Xu XL, Han Y, Wu G, Cai WL, Yuan BQ, et al. (2011) Field evaluation of effects of transgenic cry1Ab/cry1Ac, cry1C and cry2A rice on Cnaphalocrocis medinalis and its arthropod predators. Sci China Life Sci 54: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 31. Carino FO, Kenmore PE, Dyck VA (1979) The farmcop suction sampler for hoppers and predators in flooded rice fields. Int Rice Res News 4: 21–22. [Google Scholar]
- 32. Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91: 385–398. [Google Scholar]
- 33. Holling CS (1961) Principles of insect predation. Annu Rev Entomol 6: 163–182. [Google Scholar]
- 34. Romeis J, Bartsch D, Bigler F, Candolfi MP, Gielkens MMC, et al. (2008) Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat Biotechnol 26: 203–208. [DOI] [PubMed] [Google Scholar]
- 35.Li YH, Romeis J, Wu KM, Peng YF (2013) Tier-1 assays for assessing the toxicity of insecticidal proteins produced by genetically engineered plants to non-target arthropods. Insect Sci DOI: 10.1111/1744–7917.12044. Available: http://onlinelibrary.wiley.com/doi/10.1111/1744-7917.12044/abstract. Accessed 2013 Dec 6. [DOI] [PubMed]
- 36. Chen JM, Cheng JA, He JH (1994) Effects of temperature and food on the development, survival and reproduction of Cyrtorrhinus livdipennis (Reuter). Acta Entomol Sinica 37: 63–70 (in Chinese with English summary) [Google Scholar]
- 37. Chua TH, Mikil E (1989) Effects of prey number and stage on the biology of Cyrtorhinas lividipennis (Hemiptera: Miridae): a predator of Nilaparvata lagens (Homoptera: Delphacidae). Environ Entomol 18: 251–255. [Google Scholar]
- 38. Specty O, Febvay G, Grenier S, Delobel B, Piotte C, et al. (2003) Nutritional plasticity of the predatory ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae): comparison between natural and substitution prey. Arch Insect Biochem 52: 81–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.