Abstract
A substantial number of people who sustain a mild traumatic brain injury report persistent symptoms. Most common among these symptoms are headache, dizziness, and cognitive difficulties. One possible contributor to sustained symptoms may be compromised cerebrovascular regulation. In addition to injury-related cerebrovascular dysfunction, it is possible that prolonged rest after mild traumatic brain injury leads to deconditioning that may induce physiologic changes in cerebral blood flow control that contributes to persistent symptoms in some people. There is some evidence that exercise training may reduce symptoms perhaps because it engages an array of cerebrovascular regulatory mechanisms. Unfortunately, there is very little work on the degree of impairment in cerebrovascular control that may exist in patients with mild traumatic brain injury, and there are no published studies on the subacute phase of recovery from this injury. This review aims to integrate the current knowledge of cerebrovascular mechanisms that might underlie persistent symptoms and seeks to synthesize these data in the context of exploring aerobic exercise as a feasible intervention to treat the underlying pathophysiology.
Each year in the United States, as many as 3.8 million individuals sustain a mild traumatic brain injury (mTBI) in sports alone.1 Furthermore, at least 10% of Iraq/Afghanistan veterans have sustained one or more mTBIs during their military career, and more than a third of them report persistent symptoms.2 When symptoms persist beyond a month or are present chronically, the cause of these symptoms is likely multifactorial—and comorbidities such as chronic pain, depression, traumatic stress, anxiety, substance abuse, and life stress can mimic or exacerbate these symptoms. It is possible that cerebrovascular dysregulation might underlie initial, subacute, and/or chronic symptoms after mTBI. Moreover, dysregulation could result from the injury itself, or from subsequent deconditioning due to bed rest, or both. Unfortunately, there has been very little research on the degree of impairment in cerebrovascular control that may exist in patients with mTBI, especially in the subacute phase of recovery. This review aims to integrate the current knowledge of cerebrovascular mechanisms that might underlie postacute and chronic symptoms in mTBI and seeks to synthesize these data in the context of exploring aerobic exercise as a feasible intervention to treat the underlying pathophysiology.
Overview.
No organ in the body is as dependent as the brain on a steady supply of blood. However, the evolution of the skull to house the brain coupled with 2-legged motion has put fairly complex constraints on the control of cerebral blood flow, and the brain seems to lack the survival advantage of other organs, such as the liver or kidney, that are more tolerant to fluctuations in blood flow. Compensatory mechanisms, however, offset this limited autonomy, ensuring that brain perfusion is well-controlled. These include neurovascular coupling to increase flow in response to increased neuronal activity and metabolic demand, cerebral vasoreactivity to alter flow with changes in carbon dioxide (CO2) levels, and cerebral autoregulation to maintain constant flow despite changing perfusion pressure. Insults to any of these mechanisms may result in impaired cerebrovascular regulation, and might underlie some of the pathophysiologic heterogeneity associated with TBI.
There is some evidence that aerobic exercise training could reduce persistent symptoms by engaging the array of mechanisms for cerebrovascular control. For example, sustained muscle engagement during exercise leads to cortical activation in motor and sensorimotor areas, increasing cerebral metabolism, and thus engaging neurovascular coupling3 to increase flow. Increased CO2 production attendant to aerobic exercise is accompanied by greater cerebral vasoreactivity4 to regulate flow in response to hyper- and hypocapnia. In addition, the increases in systemic pressure with even low-intensity exercise must be counterregulated by effective cerebral autoregulation5 to constrain flow and prevent overperfusion. Thus, regular exercise and engagement of these mechanisms might result in a “training effect” on cerebrovascular regulation. Moreover, it is known that detraining (i.e., prolonged inactivity) results in significant deficits in cerebrovascular control,6 and emerging evidence suggests that exercise training reduces symptoms in those with mTBI.7,8 Thus, there may be beneficial adaptations to exercise training that contribute to symptom reduction via improved cerebrovascular regulation.
Neurotrauma and cerebrovascular control.
Symptoms similar to those associated with mTBI (altered cognitive function, headache, and dizziness) can arise as a result of cerebrovascular dysfunction. First, studies suggest that alterations in neurovascular coupling may relate to declines in cognitive function. Retired boxers can show evidence of cerebral hypoperfusion coupled with neurocognitive dysfunction.9 Second, individuals with migraine demonstrate excessive increases and decreases in cerebral blood flow in response to both hyper- and hypocapnia that might be associated with the development and/or persistence of headaches.10 Lastly, those with vasovagal syncope, frequently accompanied by dizziness, can demonstrate rapid changes in cerebrovascular autoregulation prodromal to frank syncope, such that autoregulation is virtually lost immediately preceding, during, and after syncope.11 In fact, there is an association between the presence of syncope, risk of fainting, and impaired autoregulation,12 and thus, impaired cerebrovascular autoregulation may be an important factor underlying dizziness. Although the mechanisms that underlie these associations are not well understood, taken together, available data suggest that dysregulation of neurovascular coupling, cerebral vasoreactivity, and cerebral autoregulation could contribute to some of the chronic symptoms of mTBI, specifically altered cognitive function, headache, and dizziness.
Neurovascular coupling.
Distribution of cerebral flow is regulated in response to the functional activity in different brain regions. That is, when activity in a brain region increases, flow to that region also increases. Evidence indicates that glia, neurons, as well as blood vessels act as an integrated unit and have a crucial role in this process. The term neurovascular unit was coined to highlight the intimate functional relationships between these cells and their coordinated pattern of reaction to injury. Moreover, because neuronal activity requires delivery of adequate oxygen and glucose to specific brain regions, cerebral blood flow and cerebral metabolic rate are normally coupled. Alterations of this “neurovascular coupling” can impair the ability of the brain to provide sufficient flow to active regions, leading to neural dysfunction. Neurovascular coupling could derive from several mechanisms. Activity-related ion content shifts, energy substrate changes, or neurotransmitters themselves can influence vasomotor tone.13 Interneurons may also mediate flow coupling via endings directly on arterioles and by secreting acetylcholine, an endothelial-dependent vasodilator.14 Alternatively, astrocytes directly contact endothelial cells and can secrete vasodilatory substances, such as epoxyeicosatrienoic acid, adenosine, nitric oxide (NO), and cyclooxygenase-2 metabolites.15 Although the exact interplay among these potential mechanisms is unclear, vasodilatory effects in the microcirculation are insufficient to effectively increase local blood flow. The vasodilatory signal must be back-propagated to upstream pial arterioles that offer the greatest resistance to flow. The signal appears to be transmitted through gap junctions of neighboring endothelial or smooth vascular muscle cells. The increase in the arterial flow might also induce further dilation as a result of increased shear stress. Hence, vascular endothelial function and smooth muscle responsiveness appear to be critical in transducing the signals into cerebral flow changes orchestrated to the period of neural activation.
Data on alterations in neurovascular coupling after mTBI are limited. Animal data suggest that after moderate to severe TBI, local cerebral blood flow decreases, and as a result of these focal impairments, neurovascular “uncoupling” occurs.16 These alterations are thought to be primarily due to alterations in neural control17 and endothelial function18 in the pial vasculature. Unfortunately, there are no comparable data in humans. One study showed that central acetylcholinesterase inhibitors (which reduce the clearance of acetylcholine, thereby increasing endothelium-dependent acetylcholine availability) may be a promising treatment to improve vigilance and attention after moderate to severe TBI in humans.19 If so, endothelial dysfunction may underlie impairment in neurovascular coupling after mTBI in humans. However, this link has not been explored.
Cerebral vasoreactivity.
Cerebral blood flow is also highly sensitive to changes in arterial CO2 level. Cerebrovascular responses to changes in CO2 are primarily mediated via changes in extracellular pH and subsequent activation of ion channels in the vascular smooth muscle. This is a key mechanism for cerebrovascular control because arterial CO2 can fluctuate widely from one breath to the next and can change significantly with everyday stressors, such as moving from supine to upright postures. Hypercapnia (i.e., high CO2) leads to vasodilation and increases in flow, whereas hypocapnia (i.e., low CO2) leads to vasoconstriction and decreases in flow. The highly sensitive flow responses to changes in CO2, termed cerebral vasoreactivity, is a vital homeostatic function that helps regulate and maintain central pH. In essence, elevations in flow with hypercapnia “wash out” CO2 from brain tissue, thereby attenuating the rise in pH, whereas declines in flow with hypocapnia attenuate the fall in brain pH. This response is rapid, occurring with an approximate 6-second delay.20 Links between systemic endothelial function and cerebral vasoreactivity have been reported,21 indicating a common pathway between peripheral flow-mediated dilation and vasoreactivity. However, the mechanism of action for CO2-mediated blood flow changes has not been entirely elucidated. Potassium channel activation may have a role in coordinating vascular tone in upstream and downstream vessels via endothelial and vascular smooth muscle effects.22 An alternative or complementary mechanism is CO2/pH-induced alterations in vasoactive factors. Key among these factors is endothelial release of NO. The extent to which NO acts as obligatory or permissive is unknown, but it seems to be an essential vasoactive factor in the response to CO2.23 Thus, similar to neurovascular coupling, endothelial function as well as smooth muscle responsiveness may be key factors in cerebral vasoreactivity.
Impaired cerebrovascular response to CO2 has been shown to predict poor outcomes in patients with severe TBI.24 CO2 reactivity is compromised in the initial days postinjury but can return to normal.25 Of note, a disruption in cerebral vasoreactivity occurs in the days immediately after a mild cortical impact injury in animals,26 and this has also been observed shortly after sports-related concussion in humans.27 However, it remains unknown whether alterations in cerebral vasoreactivity also relate to the chronic symptoms after mTBI.
Cerebral autoregulation.
A third line of defense, cerebrovascular autoregulation, counteracts the effects of arterial pressure fluctuations occurring with everyday activities. For example, changes in posture can result in as much as a 50% drop in systolic pressure and produce vasovagal syncope with brief loss of consciousness if blood flow to reticular brain cells also rapidly falls. However, this will not occur if effective “autoregulation” results in maintained blood flow via cerebrovascular resistance changes that fully counteract changes in pressure. Cerebral arteries relax when pressure decreases and constrict when pressure increases to maintain stable cerebral perfusion. As with neurovascular coupling and cerebral vasoreactivity, the exact mechanisms are not entirely known, but certain important effectors have recently been demonstrated. Autonomic control appears to be a key mechanism for adequate autoregulation. Ganglionic blockade of cardiovascular autonomic control reduces the ability of the cerebral circulation to counterregulate pressure fluctuations.28 In fact, intact sympathetic function is critical to normal cerebrovascular responses to changes in pressure29 and cholinergic control provides a counterregulatory balance to sympathetic effects.30 In addition, data in humans suggest that myogenic mechanisms counteract pressure-driven flow31 and have an important role in regulation of pressure–flow relations.32 Recent work directly addressed how sympathetic, cholinergic, and myogenic systems work in concert to shape cerebral autoregulation in healthy humans, and showed that the 3 mechanisms have distinct contributions that explain the majority of cerebral autoregulatory responses.33 Lastly, an endothelium-dependent NO mechanism may have a role,34 although this is not consistently found. Nonetheless, it is likely that adequate function at the neural, smooth muscle, and endothelial levels is required for cerebral autoregulation.
Although severity of injury is not a good predictor of autoregulatory failure,35 compromised cerebral autoregulation is a significant predictor of poor outcomes in the acute phase of severe TBI.35 For example, Lam et al.36 identified 3 distinct TBI groups with intact, transient loss, or persistent loss of cerebral autoregulation; they found that 9 of 11 with persistent loss of autoregulation died and the remaining 2 had severe disability on follow-up. In contrast to the volume of work on severe TBI, there is very limited work on the degree of impairment in cerebrovascular control in patients with mild to moderate TBI, and no work at all on the subacute phase. Early after minor cerebral contusion, global cerebral flow may be reduced, and interhemispheric flow asymmetries appear to be common.37 In fact, almost 30% of patients with mTBI have impaired or absent cerebral autoregulation within 48 hours of injury.38 One case report suggests that very mild injuries may result in sustained loss of autoregulation39; a patient who experienced a mild concussion 6 days earlier demonstrated complete absence of cerebral autoregulation. Although data are suggestive of a sustained impairment in cerebrovascular control after mTBI, there have been no systematic studies exploring a possible compromise in control mechanisms underlying symptomatology.
Current approaches to acute management, treatment, and rehabilitation.
Currently, clinical management of mTBIs, particularly those sustained by athletes, consists mainly of physical and cognitive rest during the acute phase.40–42 A primary rationale for rest, especially in the first few days after injury, is that the injured brain is believed to be in a state of neurometabolic crisis43 and rest might theoretically facilitate recovery. In addition, a rest period reduces the likelihood of another head injury during the recovery period. Although there is evidence in the animal literature that vigorous exercise in the first few days after brain injury suppresses neuromolecular markers of neurogenesis and neuroplasticity,44 evidence showing that rest and restricted physical activity result in shorter recovery times is limited, and one randomized clinical trial did not find that rest was associated with better clinical outcomes after mTBI.45 However, there are some observational studies suggesting that higher physical and cognitive activity during the acute recovery period is associated with greater postacute symptoms in concussed athletes.46 After the acute recovery phase, treatment of patients with persistent symptoms focuses on symptom management via a variety of approaches, such as medications, physical and vestibular therapy, and psychologic treatment.42
Increasingly, researchers have encouraged the use of exercise as primary or adjunctive treatment for children, adolescents, and adults who are slow to recover from mTBI,47 primarily because of the positive effects of exercise on symptom clusters (e.g., headache, fatigue, and sleep disturbance) and comorbid conditions (such as depression and anxiety). Although the biochemical and physiologic impact of mTBI may be different in children vs adults,48 there is indirect evidence that exercise might be beneficial for those with persistent or chronic symptoms after mTBI: (1) exercise promotes neuroplasticity and neurogenesis in both the healthy and injured brain,47 (2) exercise is associated with direct changes in neurotransmitter systems,49 and (3) exercise is an effective adjunctive treatment for depression and anxiety.50 The timing of exercise as an intervention is likely important: the optimal time window for exercise may differ depending on injury characteristics and symptom severity, and premature exercise may interrupt restorative mechanisms—at least in animal injury models.44 Unfortunately, the optimal timing for introduction of exercise after mTBI is not yet known. Nonetheless, compared with pharmacologic management, exercise is available at relatively low cost and mostly free of serious adverse effects. Lastly, several lines of evidence suggest a beneficial effect of mild- to moderate-intensity exercise on cerebrovascular function, potentially supporting the utility of exercise training in patients with persistent symptoms.
Exercise and cerebrovascular control.
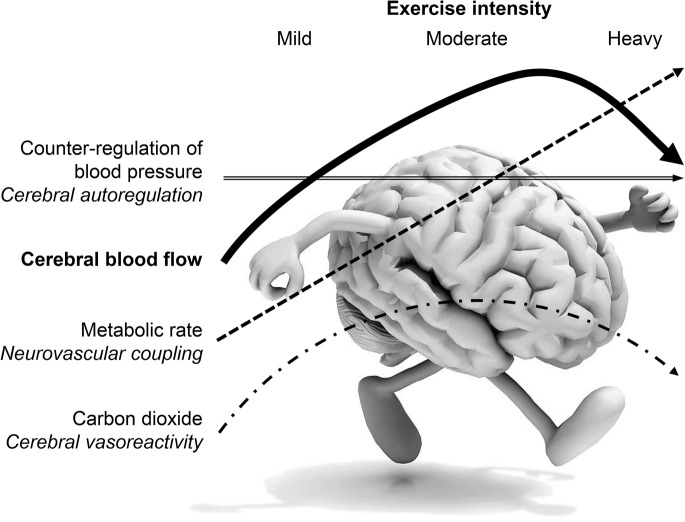
The responses of cerebral blood flow to exercise are driven by neural demand and the marked changes in arterial CO2 tension and mean arterial pressure. During exercise, increases in cerebral metabolism require increased delivery of oxygen to the brain. During mild to moderate exercise, cerebral flow increases due to cortical activation. It is thought that the vasodilation due to the exercise-induced increase in brain metabolism overrides vasoconstrictor effects of increased pressure on the cerebral vasculature.3 However, exercise-induced elevations in metabolism do not simply lead to proportional increases in global cerebral flow. Flow increases up to approximately 60% of maximal effort and returns toward baseline values at higher exercise intensities51 (see the figure). This is due to exercise intensity-dependent effects of CO2 on cerebral flow. Mild to moderate exercise is associated with a small increase in arterial CO2 that increases cerebral blood flow52 in concert with metabolism. However, with intense exercise, there is a reduction in arterial CO2 because ventilation increases exponentially with exercise intensity as pH decreases. Accordingly, intense exercise is accompanied by decreases in cerebral blood flow that ultimately interfere with adequate oxygenation of the brain and contribute to fatigue53 (see the figure). In addition to the interacting effects of metabolism and CO2, the mitigating influence of cerebral autoregulation on cerebral flow during dynamic exercise is considerable. Elevated cerebral blood flow during exercise cannot be explained simply by elevated pressure.52 It is important to note that the large increase in systolic pressure during intense exercise often exceeds the upper limit of cerebral autoregulation. Nonetheless, dynamic cerebral autoregulation appears sufficient to limit the increase in systolic cerebral blood flow velocity.54 Thus, the cerebral flow responses to exercise require the integration of the 3 primary controlling mechanisms: neurovascular coupling, cerebral vasoreactivity, and cerebral autoregulation.
Figure. Cerebral blood flow response to increasing exercise intensity and the engagement and role of the 3 mechanisms that control it.
Blood pressure increases proportionally to exercise intensity, engaging autoregulation that serves to maintain constant flow. However, at mild and moderate intensities, both metabolic rate and carbon dioxide increase, hence both neurovascular coupling and cerebrovascular reactivity result in increased cerebral blood flow. With heavy exercise intensities, there is a pronounced hypocapnia, and so the net result of the 3 controlling mechanisms is a decrease in cerebral blood flow.
Exercise training after mTBI.
As noted above, early management of mTBI consists mainly of physical and cognitive rest, with some studies suggesting a benefit.55 Most major guidelines for management of sport-related concussion call for physical rest acutely after injury, with graded return to exertion after concussion symptoms resolve.41 The limited evidence behind the recommendation for physical rest, however, is well recognized.40,41 In fact, after the acute phase, the introduction of subsymptom threshold exercise appears safe, and may be beneficial in improving symptoms.8 A 4-week intervention of cycling exercise produced significant improvement in cognitive function in patients with TBI,56 but it is unclear whether this was attributable to exercise per se or the virtual reality component of this program. Animal models suggest that exercise can promote neuroplasticity, especially in mTBI.57 However, as yet, there have been no prospective trials examining the effects of a controlled exercise training program on physiologic function and symptoms in patients with TBI. Other groups of people have shown improvements in cerebrovascular control with aerobic exercise training. For example, 7 months of aerobic training improved cerebral vasoreactivity in older (>60 years), healthy individuals.58 This may have functional significance; increases in maximal exercise capacity relate proportionately to increased cerebral blood volume in the hippocampus in older individuals, and increases in flow volume were reflected in improved cognitive function.59 Therefore, in adults and older adults with compromised cognitive function, exercise may demonstrate beneficial effects on cerebrovascular control that are reflected in cognitive improvement. However, in young, healthy individuals, exercise training may not have appreciable effects on cerebrovascular control, probably because cardiovascular function is at or near its peak capacity. Thus, the effectiveness of exercise in reducing postconcussion symptoms may be different in older and younger individuals. However, detraining does result in significant deficits in cerebral regulatory control. This may be relevant to those who have sustained mTBIs because prolonged physical rest may lead to extreme deconditioning and resultant cardiovascular declines.60 This could exacerbate impaired cerebral autoregulation; deconditioning has been shown to reduce cerebral blood flow in humans. In fact, even a single day of bed rest reduces cerebral blood flow for a substantial period of time afterward.6 Thus, independent of primary cerebrovascular dysfunction due to head injury, it seems feasible that prolonged rest after TBI leads to deconditioning that may induce physiologic changes in cerebrovascular control—and these physiologic changes could contribute to symptoms associated with the postconcussion syndrome.
Perspectives.
Persistent symptoms after mTBI in athletes, civilians, active-duty military service members, and veterans can be difficult to effectively treat. The evidence reviewed above suggests that alterations in cerebrovascular function after mTBI might partially underlie persistent symptoms, and the multifaceted nature of cerebrovascular function (neurovascular coupling, vasoreactivity, and autoregulation) and underlying physiologic mechanisms may contribute to symptom heterogeneity. For example, it is possible that potential differences in etiology of mTBI sustained from different injuries (e.g., sport-related concussion vs blast injuries) may result in different pathophysiologic alterations in cerebrovascular regulatory mechanisms, and thus, in different symptoms. Blast injury is often characterized by a series of primary (blast wave), secondary (rotational acceleration as the head moves), and tertiary (direct contusion/laceration by fragment/shrapnel) injuries, as opposed to a distinct, solitary, independent mechanism. Unfortunately, possible time-limited or persisting alterations in cerebrovascular function after mTBI remain mostly unknown, and future studies are needed to fill this gap in our knowledge.
If persistent symptoms are indeed related to cerebrovascular dysfunction, targeting this underlying pathophysiology may provide an additional treatment strategy. The evidence suggests that active exercise training may be one such strategy, because the cerebral flow responses to exercise require the integration of the major mechanisms that underlie cerebrovascular function. While physical rest initially after an mTBI is currently recommended, preliminary evidence suggests that the introduction of subsymptom exercise after the acute recovery period is safe.8 Exactly when subsymptom physical activity should be introduced and which patients might benefit most are not yet clear. Furthermore, the effect of subsymptom threshold exercise on cerebral blood flow and the association between cerebral blood flow and postconcussion symptoms, exercise tolerance, neurocognitive performance, and postural stability remain largely unknown. Given the evidence outlined above, further investigations into the effects of concussion on cerebral blood flow and the effects of exercise on patients with mTBI are warranted.
GLOSSARY
- mTBI
mild traumatic brain injury
- NO
nitric oxide
AUTHOR CONTRIBUTIONS
Can Ozan Tan, PhD: drafted and revised the manuscript for content, including medical writing for content. William P. Meehan III, MD: revised the manuscript for content, including medical writing for content. Grant L. Iverson, PhD: revised the manuscript for content, including medical writing for content. J. Andrew Taylor, PhD: drafted and revised the manuscript for content, including medical writing for content. All authors have seen and approved the final version of the manuscript.
STUDY FUNDING
Dr. Meehan is supported by a grant from the NFL Players Association.
DISCLOSURE
C. Tan reports no disclosures relevant to the manuscript. W. Meehan receives royalties from ABC-Clio publishing for the sale of his book, Kids, Sports, and Concussion: A guide for coaches and parents and royalties from Wolters Kluwer for working as an author for UpToDate. G. Iverson has been reimbursed by the government, professional scientific bodies, and commercial organizations for discussing or presenting research relating to mTBI and sport-related concussion at meetings, scientific conferences, and symposiums. He has a clinical practice in forensic neuropsychology involving individuals who have sustained mTBIs. He is a coinvestigator, collaborator, or consultant on grants relating to mTBI funded by several organizations. J. Taylor reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). Nonfatal traumatic brain injuries from sports and recreation activities: United States, 2001–2005. MMWR Morb Mortal Wkly Rep 2007;56:733–737. [PubMed] [Google Scholar]
- 2.Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol 2008;167:1446–1452. [DOI] [PubMed] [Google Scholar]
- 3.Linkis P, Jorgensen LG, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean flow velocity. J Appl Physiol 1995;78:12–16. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen P, Stie H, Nielsen B, Nybo L. Enhanced cerebral CO2 reactivity during strenuous exercise in man. Eur J Appl Physiol 2006;96:299–304. [DOI] [PubMed] [Google Scholar]
- 5.Brys M, Brown CM, Marthol H, Franta R, Hilz MJ. Dynamic cerebral autoregulation remains stable during physical challenge in healthy persons. Am J Physiol Heart Circ Physiol 2003;285:H1048–H1054. [DOI] [PubMed] [Google Scholar]
- 6.Kawai Y, Murthy G, Watenpaugh DE, Breit GA, Deroshia CW, Hargens AR. Cerebral blood flow velocity in humans exposed to 24 h of head-down tilt. J Appl Physiol 1993;74:3046–3051. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon I, Galli C, Friedman D, Grilli L, Iverson GL. Active rehabilitation for children who are slow to recover following sport-related concussion. Brain Inj 2009;23:956–964. [DOI] [PubMed] [Google Scholar]
- 8.Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Curr Sports Med Rep 2013;12:370–376. [DOI] [PubMed] [Google Scholar]
- 9.Bailey DM, Jones DW, Sinnott A, et al. Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin Sci 2013;124:177–189. [DOI] [PubMed] [Google Scholar]
- 10.Chan ST, Tam Y, Lai CY, et al. Transcranial Doppler study of cerebrovascular reactivity: are migraineurs more sensitive to breath-hold challenge? Brain Res 2009;1291:53–59. [DOI] [PubMed] [Google Scholar]
- 11.Ocon AJ, Kulesa J, Clarke D, Taneja I, Medow MS, Stewart JM. Increased phase synchronization and decreased cerebral autoregulation during fainting in the young. Am J Physiol Heart Circ Physiol 2009;297:H2084–H2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gur AY, Auriel E, Korczyn AD, et al. Vasomotor reactivity as a predictor for syncope in patients with orthostatism. Acta Neurol Scand 2012;126:32–36. [DOI] [PubMed] [Google Scholar]
- 13.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 2006;100:328–335. [DOI] [PubMed] [Google Scholar]
- 14.Van Beek AH, Claassen JA. The cerebrovascular role of the cholinergic neural system in Alzheimer's disease. Behav Brain Res 2011;221:537–542. [DOI] [PubMed] [Google Scholar]
- 15.Jakovcevic D, Harder DR. Role of astrocytes in matching blood flow to neuronal activity. Curr Top Dev Biol 2007;79:75–97. [DOI] [PubMed] [Google Scholar]
- 16.Richards HK, Simac S, Piechnik S, Pickard JD. Uncoupling of cerebral blood flow and metabolism after cerebral contusion in the rat. J Cereb Blood Flow Metab 2001;21:779–781. [DOI] [PubMed] [Google Scholar]
- 17.Unterberg AW, Stroop R, Thomale UW, Kiening KL, Pauser S, Vollmann W. Characterisation of brain edema following “controlled cortical impact injury” in rats. Acta Neurochir Suppl 1997;70:106–108. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell WL, Irvine A, Adams JH, Graham DI, Gennarelli TA. Response of cerebral microvasculature to brain injury. J Pathol 1988;155:327–335. [DOI] [PubMed] [Google Scholar]
- 19.Tenovuo O. Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury: clinical experience in 111 patients. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:61–67. [DOI] [PubMed] [Google Scholar]
- 20.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 2009;296:R1473–R1495. [DOI] [PubMed] [Google Scholar]
- 21.Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2006;291:H1856–H1861. [DOI] [PubMed] [Google Scholar]
- 22.Brian JE, Jr. Carbon dioxide and the cerebral circulation. Anesthesiology 1998;88:1365–1386. [DOI] [PubMed] [Google Scholar]
- 23.Smith JJ, Lee JG, Hudetz AG, Hillard CJ, Bosnjak ZJ, Kampine JP. The role of nitric oxide in the cerebrovascular response to hypercapnia. Anesth Analg 1997;84:363–369. [DOI] [PubMed] [Google Scholar]
- 24.Enevoldsen EM, Jensen FT. Autoregulation and CO2 responses of cerebral blood flow in patients with acute severe head injury. J Neurosurg 1978;48:689–703. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Kelly DF, Oertel M, et al. Carbon dioxide reactivity, pressure autoregulation, and metabolic suppression reactivity after head injury: a transcranial Doppler study. J Neurosurg 2001;95:222–232. [DOI] [PubMed] [Google Scholar]
- 26.Golding EM, Steenberg ML, Contant CF, Jr, Krishnappa I, Robertson CS, Bryan RM, Jr. Cerebrovascular reactivity to CO(2) and hypotension after mild cortical impact injury. Am J Physiol 1999;277:H1457–H1466. [DOI] [PubMed] [Google Scholar]
- 27.Len TK, Neary JP, Asmundson GJ, Goodman DG, Bjornson B, Bhambhani YN. Cerebrovascular reactivity impairment after sport-induced concussion. Med Sci Sports Exerc 2011;43:2241–2248. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 2002;106:1814–1820. [DOI] [PubMed] [Google Scholar]
- 29.Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke 2010;41:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. J Physiol 2012;590:6343–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzeng YC, Chan GS, Willie CK, Ainslie PN. Determinants of human cerebral pressure–flow velocity relationships: new insights from vascular modelling and Ca(2)(+) channel blockade. J Physiol 2011;589:3263–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan CO, Hamner JW, Taylor JA. The role of myogenic mechanisms in human cerebrovascular regulation. J Physiol 2013;591:5095–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamner JW, Tan CO. Relative contributions of sympathetic, cholinergic, and myogenic mechanisms to cerebral autoregulation. Stroke 2014;45:1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White RP, Vallance P, Markus HS. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clin Sci 2000;99:555–560. [PubMed] [Google Scholar]
- 35.Kirkness CJ, Mitchell PH, Burr RL, Newell DW. Cerebral autoregulation and outcome in acute brain injury. Biol Res Nurs 2001;2:175–185. [DOI] [PubMed] [Google Scholar]
- 36.Lam JM, Hsiang JN, Poon WS. Monitoring of autoregulation using laser Doppler flowmetry in patients with head injury. J Neurosurg 1997;86:438–445. [DOI] [PubMed] [Google Scholar]
- 37.Arvigo F, Cossu M, Fazio B, et al. Cerebral blood flow in minor cerebral contusion. Surg Neurol 1985;24:211–217. [DOI] [PubMed] [Google Scholar]
- 38.Junger EC, Newell DW, Grant GA, et al. Cerebral autoregulation following minor head injury. J Neurosurg 1997;86:425–432. [DOI] [PubMed] [Google Scholar]
- 39.Strebel S, Lam AM, Matta BF, Newell DW. Impaired cerebral autoregulation after mild brain injury. Surg Neurol 1997;47:128–131. [DOI] [PubMed] [Google Scholar]
- 40.Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013;80:2250–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport, Zurich, 2012. J Athl Train 2013;48:554–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meehan WP, III. Medical therapies for concussion. Clin Sports Med 2011;30:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- 44.Griesbach GS. Exercise after traumatic brain injury: is it a double-edged sword? PM R 2011;3(6 suppl 1):S64–S72. [DOI] [PubMed] [Google Scholar]
- 45.de Kruijk JR, Leffers P, Meerhoff S, Rutten J, Twijnstra A. Effectiveness of bed rest after mild traumatic brain injury: a randomised trial of no versus six days of bed rest. J Neurol Neurosurg Psychiatry 2002;73:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majerske CW, Mihalik JP, Ren D, et al. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J Athl Train 2008;43:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med 2008;10:128–140. [DOI] [PubMed] [Google Scholar]
- 48.Kochanek PM. Pediatric traumatic brain injury: quo vadis? Dev Neurosci 2006;28:244–255. [DOI] [PubMed] [Google Scholar]
- 49.Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand 1989;137:1–13. [DOI] [PubMed] [Google Scholar]
- 50.Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev 2007;27:359–367. [DOI] [PubMed] [Google Scholar]
- 51.Moraine JJ, Lamotte M, Berre J, Niset G, Leduc A, Naeije R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur J Appl Physiol Occup Physiol 1993;67:35–38. [DOI] [PubMed] [Google Scholar]
- 52.Jorgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol 1992;72:1123–1132. [DOI] [PubMed] [Google Scholar]
- 53.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol 2008;104:306–314. [DOI] [PubMed] [Google Scholar]
- 54.Ogoh S, Dalsgaard MK, Yoshiga CC, et al. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol 2005;288:H1461–H1467. [DOI] [PubMed] [Google Scholar]
- 55.Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP, III. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 2014;133:e299–e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Arch Phys Med Rehabil 1999;80:661–667. [DOI] [PubMed] [Google Scholar]
- 57.Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma 2007;24:1161–1171. [DOI] [PubMed] [Google Scholar]
- 58.Vicente-Campos D, Mora J, Castro-Pinero J, Gonzalez-Montesinos JL, Conde-Caveda J, Chicharro JL. Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J Sports Med Phys Fitness 2012;52:537–544. [PubMed] [Google Scholar]
- 59.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 2007;104:5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mossberg KA, Ayala D, Baker T, Heard J, Masel B. Aerobic capacity after traumatic brain injury: comparison with a nondisabled cohort. Arch Phys Med Rehabil 2007;88:315–320. [DOI] [PubMed] [Google Scholar]