Abstract
Objectives
This in vitro study aimed to investigate the ability of Candida albicans (C. albicans) and Enterococcus faecalis (E. faecalis) to penetrate dentinal tubules of instrumented and retreated root canal surface of split human teeth.
Materials and Methods
Sixty intact extracted human single-rooted teeth were divided into 4 groups, negative control, positive control without canal instrumentation, instrumented, and retreated. Root canals in the instrumented group were enlarged with endodontic instruments, while root canals in the retreated group were enlarged, filled, and then removed the canal filling materials. The teeth were split longitudinally after canal preparation in 3 groups except the negative control group. The teeth were inoculated with both microorganisms separately and in combination. Teeth specimens were examined by scanning electron microscopy (SEM), and the depth of penetration into the dentinal tubules was assessed using the SMILE view software (JEOL Ltd).
Results
Penetration of C. albicans and E. faecalis into the dentinal tubules was observed in all 3 groups, although penetration was partially restricted by dentin debris of tubules in the instrumented group and remnants of canal filling materials in the retreated group. In all 3 groups, E. faecalis penetrated deeper into the dentinal tubules by way of cell division than C. albicans which built colonies and penetrated by means of hyphae.
Conclusions
Microorganisms can easily penetrate dentinal tubules of root canals with different appearance based on the microorganism size and status of dentinal tubules.
Keywords: Candida albicans, Dentinal tubules, Enterococcus faecalis, Root canal infection, Scanning electron microscopy
Introduction
Bacteria have been shown to be the main causative factor of pulpal and periapical disease.1,2,3 When complex microflora invade the root canal space, there is usually colonization inside the dentinal tubules, isthmus, accessory canals and apical ramification that cannot then be easily reached by endodontic instruments or intracanal medicaments.4 Additionally, persistence inside the root canal space may play a significant role in the failure of the endodontic treatment.5,6 The ability of microorganisms to penetrate the dentinal tubules has been reported to range from 10 to 150 µm for yeasts, and up to the cementodentinal junction in the case of heavily infected root canals of extracted teeth.7,8 Orstavik and Haapasalo found that Enterococcus faecalis penetrated deeply in dentinal tubules, up to 400 µm in vitro.9 This invasion can occur within 2 - 3 weeks. The penetration of microorganisms into the dentinal tubules of intact dentin has been observed by light and scanning electron microscopy (SEM).3 In addition, an in vitro observation of infected dentin after instrumentation has been reported.10,11,12 However, dentin of re-treated canals have not been evaluated previously. Thus, the aim of the present study was to evaluate the ability of selected microorganisms to penetrate into the dentinal tubules of instrumented and re-treated split root canal walls using SEM.
Materials and Methods
Preparation of microorganisms
Two microorganisms were selected for this study, Candida albicans (C. albicans) and Enterococcus faecalis (E. faecalis). Stock cultures of clinically isolated C. albicans (ATCC 66027, ATCC, Manassas, VA, USA) were maintained on Sabouraud agar plates (NIS-SUI Co., Tokyo, Japan). A suspension was prepared by transferring three colonies from the Sabouraud agar plate using a sterile 4 mm diameter platinum loop to 10 mm Sabouraud dextrose broth (SDB, Merck, Darmstadt, Germany) in a sterile 10 mL screw-cap tube, followed by incubation for 2 days at 37℃. Six test tubes were prepared. E. faecalis (ATCC 29212, ATCC) was maintained by subculturing on tryptic soy agar (Difco Laboratories, Detroit, MI, USA). The organism was scraped from the agar plate and resuspended in tryptic soy broth (TSB, Merck). Cultures were grown overnight in 10 mL brain heart infusion broth (BHI, Oxoid Ltd., Basingstoke, UK) at 37℃, in aerobic chamber.
Preparation of teeth
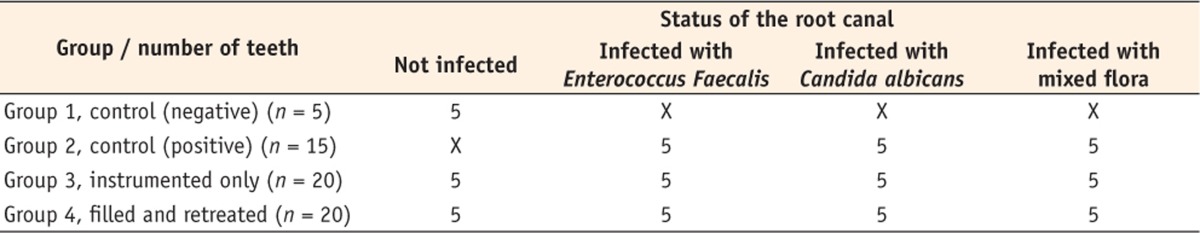
Sixty intact extracted human single-rooted teeth were used. All teeth were radiographed mesiodistally and buccolingually to ensure that they were free of root resorption or calcification. A magnifying dental loupe (Orascoptic, Middleton, WI, USA) of ×2.5 magnification was used to inspect the roots for cracks, external resorption, or caries. The selected teeth were stored for 2 days at room temperature in 5.25% NaOCl to remove organic debris, washed with distilled water, and immersed in 0.9% saline solution until use. The teeth were divided randomly into 2 experimental groups, and negative and positive control groups (Table 1).
Table 1.
The four groups according to the status of the root canal (n = number of teeth)
Experimental procedures
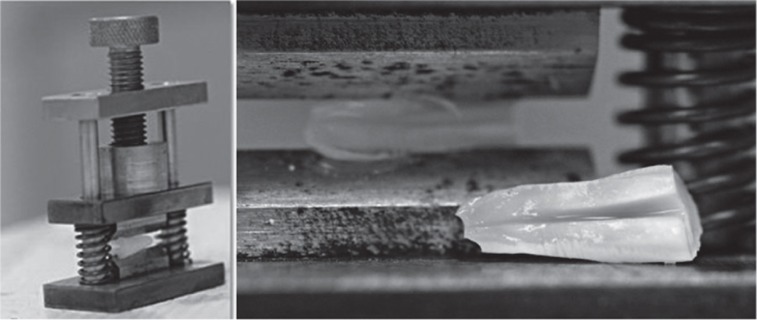
The teeth from each group were cut at the cementoenamel junction and 3 mm from the root apex using a carborundum disk under a water spray to give approximately 10 mm of root length. A longitudinal groove was cut along the entire length of the root in the control group, and then they were split into two halves with a tool specifically designed and manufactured for this purpose by an engineer. This tool was constructed of steel with hardened tool steel blade (Figure 1). Pressure was applied manually on the grooved tooth until the sample split. The blades were designed to leave a gap to ensure the root canal was not contaminated. This procedure was better than using a disc where debris accumulates in the cut dentin surface that plugs the opening of dentinal tubules, preventing penetration by the microorganisms.13 The split root canals of the negative control group received no treatment. They were immediately fixed in 2.5% phosphate-buffered glutaraldehyde solution for 9 days according to Sen et al.7 Split root canals of the positive control group were placed in a Petri dish and infected with 0.5 mL of the microorganisms (E. faecalis, C. albicans, and a mixture of both bacteria) using a micropipette followed by placement in an incubator at 37℃. The infected canals were refreshed with 0.5 mL culture media every day for 4 weeks.
Figure 1.
The tool used to split the root into two halves.
The instrumented group. Root canal patency was verified by placing a #15 K-file (Kerr, Romulus, MI, USA) through the apical foramen. The working length was established by subtracting 1 mm from this measurement. Each canal was instrumented using a high-torque motor at 350 rpm with a 0.04 K3 nickel-titanium (NiTi) rotary instrument (SybronEndo, Orange, CA, USA) to ISO size 40, using the crown-down technique. RC-Prep gel (Dentsply Maillefer, Ballaigues, Switzerland) was used as a lubricant. In total, 20 mL 1.0% NaOCl was used as an irrigant for each canal. The root canals were flushed with 3 mL normal physiological saline and dried with sterile paper points.
The root canal filled and retreated group. The root canal of the teeth in this group were prepared similar to the instrumented group. They were filled with gutta-percha and AH26 cement (De Tray Dentsply, Milford, DE, USA) using the lateral cold condensation technique. All teeth were placed in an incubator for 2 weeks to allow complete setting of AH26 root canal cement. The gutta-percha filling was removed with Gates Glidden bur, and the canal walls were cleaned with NiTi files. The canals were flushed with 10 mL 1.0% NaOCl followed by 3 mL normal physiological saline, and dried with sterile paper points.
At the end of the experiment, the root surfaces of the instrumented and root canal-filled groups were washed with 5.25% NaOCl followed by sterile saline. The roots were split into two halves, similar to the control groups. The infection procedure in the positive group was followed for the instrumented and retreated root canal groups. On the last day of the fourth week, half of the teeth in the positive control, the instrumented, and root canal-filled and retreated groups were fixed in 2.5% phosphate-buffered glutaraldehyde solution for 9 days.
SEM preparation
After dehydration through an ascending alcohol series, the specimens were sputter-coated with gold to a thickness of 200 nm. The presence of microorganisms in the root canal walls and the adjacent dentinal tubules were examined using an SEM (JSM-6360LV, JEOL Ltd, Tokyo, Japan) at an accelerating voltage of 10 kV. The maximum depth of microorganism penetration into dentinal tubules of all samples was measured using the Smile view software version 2.03 (JEOL Ltd). The evaluation is based on the nature of dentin surface and the status of dentinal tubules. This research is approved by the College of Dentistry Research Center (FR-0006).
Results
Presence of E. faecalis and C. albicans through the tubules was observed in all the samples except the negative control. The penetration of the dentinal tubules was by way of cell division for E. faecalis. C. albicans builds colonies along the dentin surface of the root canal and the penetration was by hyphae. Infection was extensive in most of the tubules.
Negative control
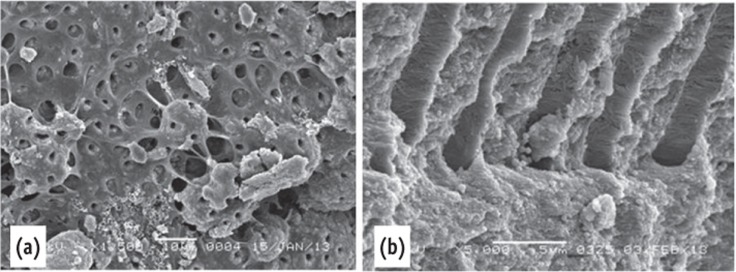
The negative control specimens showed the normal appearance of open dentinal tubules, dentin structure, pulp tissue and collagen fibers (Figure 2). No microorganisms were seen in the canal walls.
Figure 2.
Scanning electron microscope images of the negative control. (a) The dentin surface and the dentinal tubules were covered by pulp tissue; (b) Collagen fibers were seen inside the dentinal tubules.
Positive control
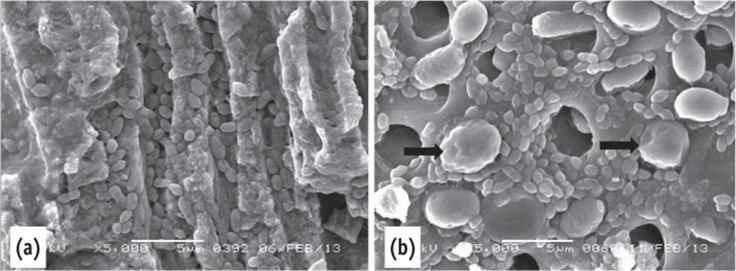
Aggregation of bacteria were observed in all areas of the canal walls. Most of the dentinal tubules were occluded by microorganisms. The size of E. faecalis is very small compared to the diameter of the dentinal tubules, allowing them to penetrate deeply (Figure 3a). The size of C. albicans is larger than E. faecalis and roughly similar to the opening of the dentinal tubules (Figure 3b). Both microorganisms were seen on the dentin surface together.
Figure 3.
Scanning electron microscope images of the positive control. (a) Deep penetration of E. faecalis into dentinal tubules. Note the size of the E. faecalis and the C. albicans; (b) The C. albicans blocked the opening of the dentinal tubules (arrow) mixed microorganisms.
Instrumented root canals
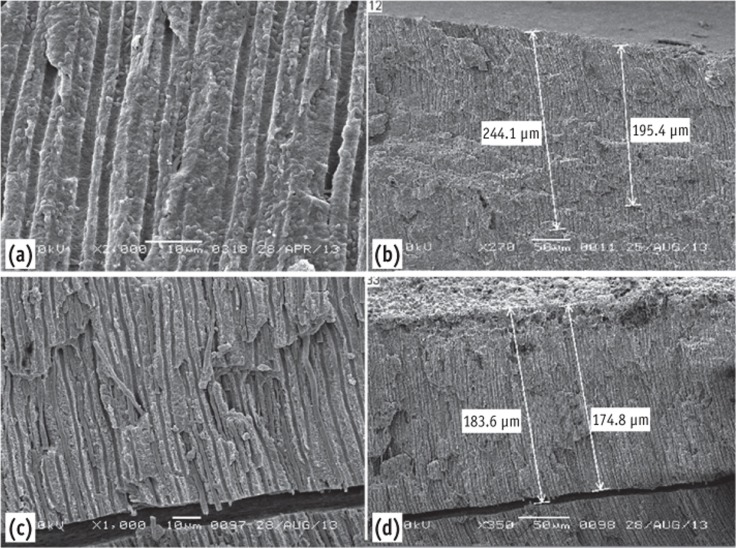
The dentin surface and dentinal tubules of the instrumented non-infected samples were clearly seen in the coronal third. Remnants of pulp tissue and dentin debris were seen at the middle and apical third of the dentin surface, covering some of the openings of the dentinal tubules. Both microorganisms were seen on the instrumented dentin surface and inside the dentinal tubules. E. faecalis penetrated deeper (up to 244 µm) into the dentinal tubules (Figures 4a and 4b), while C. albicans penetrated up to 184 µm (Figures 4c and 4d).
Figure 4.
(a) Scanning electron microscope images of the instrumented group showing E. faecalis inside the dentinal tubules; (b) It can be traced up to 244 µm - low magnification; (c) C. albicans inside the dentinal tubules; (d) Penetrated up to 184 µm.
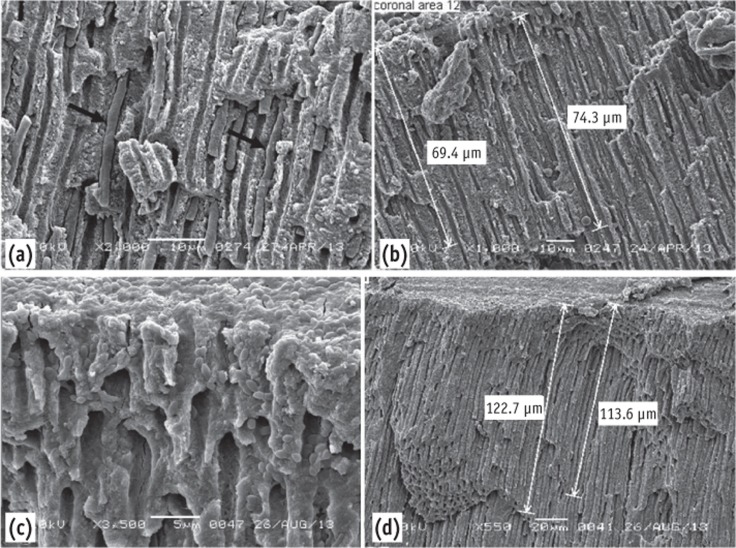
Re-treated root canals
Remnants of root canal-filling materials were seen blocking the openings of a few dentinal tubules. Both microorganisms in aggregation were seen on the re-treated (re-instrumented) dentin surface. C. albicans penetrated the dentinal tubules up to 74 µm (Figures 5a and 5b) and E. faecalis up to 123 µm (Figures 5c and 5d).
Figure 5.
Scanning electron microscope images of the re-treated group. (a) Note the depth of penetration of the dentinal tubules by C. albicans; (b) It can be traced up to 74 µm; (c) E. faecalis in dentinal tubules; (d) Penetrated up to 123 µm.
Discussion
Endodontic infections have been found to be polymicrobial.14,15 Their presence in the root canal system contributes to persistent clinical signs and symptoms. C. albicans and E. faecalis are common resistant species isolated from failed root canal treated teeth with persistent periapical lesions.16,17 Kim et al. suggested the ability of the E. faecalis to suppress IL-2 and IL-4 production by lymphocytes to be one of the factors.18 C. albicans is usually associated with bacteria, especially E. faecalis.19 The selection of these pathogens as test organisms was because of their clinical importance and extensive use in previous studies.2,20 Additionally, E. faecalis is usually viable at high pH levels, and is capable of invading dentinal tubules and surviving as a single species, where it adheres to collagen in the presence of human serum.21,22 Penetration of E. faecalis into dentinal tubules was seen in all infected samples, as far as 244 µm. These observations were similar to previous studies.11,12 This bacterial invasion of dentinal tubules by E. faecalis is an active process mediated by cell division.23
C. albicans adheres to various surfaces and has a particular affinity for dentin and the smear layer.24 It uses dentin as a nutrient source, due to its collagenolytic activity.25 The SEM analysis showed that C. albicans builds colonies along the dentin surface of the root canal walls and penetrates the dentinal tubules with its hyphae only. It was noticed that the hyphae of C. albicans in the present study could spread inside the dentinal tubule as far as 183 µm. This observation contrasts with other reports of poor penetration of dentinal tubules by C. albicans, even though there was heavy colonization on the dentin surfaces in those studies.24,26,27,28 These differences could be attributable to the different experimental designs. Bacterial penetration into dentinal tubules was confirmed after 4-weeks incubation period in the present study. The incubation period was similar to that used by Haapasalo and Orstavik, and shorter than 60 days used by Gurgel-Filho et al.3,12 Growth of the 2 microorganisms was seldom seen in one tubule. This could be due to their size differences.
Remnants of dentin debris and root canal filling materials, and uninstrumented surfaces were noted in the instrumented and root canal-filled groups. NiTi rotary files were used to prepare and re-treat the root canal filling. According to Jeon et al. and Hülsmann et al., complete cleaning of all root canal walls and removing root canal filling materials cannot be achieved by most rotary and hand instrumentation techniques.29,30 This usually leaves areas of the canal wall uninstrumented, which will alter the penetration of the dentinal tubules by the microorganisms. Inevitably, because the canal is not instrumented in these regions, infected inner layer of dentin will remain. The use of RC-Prep and NaOCl irrigation in the instrumented group removed pulp tissue from the dentin surface, the dentinal tubules contents, and the smear layer, allowing them to be more readily penetrated by the microorganisms versus the uncleaned dentin surface (positive group) or the surface with remnants of filling materials (root canal-filled group). These observations are similar to those of Sen et al.24 This contradict with Biesterfeld & Taintor who reported that the wax in the RC-Prep material that was left on the root canal walls was hard to remove despite further instrumentation and irrigation.31 The smear layer of the teeth that were root-filled and then had the root fillings removed was not removed. This did not alter the penetration of the tested microorganisms into the dentinal tubules. This observation is similar to the observation by Meryon and Brook.32
The ability of E. faecalis and C. albicans to migrate deeply inside the dentinal tubules was clearly demonstrated in the current SEM study. The extent of the microorganism migration was similar to that of previous studies.33,34 It was noticed that the depth of migration is dependent on incubation time, the nature of the dentin surface, and the status of the dentinal tubules. This was observed in the current study, where the presence of remnant root canal-filling materials in some areas affected the ability of C. albicans and E. faecalis cells to penetrate the dentinal tubules. In addition, variation on penetration depth could be attributed to the orientation of the dentinal tubules.34
Conclusions
Microorganisms can easily penetrate dentinal tubules of root canals with different appearance based on the microorganism size and dentinal tubules status.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Akpata ES, Blechman H. Bacterial invasion of pulpal dentin wall in vitro. J Dent Res. 1982;61:435–438. doi: 10.1177/00220345820610021401. [DOI] [PubMed] [Google Scholar]
- 3.Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–1379. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 4.Ricucci D, Siqueira JF., Jr Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. J Endod. 2010;36:1–15. doi: 10.1016/j.joen.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Engström B, Lundberg M. The correlation between positive culture and the prognosis of root canal therapy after pulpectomy. Odontol Revy. 1965;16:193–203. [PubMed] [Google Scholar]
- 6.Nair PN, Sjögren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscope follow-up study. J Endod. 1990;16:580–588. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 7.Sen BH, Piskin B, Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol. 1995;11:6–9. doi: 10.1111/j.1600-9657.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 8.Armitage GC, Ryder MI, Wilcox SE. Cemental changes in teeth with heavily infected root canals. J Endod. 1983;9:127–130. doi: 10.1016/S0099-2399(83)80030-2. [DOI] [PubMed] [Google Scholar]
- 9.Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressing of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6:142–149. doi: 10.1111/j.1600-9657.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 10.Valera MC, de Moraes Rego J, Jorge AO. Effect of sodium hypochlorite and five intracanal medications on Candida albicans in root canals. J Endod. 2001;27:401–403. doi: 10.1097/00004770-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Saleh IM, Ruyter IE, Haapasalo M, Ørstavik D. Survival of Enterococcus faecalis in infected dentinal tubules after root canal filling with different root canal sealers in vitro. Int Endod J. 2004;37:193–198. doi: 10.1111/j.0143-2885.2004.00785.x. [DOI] [PubMed] [Google Scholar]
- 12.Gurgel-Filho ED, Vivacqua-Gomes N, Gomes BP, Ferraz CC, Zaia AA, Souza-Filho FJ. In vitro evaluation of the effectiveness of the chemomechanical preparation against Enterococcus faecalis after single- or multiple-visit root canal treatment. Braz Oral Res. 2007;21:308–313. doi: 10.1590/s1806-83242007000400005. [DOI] [PubMed] [Google Scholar]
- 13.Michelich VJ, Schuster GS, Pashley DH. Bacterial Penetration of human dentin in vitro. J Dent Res. 1980;59:1398–1403. doi: 10.1177/00220345800590080701. [DOI] [PubMed] [Google Scholar]
- 14.Siqueira JF., Jr Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34:1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee YJ, Kim MK, Hwang HK, Kook JK. Isolation and identification of bacteria from the root canal of the teeth diagnosed as the acute pulpitis and acute periapical abscess. J Korean Acad Conserv Dent. 2005;30:409–422. [Google Scholar]
- 16.Siqueira JF, Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008;34:1291–1301. doi: 10.1016/j.joen.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 17.Poptani B, Sharaff M, Archana G, Parekh V. Detection of Enterococcus faecalis and Candida albicans in previously root-filled teeth in a population of Gujarat with polymerase chain reaction. Contemp Clin Dent. 2013;4:62–66. doi: 10.4103/0976-237X.111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Jang SW, Shon WJ, Lee ST, Kim CH, Lee WC, Lim SS. Effects of Enterococcus faecalis sonicated extracts on IL-2, IL-4 and TGF-β1 production from human lymphocytes. J Korean Acad Conserv Dent. 2005;30:1–6. [Google Scholar]
- 19.Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J. 2001;34:429–434. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 20.Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- 21.Kim HJ, Park SH, Cho KM, Kim JW. Evaluation of time-dependent antimicrobial effect of sodium dichloroisocyanurate (NaDCC) on Enterococcus faecalis in the root canal. J Korean Acad Conserv Dent. 2007;32:121–129. [Google Scholar]
- 22.Love RM. Enterococcus faecalis-a mechanism for its role in endodontic failure. Int Endod J. 2001;34:399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 23.Zapata RO, Bramante CM, de Moraes IG, Bernardineli N, Gasparoto TH, Graeff MS, Campanelli AP, Garcia RB. Confocal laser scanning microscopy is appropriate to detect viability of Enterococcus faecalis in infected dentin. J Endod. 2008;34:1198–1201. doi: 10.1016/j.joen.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Sen BH, Safavi KE, Spångberg LS. Growth patterns of Candida albicans in relation to radicular dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:68–73. doi: 10.1016/s1079-2104(97)90298-5. [DOI] [PubMed] [Google Scholar]
- 25.Hagihara Y, Kaminishi H, Cho T, Tanaka M, Kaita H. Degradation of human dentine collagen by an enzyme produced by the yeast Candida albicans. Arch Oral Biol. 1988;33:617–619. doi: 10.1016/0003-9969(88)90138-0. [DOI] [PubMed] [Google Scholar]
- 26.Sen BH, Safavi KE, Spångberg LS. Colonisation of Candida albicans on cleaned human dental hard tissues. Arch Oral Biol. 1997;42:513–520. doi: 10.1016/s0003-9969(97)00026-5. [DOI] [PubMed] [Google Scholar]
- 27.Waltimo TM, Ørstavik D, Sirén EK, Haapasalo MP. In vitro yeast infection of human dentin. J Endod. 2000;26:207–209. doi: 10.1097/00004770-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Siqueira JF, Jr, Rôças IN, Lopes HP, Elias CN, de Uzeda M. Fungal infection of the radicular dentin. J Endod. 2002;28:770–773. doi: 10.1097/00004770-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Jeon IS, Kum KY, Park SH, Yoon TC. Scanning electron microscopic study on the efficacy of root canal wall debridement of rotary Ni-Ti instruments with different cutting angle. J Korean Acad Conserv Dent. 2002;27:577–586. [Google Scholar]
- 30.Hülsmann M, Schade M, Schäfers F. A comparative study of root canal preparation with HERO 642 and Quantec SC rotary Ni-Ti instruments. Int Endod J. 2001;34:538–546. doi: 10.1046/j.1365-2591.2001.00431.x. [DOI] [PubMed] [Google Scholar]
- 31.Biesterfeld RC, Taintor JF. A comparison of periapical seals of root canals with RC-Prep or Salvizol. Oral Surg Oral Med Oral Path. 1980;49:532–537. doi: 10.1016/0030-4220(80)90079-1. [DOI] [PubMed] [Google Scholar]
- 32.Meryon SD, Brook AM. Penetration of dentine by three oral bacteria in vitro and their associated cytotoxicity. Int Endod J. 1990;23:196–202. doi: 10.1111/j.1365-2591.1990.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 33.Ando N, Hoshino E. Predominant obligate anaerobes invading the deep layers of root canal dentine. Int Endod J. 1990;23:20–27. doi: 10.1111/j.1365-2591.1990.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 34.Peters LB, Wesselink PR, Buijs JF, van Winkelhoff AJ. Viable bacteria in root dentinal tubules of teeth with apical periodontitis. J Endod. 2001;27:76–81. doi: 10.1097/00004770-200102000-00002. [DOI] [PubMed] [Google Scholar]