Abstract
Objective:
To determine whether, and to what degree, preclinical Alzheimer disease (AD) confounds studies of healthy aging where “healthy” is based on cognitive normality alone.
Methods:
We examined the effects of preclinical AD in cognitively normal older individuals using resting-state functional connectivity MRI. We investigated 2 groups of cognitively normal participants: one group with evidence of preclinical AD as assessed by CSF markers of AD and the other group with normal CSF biomarkers.
Results:
There were significant interactions between age and biomarker status in the default-mode, dorsal attention, and salience resting-state networks. In the group with evidence of preclinical AD, there were dramatic changes in functional connectivity with age. In the group without evidence of preclinical AD, those changes were greatly attenuated. In most regions with significant interactions of age and biomarker status, the age-related change in functional connectivity in the normal biomarker group was indistinguishable from zero.
Conclusions:
These results suggest that preclinical AD accounts for a substantial portion of the reported effects of aging in the extant functional connectivity literature.
Healthy aging is associated with decreased functional connectivity, as measured by resting-state functional MRI (rs-fMRI).1–4 However, previous studies of aging have not controlled for undiagnosed preclinical Alzheimer disease (AD). AD is of particular interest because it is very prevalent in older individuals5 and has reliable CSF and imaging biomarkers.6 Nevertheless, prior rs-fMRI studies of aging have not excluded participants with such biomarkers, thereby potentially confounding the effects of aging and preclinical disease. A predictable consequence of this confound would be overestimation of the effects of “healthy aging.” It is important to recognize that amyloid deposition disrupts default mode network (DMN) functional connectivity in cognitively normal individuals.7–10 Moreover, an APOE ε4 allele, a strong AD risk factor, disrupts functional connectivity in the DMN and the salience network (SAL).11–13
Previous studies have reported decreased functional connectivity within the DMN in advanced age.1–4 Critically, the DMN is also affected in AD.14 In addition, other resting-state networks (RSNs), including the SAL, are frequently implicated in aging and dementia, including AD.15,16 Despite compelling evidence of this confound, less than half of recent aging studies acknowledge it and none address it (see appendix e-1 on the Neurology® Web site at Neurology.org). The goal of this study is to explicitly demonstrate the magnitude and topography of this confound. To frame our analysis in quantitative terms, we cross-sectionally estimate the effect of age in rs-fMRI measures in subjects with and without CSF evidence of preclinical AD.
METHODS
Subjects.
Participants were drawn from studies of aging and dementia at the Knight Alzheimer's Disease Research Center at Washington University in St. Louis. Participants were community-dwelling volunteers who consented to clinical examination, lumbar puncture, and imaging studies. Inclusion criteria for this study were cognitive normality as defined by a Clinical Dementia Rating (CDR) of zero17 and additional testing (including lumbar puncture and neuroimaging) performed within 1 year of CDR evaluation. These participants did not present with objective memory problems and underwent lumbar puncture and imaging for strictly research purposes.
Standard protocol approvals, registrations, and patient consents.
All studies were approved by the Washington University School of Medicine Human Research Protection Office. Informed consent was obtained from all participants. A subset of these participants was included in previous studies.16,18,19
Lumbar puncture and CSF analysis.
CSF was assayed for β-amyloid 1–42 (Aβ42) and tau as previously described (see appendix e-1). CDR0 participants who were Aβ42+ only or Aβ42+ and tau+ (National Institute on Aging preclinical stages 1 and 2, respectively20,21) were classified as CDR0+, while those with normal CSF biomarkers were classified as CDR0−. Participants who were CSF Aβ42−, tau+21 were not studied.
Imaging acquisition, preprocessing, and quality assurance.
Functional and structural imaging as well as initial preprocessing was performed as previously described,16 but modified to avoid recently described biases.22 Reduction of head motion artifact was accomplished by regression of the time series derived by retrospective realignment, and nuisance regressors extracted from white matter, CSF, and the global signal. In addition, high movement frames were excluded from functional connectivity analyses (see appendix e-1 for details and summary statistics).
Volumetric data.
Estimates of total gray matter volume were calculated using FreeSurfer (http://surfer.nmr.mgh.harvard.edu).23 The total gray matter value was calculated as the volume of the FreeSurfer-derived gray matter mask. Total gray matter volume was used as a regressor of no interest in the statistical analyses. This measure is not spatially specific and accounts only for global atrophy.
Statistical analyses.
This analysis is principally concerned with identifying sets of voxels where the size of the aging effect varies depending on biomarker status (CDR0− vs CDR0+). We investigated 2 seed regions: the posterior cingulate cortex (PCC) representing the DMN and the dorsal anterior cingulate cortex representing the SAL. We fit the following model at each voxel:
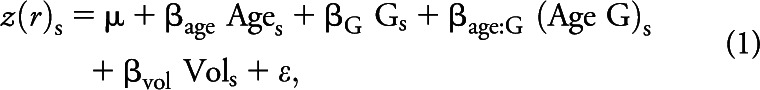 |
where z(r)s indicates the Fisher z-transformed correlation between a seed region of interest (ROI) and 1 voxel in subject s. G is a binary variable representing biomarker status (CDR0−, CDR0+). Model parameters included the voxelwise mean over subjects, μ, and regression coefficients (β) estimating the effects of FreeSurfer-derived gray matter volume, age, biomarker status, and the interaction of age and biomarker status. For the effect of interest (age by biomarker status), parametric volumes of F and p statistics were computed. These maps were voxelwise thresholded at p < 0.01 and cluster thresholded at a corrected p < 0.01.24 Voxelwise significance was determined from the linear model. Cluster-wise significance was determined by simulating the null hypothesis (no CDR0− vs CDR0+ difference) using permutation resampling. Thus, over 1,000 resamplings, the size of clusters surpassing a range of voxelwise βage thresholds was systematically tabulated.25–27 The critical F was 4.2 and the critical cluster size was 95 voxels. We then extracted mean βage corresponding to the CDR0− and CDR0+ groups in voxels within significant clusters.
Statistical analyses: Composite scores.
The previous analyses focused on relationships between ROIs and voxels. We have previously defined composite scores as measures of RSN integrity based on correlations averaged over ROI pairs within and between networks.16 These measures are calculated by evaluating z(r) between a priori defined region pairs and averaging these quantities over all ROI pairs within a given RSN. In the present analyses, composite z(r) scores were obtained for 5 RSNs. Examined RSNs included (1) DMN, (2) dorsal attention network (DAN), (3) control network (CON), (4) SAL, and (5) sensorimotor network (SMN). These composite scores were calculated for each participant and subjected to the following model:
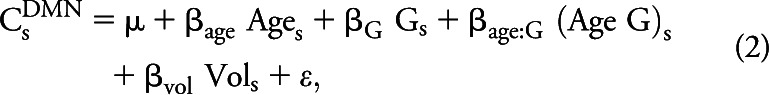 |
where C represents the network composite score (e.g., DMN) in subject s. Composite scores were also calculated across RSNs, e.g., between the DMN and DAN. Across-RSN composite scores are notated, e.g., as DMN-DAN. Comparisons of composite scores were Bonferroni-corrected for multiple comparisons.
RESULTS
Participants.
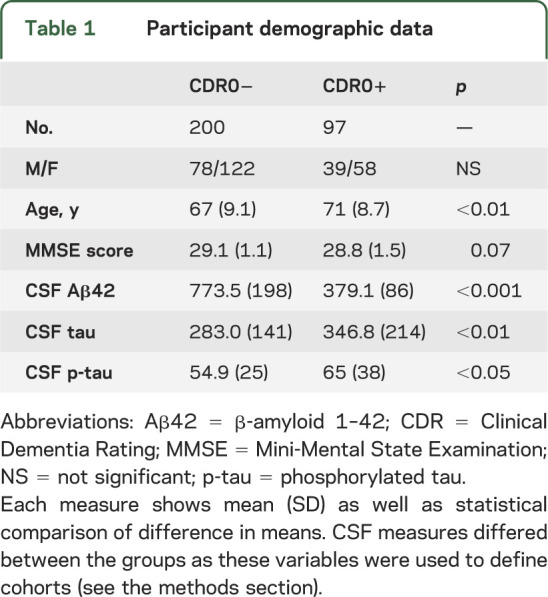
Ages ranged from 45 to 95 years. In study participants, motion-related quality-assurance measures were similar across groups (p > 0.1). The CDR0− (n = 200) and CDR0+ (n = 97) groups differed slightly in age (table 1) but were well distributed across the age ranges (see appendix e-1, figure e-1). We therefore elected to maintain the larger groups as opposed to reducing the number of participants to perfectly match the groups for age. This allows for the most accurate linear fit of the data (see appendix e-1).
Table 1.
Participant demographic data
Presence of AD biomarkers modulates age effects in the DMN and SAL.
We first investigated the interaction of age and biomarker status on functional connectivity in the DMN and SAL in both CDR0− and CDR0+ groups. Figure 1 shows functional connectivity results obtained using the PCC as seed region for the DMN (figure 1A) and the dorsal anterior cingulate cortex as seed for the SAL (figure 1B). Significant interactions of age and biomarker status were present across several brain regions, but had similar effects in each significant cluster. In the CDR0− group, the cross-sectional change in functional connectivity per year (βage) was near 0. In the CDR0+ group, these changes were of greater magnitude. In region pairs belonging to the same network (e.g., PCC and medial prefrontal cortex), the change was positive correlations approaching 0 (from above) with age. Conversely, in regions with different network memberships (e.g., PCC and left frontal), the change was negative correlations approaching 0 (from below) with age.
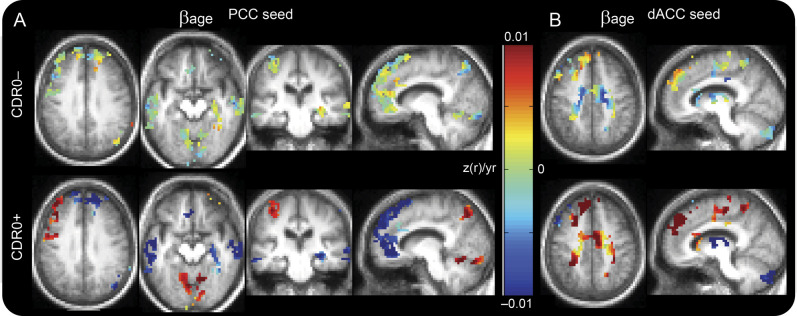
Figure 1. Preclinical AD accounts for a large fraction of observed effects of age.
Maps of βage in the DMN (PCC seed) and SAL (dACC seed). βage for each group (CDR0− and CDR0+) extracted from equation 1. Only voxels belonging to clusters exhibiting a significant interaction of age and presence of pathology are shown. For each cluster, the CDR0− group shows βage values near 0 (green) indicating change with age and the CDR0+ group shows large magnitude changes with age (dark blue and dark red). (A) Changes associated with a PCC seed. (B) Changes associated with a dACC seed. AD = Alzheimer disease; CDR = Clinical Dementia Rating; dACC = dorsal anterior cingulate cortex; DMN = default mode network; PCC = posterior cingulate cortex; SAL = salience network.
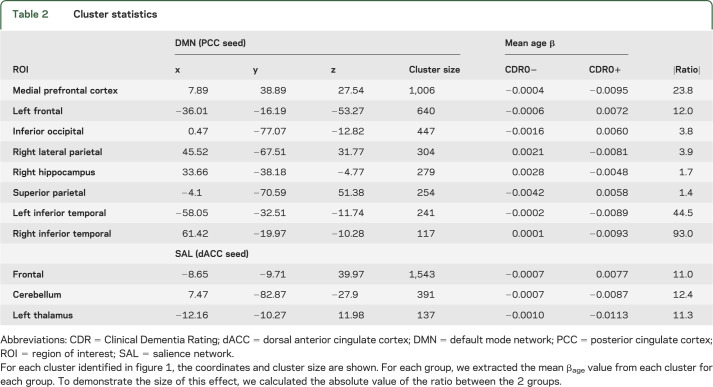
To more quantitatively investigate the interaction of age and the presence of pathology, we identified the significantly affected clusters and extracted the mean βage value in each cluster. Those data are presented in table 2. In each case, the magnitude of the age-related change is larger in the CDR0+ group; the multiplicative factor ranges from approximately 4 to 90. To demonstrate this graphically, we extracted the mean Fisher z-transformed correlation coefficient within each cluster for each subject and plotted them against their age (figure 2). It is graphically apparent that the slope of the age vs functional connectivity regression line is steeper in the CDR0+ group compared with the CDR0− group.
Table 2.
Cluster statistics
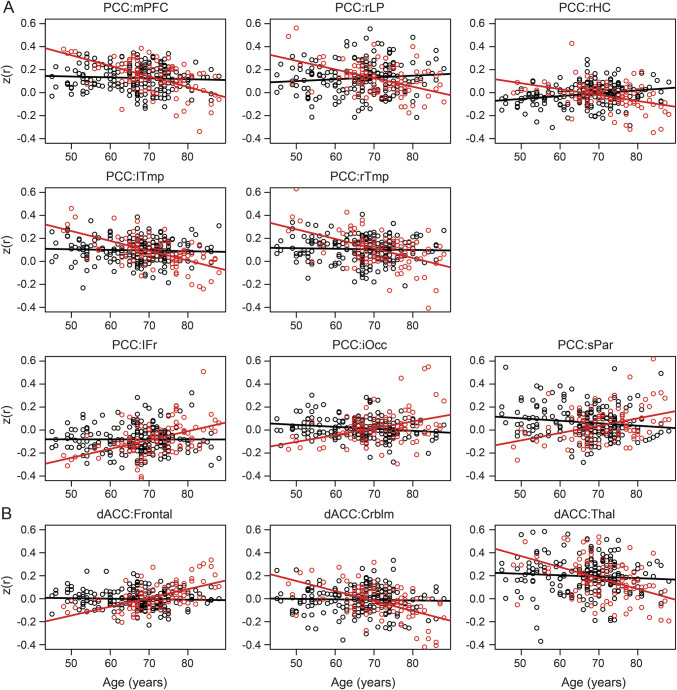
Figure 2. The slope of the aging effect is reduced in the CDR0− group.
Scatterplots for each participant separated according to group (CDR0− vs CDR0+). (A) Values averaged across voxels in significant clusters scattered against age of each participant. For each ROI pair (PCC as seed), the slope in the CDR0+ (red) group is significantly steeper than the slope in the CDR0− (black) group as determined by a significant interaction of age × biomarker status (table 2). (B) Similar to panel A, but showing effects between the dACC and the significant clusters identified therein. As with the PCC, in each ROI pair, the slope of the CDR0+ regression is steeper than the CDR0− regression (table 2). CDR = Clinical Dementia Rating; Crblm = cerebellum; dACC = dorsal anterior cingulate cortex; iOcc = inferior occipital; lFr = left frontal; lTmp = left temporal; mPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; rHC = right hippocampus; rLP = right lateral parietal; ROI = region of interest; rTmp = right temporal; sPar = superior parietal; Thal = thalamus.
Size of aging effects in CDR0− is small in regions with interaction.
The previous results (figure 2) suggest that the remaining effect of age after accounting for preclinical AD is small. We sought to determine whether these changes were statistically distinguishable from the null hypothesis that functional connectivity in these regions does not change with age. The value of βage in the CDR0− group for each significant cluster is plotted with the 95% confidence interval of that estimate (figure e-2). It is important to note that for most of these clusters, the estimate of the slope of the functional connectivity vs age line overlaps zero, indicating the inability to demonstrate an effect of age, once the effects of preclinical AD are excluded in this set of regions.
Age-related decline in multiple RSNs.
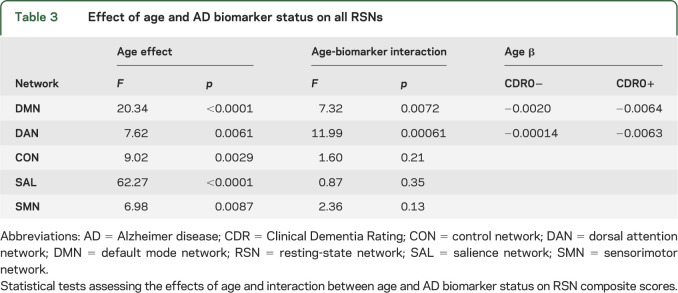
The analyses described so far focused on rs-fcMRI effects within the DMN and SAL, which were studied based on previous literature. To test whether other networks were similarly affected, we calculated RSN composite scores for the 5 previously reported RSNs16 and determined the effect of age and presence of pathology (or an interaction) on composite scores (equation 2). We found significant interactions of age and biomarker status in the DMN and DAN, but not in the other networks (table 3). It is likely that the SAL does not show a significant interaction at the composite score level because the interaction effect is focused in only a few brain regions and does not survive averaging over a priori RSN definitions. The CON, SAL, and SMN showed effects of age alone, but no significant age by biomarker status interaction.
Table 3.
Effect of age and AD biomarker status on all RSNs
Age-related declines between RSNs.
Previous work suggests that large-scale neural dysfunction can manifest as altered across-RSN functional connectivity.16 To determine whether age by biomarker status interaction effects were also manifested across RSNs, we tested the model represented in equation 2 using cross-RSN composite scores. We found significant effects of age between the DMN-DAN (F1,293 = 15.21, p = 0.00012) but no significant interaction between age and biomarker group (all other p values >0.05).
Supporting analyses.
Two analytic decisions potentially affect our results: (1) the use of global signal regression (GSR) as a preprocessing step, and (2) the use of slightly age nonmatched groups. GSR is a potent noise-reduction strategy,28,29 but objections have been raised regarding the introduction of bias. In appendix e-1, we replicated the main finding of this report (figure 1) without the use of GSR as a preprocessing step (see appendix e-1, figure e-3). This result demonstrates that our principal finding is robust to this processing choice, although omitting GSR increases noise.
In the sample presented here, the ages of the CDR0− and CDR0+ groups differ significantly, but they span a similar range. To ensure that the reported results are not driven by the mismatch in age, we selected a subset of the CDR0− group that most closely matched the age of the CDR0+ group. This significantly reduced the number of subjects in the sample and also reduced statistical power. However, the interaction of age and biomarker status on RSN composite scores in the DMN and DAN demonstrated in table 3 remained significant in the age-matched sample (see appendix e-1, figure e-4, and table e-1).
DISCUSSION
This study demonstrates that age-related alterations in functional connectivity of the DMN and SAL are strongly influenced by the presence of CSF biomarkers of AD. In quantitative terms, regression coefficients expressing the age dependence of rs-fcMRI measures were increased by a factor of 4 or more in individuals with AD biomarkers. Given that previous studies have shown that amyloid deposition, as imaged by Pittsburgh compound B (PiB)-PET, is associated with decreased DMN functional connectivity,7–10 it might be argued that the present results are predictable. Nevertheless, although preclinical AD has been acknowledged as a potential confound in studies of “healthy aging”30 it is not often taken into account. The data presented here demonstrate that failure to do so results in a significant overestimation of the aging effect in some regions.
The concept of amyloid deposition modulating functional connectivity is not new. Indeed, many studies have investigated the effects of amyloid10,31 and APOE ε412,13 on functional connectivity in cognitively normal older adults. These studies have demonstrated decreased functional connectivity but have not investigated how risk factors interacted with age. One study did present data parametric in age and PiB status1; however, that study did not estimate the effect of age separately for the PiB− and PiB+ groups. Thus, our study describes the size of the age by amyloid status interaction for the first time. Our findings are consistent with current hypotheses concerning preclinical AD32 and accelerated aging profiles in symptomatic AD.33 These biomarker-related increases in age-related changes in functional connectivity may be insufficient to cause cognitive deficits. Alternatively, clinical measures (such as the CDR used here) do not reliably detect subtle derangements of cognition. This work underscores the importance of accounting for both age and amyloid status in studies of aging and dementia.
We observed significant interactions between age and biomarker status in the DMN, DAN, and SAL, but found effects of age alone in other RSNs (CON, SMN) and between-RSN (DMN-DAN) functional connectivity. These measures were affected by aging but those effects were not modulated by the presence or absence of AD pathology. We previously reported that the CON and SMN are affected by AD.16 Here, there was no identifiable effect of biomarker status on either network. Thus, changes in these networks may be a later manifestation of AD. Similar statements apply to between-RSN rs-fcMRI measures. These results indicate that the effects of aging and early AD are dissociable. Moreover, progressive involvement of RSNs may index progression of the disease.
The presence of AD biomarkers significantly increased the size of the aging effect in the DMN, DAN, and SAL. In each case reported here, the magnitude of the age-related effect was larger in the CDR0+ group. In fact, the size of the aging effect in many regions was indistinguishable from zero in the CDR0− group. This suggests that in the regions where there is a significant interaction of biomarker status and age, the effect of age alone may be minimal. This is not to say there are not significant effects of aging; indeed, we detected main effects of age with no modulating influence of AD pathology. The goal of this study was not to characterize every age effect. We endeavored to identify sets of brain regions where the effect of age was amplified by the presence of AD pathology.
After the removal of the effects of detectable AD pathology, there remain significant, nonzero effects of age. These changes exist in the absence of cognitive decline or detectable neurodegeneration. While it is possible that these effects represent some nonneural process (e.g., vascular), converging evidence indicates that healthy aging is associated with reorganization of functional networks.34–36 However, these inferences are based on studies often confounded by preclinical AD. Future studies on functional reorganization in CDR0 cohorts, screened to exclude preclinical AD, coupled with sensitive neuropsychological assessment, may provide insight into the nature of these changes.
The primary finding of this work is that the failure to account for participants who are in the presymptomatic stages of AD leads to an overestimation of the age effect in functional connectivity measures. We suggest that the increased effect of age in the DMN, DAN, and SAL represents the earliest rs-fcMRI manifestations of AD. Future studies should investigate the prodromal periods of other neurodegenerative diseases, the effects of focal atrophy on these observed effects, and the longitudinal nature of these changes.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge the support of the Clinical, Biomarker, Imaging, Informatics, Biostatistics, and Genetics Cores of the Knight Alzheimer's Disease Research Center at Washington University in St. Louis.
GLOSSARY
- Aβ42
β-amyloid 1–42
- AD
Alzheimer disease
- CDR
Clinical Dementia Rating
- CON
control network
- DAN
dorsal attention network
- DMN
default mode network
- GSR
global signal regression
- PCC
posterior cingulate cortex
- PiB
Pittsburgh compound B
- ROI
region of interest
- rs-fcMRI
resting-state functional connectivity MRI
- RSN
resting-state network
- SAL
salience network
- SMN
sensorimotor network
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
M.R.B.: study concept and design, analysis and interpretation, critical revision of manuscript. J.B.T.: analysis and interpretation, critical revision of manuscript. A.Z.S.: analysis and interpretation, critical revision of manuscript. L.W.: analysis and interpretation, critical revision of manuscript. A.M.F.: collection of data, analysis and interpretation. T.B.: collection of data, study supervision. J.C.M.: study concept and design, critical revision of manuscript, study supervision. B.M.A.: study concept and design, analysis and interpretation, critical revision of manuscript, study supervision.
STUDY FUNDING
Supported by the MSTP training grant to WUSTL (M.R.B.), and grants P01AG026276, P01AG03991, PSOAG05681, and U19AG032438 from the National Institute on Aging (J.C.M.). Also supported by grant NSO6833 from the NINDS and by P30NSO48056 from NIMH (A.Z.S.). Finally, this study was supported by grants RO1NRO14449, RO1NRO12657, and R01NR012907, and by the Alzheimer's Association (B.M.A.). Additional support was provided through the generous support of Fred Simmons and Olga Mohan and the McDonnell Center for Systems Neuroscience. Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
DISCLOSURE
M. Brier, J. Thomas, A. Snyder, and L. Wang report no disclosures relevant to the manuscript. A. Fagan consults for Roche and Lilly USA. T. Benzinger has a research grant from Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly), and has received other support from the National Multiple Sclerosis Society (travel for grant review). J. Morris has participated in drug trials with Janssen Immunotherapy, Pfizer, and Eli Lilly, and has received honoraria from the Charles A. Dana Foundation, Eli Lilly, and Eisai. B. Ances reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Andrews-Hanna JR, Snyder AZ, Vincent JL, et al. Disruption of large-scale brain systems in advanced aging. Neuron 2007;56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA 2010;107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch W, Teipel S, Mueller S, et al. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage 2010;51:280–287. [DOI] [PubMed] [Google Scholar]
- 4.Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry 2012;471:549–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003;62:1087–1095. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drzezga A, Becker JA, Van Dijk KR, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 2011;134:1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedden T, Van Dijk KR, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci 2009;29:12686–12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mormino EC, Smiljic A, Hayenga AO, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex 2011;21:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 2010;67:584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA 2009;106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machulda MM, Jones DT, Vemuri P, et al. Effect of APOE epsilon4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol 2011;68:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheline YI, Morris JC, Snyder AZ, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci 2010;30:17035–17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 2004;101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 2010;133:1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brier MR, Thomas JB, Snyder AZ, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci 2012;32:8890–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Roe CM, Snyder AZ, et al. Alzheimer disease family history impacts resting state functional connectivity. Ann Neurol 2012;72:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brier MR, Thomas JB, Fagan AM, et al. Functional connectivity and graph theory in preclinical Alzheimer's disease. Neurobiol Aging 2014;35:757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 2012;71:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 2013;82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004;23:S69–S84. [DOI] [PubMed] [Google Scholar]
- 24.Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 1999;18:32–42. [DOI] [PubMed] [Google Scholar]
- 25.Hayasaka S, Nichols TE. Combining voxel intensity and cluster extent with permutation test framework. Neuroimage 2004;23:54–63. [DOI] [PubMed] [Google Scholar]
- 26.Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res 2003;12:419–446. [DOI] [PubMed] [Google Scholar]
- 27.Nichols T, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2001;15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014;84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev 2013;37:384–400. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Brier MR, Snyder AZ, et al. Cerebrospinal fluid Abeta42, phosphorylated tau181, and resting-state functional connectivity. JAMA Neurol 2013;70:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009;30:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones DT, Machulda MM, Vemuri P, et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 2011;77:1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage 2009;44:715–723. [DOI] [PubMed] [Google Scholar]
- 35.Meunier D, Stamatakis EA, Tyler LK. Age-related functional reorganization, structural changes, and preserved cognition. Neurobiol Aging 2014;35:42–54. [DOI] [PubMed] [Google Scholar]
- 36.Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex Epub 2014 Feb 13. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.