Abstract
Introduction
The aim of the study was to detect the effect of laparoscopic greater curvature plication (LGCP) on peripheral blood lymphocyte subsets (helper and cytotoxic T lymphocytes – CD4+ and CD8+ T cells respectively), leptin level and weight loss in morbidly obese patients.
Material and methods
Morbidly obese patients (n = 20, age range: 25–50 years, body mass index (BMI) range: 37–45 kg/m2) who underwent LGCP were enrolled in a prospective study to determine the percentages of their peripheral blood T cells (CD4+ and CD8+) before and 4 months postoperatively using flow cytometry. Also, the level of their leptin before and 4 months postoperatively was established using enzyme-linked immunosorbent assay (ELISA). The data are expressed as the percentage of total lymphocytes ± the standard error of the mean.
Results
A decrease in the BMI and loss of weight (31.20 ±1.2%) were confirmed 4 months postoperatively since BMI was 44.71 ±4.3 (range: 37–45) kg/m2 preoperatively, and decreased to 31.80 ±1.1 (range: 24–33) kg/m2 after surgery. The mean percentage of CD4+ and CD8+ T lymphocytes significantly decreased postoperatively (38.2 ±1.5 before and 29.3 ±2.6 after operation for CD4+, 17.3 ±1.8 preoperatively and 9.5 ±1.7 postoperatively for CD8+, p < 0.05). The mean leptin level was 43.01 ±22.01 preoperatively while postoperatively it was 24.8 ±11.1 (p < 0.05), so the leptin level substantially decreased compared to its preoperative values.
Conclusions
This study found that weight loss after LGCP in morbidly obese patients led to decreases in levels of leptin and circulating immune cells compared to their preoperative values.
Keywords: bariatric surgery, laparoscopic greater curvature plication, morbid obesity, immunity, leptin
Introduction
Obesity is a worldwide health problem with several comorbidities including respiratory diseases, cardiovascular diseases, gallstones, osteoarthritis and reproductive disorders [1]. Furthermore, obesity is associated with decreased immunocompetence [2]. Several studies have revealed an increase in the incidence of infections and many types of cancer in obese individuals [3, 4].
Bariatric surgery is the only effective treatment for morbid obesity. It can also improve the obesity-related disorders [5], and is associated with a decrease in overall mortality [6, 7]. Since 2006, the LGCP technique had been evaluated to eliminate adjustable gastric band (AGB) and vertical sleeve gastrectomy (VSG) associated complications by restriction without gastric stapling resection or using an implant.
In obesity, low grade inflammation is the product of the activated innate immune system, with activated tissue based innate immunity and circulating immune cells [8, 9]. Moreover, morbid obesity is characterized by infiltration of adipose tissue with macrophages [9]. Recent studies have revealed the presence of multiple leucocyte subsets such as mast cells and T-cells that are present in adipose tissue and regulate inflammation [10–13].
CD4+ and CD8+ T lymphocytes are types of white blood cells known as T cells which have surface markers known as CD4 and CD8 respectively. CD4 cells are commonly known as T-helper cells as they help to detect and fight off bacterial and viral infection while CD8 cells detect and try to fight off infections caused by viruses or diseases such as cancer and are known as cytotoxic T cells [14]. Controversial outcomes were recorded for immunological parameters including the level of immune cells in obesity [15, 16]. Recently, it was found that obesity reduces thymopoiesis and eases immune surveillance [17].
It was proved that bariatric procedures result in short- and long-term weight loss in response to postoperative neurohormonal alterations [18]. Leptin is an adipocyte-derived hormone that is produced in proportion to the body fat. Plasma leptin concentration is an indicator of the total amount of fat present in the body, and lower plasma leptin levels have been reported consistently among weight-losing patients [19, 20]. Recent studies confirmed that leptin has a direct impact on the immune system [21].
Material and methods
This prospective study included 20 patients who underwent LGCP to assess the effect of this operation on excess weight loss, leptin level as well as on the percentages of their peripheral blood T cells (CD4+ and CD8+ T cells) before and four months postoperatively using flow cytometry.
Surgical procedures were carried out on 20 patients from June 2010 to July 2012 in El Fayoum University Hospital using the National Institute of Health's (NIH) inclusion criteria for bariatric surgery (patients with BMI > 40 kg/m2 or over 35 kg/m2 with at least one comorbidity) and an informed consent from all cases were taken. The study included 20 patients: 15 (75%) females aged 25–46 years and 5 (25%) males aged 38–50 years who were considered clinically obese with a mean BMI 44.71 ±4.3 (37–45) kg/m2, mean age 39.5 ±9.5 (25–50) years. The body weight for all patients was stable for 4 months prior to the study.
Patients had full history taking, especially for family history of similar condition, BMI, age, social habits of smoking, alcohol consumption, present medical history of any drug intake especially steroids, salicylic acid and non-steroidal anti-inflammatory drugs (NSAIDs), etc. Also, their previous history of any deep venous thrombosis (DVT), any postsurgical morbidities in the abdomen, or any current clinical disease in the abdomen (e.g. hernia, postsurgical scarring, etc) was followed.
The results of their preoperative laboratory tests including complete blood count (CBC), blood sugar, T3, T4, thyroid stimulating hormone (TSH), liver as well as kidney functions, their coagulation profile and of their preoperative abdominopelvic ultrasound were obtained. Upper gastrointestinal tract (GIT) endoscopy was done preoperatively for all patients to exclude gastritis. Moreover, pulmonary function tests and ECG followed by anesthetic consultation were carried out for all patients before surgery. Fifteen of the patients were smokers, with no alcohol consumption. Their imaging studies revealed non-cancerous abdominopelvic ultrasound and chest X-ray. Additionally, their preoperative laboratory tests revealed mild anemia in 6 patients. We excluded patients who showed unfitness for general anesthesia and major abdominal surgery, patients who were sweet or alcohol addicts and those with end stage obesity comorbid diseases such as advanced diabetes or advanced atherosclerosis. Also, patients with psychological instability, with fear of operations, drug addiction and those with lack of motivation for weight loss were excluded.
Surgical procedure
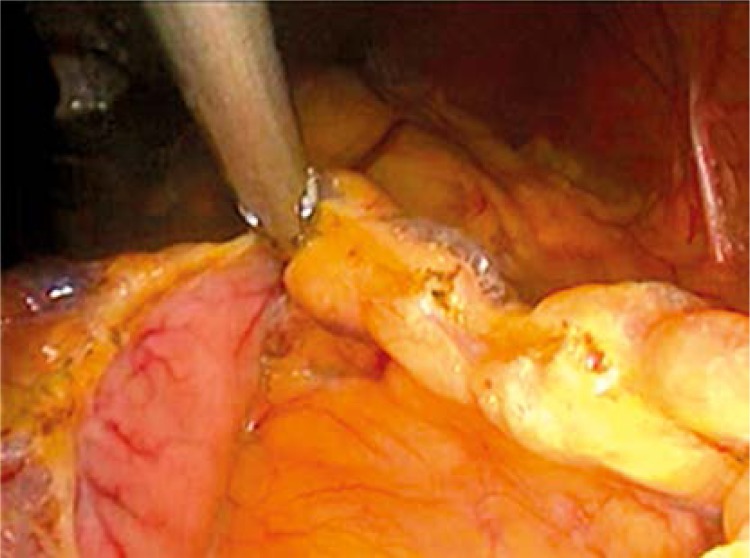
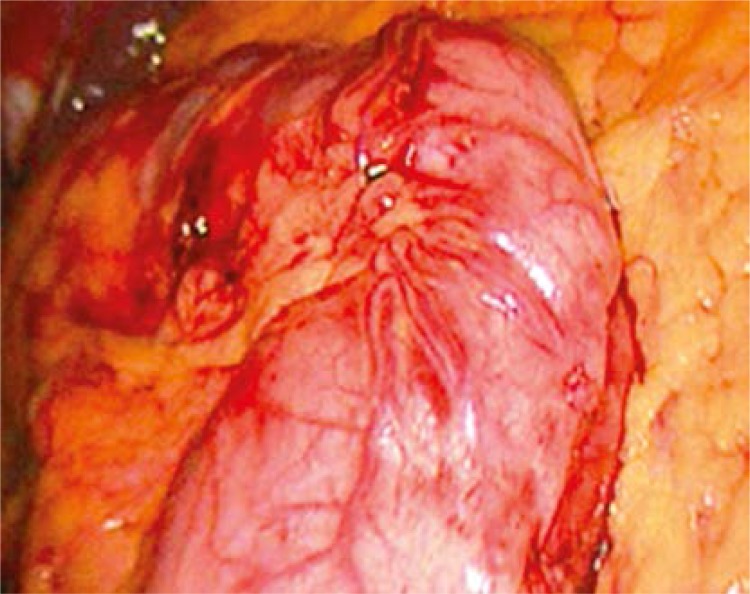
The LGCP procedure was done under general anesthesia, and was started by division of the greater curvature blood supply using the Harmonic scalpel distally until the pylorus and then proximally until the angle of His. Then the stomach was folded into itself over a 32-Fr bougie, applying a first row of extramucosal stitches of 2-0 vicryl. This row guided another one created with extramucosal running suture lines of 2-0 prolene. Methylene blue was injected intraoperatively to check for leakage. The patients started eating on the 10th day postoperatively and the follow-up was carried out (Figures 1 and 2).
Figure 1.
Division of the vascular supply of the greater curvature of the stomach
Figure 2.
Plicated stomach
Immune cell preparation and flow cytometry analysis
Whole blood was collected from all chosen individuals before and following the surgical procedure in acid citrate dextrose (ACD). Simultaneous collections were made in tubes containing potassium-EDTA for total lymphocyte count and allowed determination of absolute counts. Evaluation of CD4 and CD8 lymphocytes was performed by three-color immunofluorescence flow cytometry using a monoclonal antibody (MAb) panel for the desired cell surface proteins including fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated MAb to CD4 and CD8 (Beckman Coulter Electronics, Hialeah, FL). All antibodies were prepared according to the manufacturer's directions and then were incubated with whole blood for 5 min at 25°C before red cell lysis and fixation using Immunoprep reagents and Q-prep equipment (Beckman Coulter), as directed by the manufacturer. Cells were then stored at 4°C for up to 24 h before analysis by a flow cytometer (EPICS XL; Beckman Coulter). The data were expressed as the percentage of total lymphocytes ± the standard error of the mean.
Leptin level and ELISA
Serum samples were collected before and after surgery and stored at –20°C. Leptin concentration was measured in serum using a commercially available ELISA kit from Millipore, CA. according to the manufacturer's instructions.
Statistical analysis
Differences between means and the effects of treatments were determined by one-way ANOVA using Tukey's test. Value of p < 0.05 was considered statistically significant.
Results
The mean operative time was 65.6 ±10.2 (60–120 min); there were no conversions. Postoperative complications were restricted to 5% in the form of nausea and vomiting and were treated within 12 days, but there no intraoperative complications. Mean hospital stay was 8.2 ±3.5 (range: 7–12) days, loss of weight was 31.20 ±1.2%, preoperative BMI was 44.71 ±4.3 (range: 37–45) kg/m2 while postoperatively it was 31.80 ±1.1 (range: 24–33) kg/m2. The mean percentage of CD4+ T lymphocytes was 38.2 ±1.5 preoperatively and 29.3 ±2.6 after the operation, p < 0.05. So, there was a postoperative decrease in the percentage of CD4+ T lymphocytes. Regarding CD8+ T, the mean percentage was 17.3 ±1.8 and 9.5 ±1.7 pre- and postsurgically, respectively, p < 0.05. This in turn indicates that there was a postoperative decrease in the percentage of CD8+ T lymphocytes (Table I). The mean leptin level was 43.01 ±22.01 preoperatively and decreased to 24.80 ±11.10 postoperatively, p < 0.05 (Table I).
Table I.
Patients’ characteristics
| Data | Result | Value of p |
|---|---|---|
| Age [years] | 39.5 ±9.5 | |
| Gender: | ||
| Females | 15 (75%) | |
| Males | 5 (25%) | |
| BMI (preoperative) [kg/m2] | 44.71 ±4.3 | |
| BMI (postoperative) [kg/m2] | 31.80 ±1.1 | |
| Weight loss [%] | 31.20 ±1.2 | |
| Operative time [min] | 65.6 ±10.2 | |
| Hospital stay [days] | 8.2 ±3.5 | |
| CD4+ T lymphocytes percentage (preoperatively) | 38.2 ±1.5 | < 0.05 |
| CD4+ T lymphocytes percentage (postoperatively) | 29.3 ±2.6 | |
| CD8+T lymphocytes percentage (preoperatively) | 17.3 ±1.8 | < 0.05 |
| CD8+T lymphocytes percentage (postoperatively) | 9.5 ±1.7 | |
| Leptin level (preoperatively) [ng/ml] | 43.01 ±22.01 | < 0.05 |
| Leptin level (postoperatively) [ng/ml] | 24.80 ±11.10 |
Data are expressed as mean values ± SD (standard deviation). Body mass index (BMI), helper T lymphocyte (CD4+) and cytotoxic T lymphocytes (CD8+)
Discussion
In this study, LGCP showed satisfactory weight loss, which is consistent with other findings [22]. Moreover, there were no major complications, contrasting with other surgical procedures which showed leakage and bleeding [22, 23].
This study of morbidly obese patients showed that dietary energy restriction and weight loss due to surgery reduced the percentages of CD4+ and CD8+ T cells, p < 0.05. This is considered statistically significant compared to other studies which did not a show substantial change in these peripheral blood T cells’ subsets [24]; this might be due to the more efficient impact of this type of bariatric surgery on the circulating immune cells. Consequently, this study observed decreased CD4+ and CD8+ T lymphocytes and suggests that this might lead to attenuated activation of circulating immune cells after weight loss by LGCP. Limitations of this study include lack of a control group undergoing weight loss without surgery (with diet and exercise).
Data on the effect of weight loss after bariatric surgery on inflammation and immune cells are emerging, suggesting the reduction in the circulating inflammatory markers [25]; another study also showed that immune cell activation can be regulated by acute energy restriction [26]. The mechanism by which weight loss and energy restriction in obesity ameliorate proinflammatory activation of immune cells is unclear.
Plasma leptin level rises upon deposition of fat and stimulates hypothalamic leptin receptors [27]. The obtained results demonstrated a significant reduction of leptin level postoperatively, which is recorded in gastric bypass surgery [28]. The normalization of leptin levels, which may reflect a surgically induced improvement of leptin sensitivity, may play a role, directly or indirectly, in the induction of weight loss after surgery.
Additionally, decreased CD4+ and CD8+ T cells might be due to the reduced leptin level, which was proved to be the key element linking nutritional status with T-cell function [2]. It has been reported that leptin can affect the immune cells’ proliferation through modulating the release of pro-inflammatory cytokines [29–31] and acute leptin reduction decreased lymphocyte numbers in a fasted mouse model [32, 33]. Furthermore, it was recorded that leptin can regulate the inflammatory response through affecting leptin receptors, which has been detected in human CD4+ and CD8+ T cells [34, 35].
The obesity-related white adipose tissue (WAT) inflammation was formerly thought to be a totally macrophage-dependent phenomenon which leads to insulin resistance. Recently, there is evidence for the vital role of lymphocytes, especially T cells, in the WAT, for the development of insulin resistance. It has been reported that CD8+ cells are the main T cell subset in the visceral WAT of obese humans and mice [11, 36, 37], suggesting that this cell type may collaborate with macrophages and contribute to the inflammation of the obese WAT. It was shown that CD4+ T cells may also control insulin sensitivity [12], which was supported by an increased number of activated CD4+ cells in morbidly obese humans [38]. Leptin receptors were found on T cells. So, leptin can influence T cell activation [39]. Thus, the interrelation between CD4, CD8 T cells and leptin may exert an impact on WAT inflammation and insulin resistance that might be ameliorated by LGCP, as shown in the current study.
In conclusion, LGCP-induced weight loss in morbidly obese patients led to a significant reduction of leptin level with a subsequent decrease in the percentage of CD4+ T and CD8+ T cells, which may lead to greater regulation of chronic inflammation in morbidly obese patients.
Acknowledgments
We thank the patients and their parents for their cooperation.
References
- 1.World Health Organization (WHO) Report of a WHO consultation on obesity. Geneva, Switzerland: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 2.Martí A, Marcos A, Martínez JA. Obesity and immune function relationships. Obes Rev. 2001;2:131–40. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–40. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 5.Choban PS, Jackson B, Poplawski S, Bistolarides P. Bariatric surgery for morbid obesity: why, who, when, how, where, and then what? Cleve Clin J Med. 2002;69:897–903. doi: 10.3949/ccjm.69.11.897. [DOI] [PubMed] [Google Scholar]
- 6.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 7.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 8.van Oostrom AJ, van Wijk JP, Sijmonsma TP, Rabelink TJ, Castro Cabezas M. Increased expression of activation markers on monocytes and neutrophils in type 2 diabetes. Neth J Med. 2004;62:320–5. [PubMed] [Google Scholar]
- 9.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–5. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 12.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janeway CA, Travers M, Walport M, Shalomchik MJ. Immunobiology: the immune system in health and disease. 6th ed. New York: Garland Science Publishing Incl.; [Google Scholar]
- 15.O'Rourke RW, Kay T, Scholz MH, et al. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes Surg. 2005;15:1463–8. doi: 10.1381/096089205774859308. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Isoda F, Ishihara Y, Kimura M, Yamakawa Y. T lymphopaenia in relation to body mass indexand TNF-alpha in human obesity: adequate weight reduction can be corrective. Clin Endocrinol. 2001;54:347–54. [PubMed] [Google Scholar]
- 17.Yang H, Youm YH, Vandanmagsar B, et al. Obesity accelerates thymic aging. Blood. 2009;114:3803–12. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calcaterra V, Cena H, Nakib G, et al. Robotic-assisted gastroplication in a morbidly obese adolescent: early improvement in metabolic and neurohormonal parameters. Pediatr Rep. 2012;4:e36. doi: 10.4081/pr.2012.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace AM, Sattar N, McMillan DC. Effect of weight loss and the inflammatory response on leptin concentrations in gastrointestinal cancer patients. Clin Cancer Res. 1998;4:2977–9. [PubMed] [Google Scholar]
- 20.SchSchols AM, Creutzberg EC, Buurman WA, et al. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1220–6. doi: 10.1164/ajrccm.160.4.9811033. [DOI] [PubMed] [Google Scholar]
- 21.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–42. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 22.Talebpour M, Amoli BS. Laparoscopic total gastric vertical plication in morbid obesity. J Laparoendosc Adv Surg Tech A. 2007;17:793–8. doi: 10.1089/lap.2006.0128. [DOI] [PubMed] [Google Scholar]
- 23.Andraos Y, Ziade D, Achcouty D, Awad M. Early complications of 120 laproscopic greater curvature plication procedures. Bariatr Times. 2011;8:10–5. [Google Scholar]
- 24.Merhi ZO, Durkin HG, Feldman J, Macura J, Rodriguez C, Minkoff H. Effect of baniatric surgery on peripheral blood lymphocytes subsets in women. Surg Obes Rel Dis. 2009;5:165–71. doi: 10.1016/j.soard.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Kopp HP, Kopp CW, Festa A, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscl Thromb Vasc Biol. 2003;23:1042–7. doi: 10.1161/01.ATV.0000073313.16135.21. [DOI] [PubMed] [Google Scholar]
- 26.Fraser DA, Thoen J, Reseland JE, Førre O, Kjeldsen-Kragh J. Decreased CD4+ lymphocyte activation and increased interleukin-4 production in peripheral blood of rheumatoid arthritis patients after acute starvation. Clin Rheumatol. 1999;18:394–401. doi: 10.1007/s100670050125. [DOI] [PubMed] [Google Scholar]
- 27.Hickey MS, Pories WJ, MacDonald KG, et al. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg. 1998;227:637–44. doi: 10.1097/00000658-199805000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 29.Assals HS, Fath-Allah M, Elsherbiny A. Serum leptin and adiponectin in obese diabetic and non-diabetic. J Med Sci. 2007;7:865–9. [Google Scholar]
- 30.Otero M, Lago R, Gomez C, et al. Towards a proinflammatory and immunomodulatory emerging role of leptin. Rheumatology. 2006;45:944–50. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- 31.Paz-Filho G, Mastronardi C, Franco CB, Wang KB, Wong M, Licinio J. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metab. 2012;56:597–607. doi: 10.1590/s0004-27302012000900001. [DOI] [PubMed] [Google Scholar]
- 32.Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–9. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–71. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 34.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 36.Duffaut C, Zakaroff-Girard A, Bourlier V, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–14. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 37.Koenen TB, Stienstra R, van Tits LJ, et al. The inflammasome and caspase-1 activation: a new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology. 2011;152:3769–78. doi: 10.1210/en.2010-1480. [DOI] [PubMed] [Google Scholar]
- 38.van der Weerd K, Dik WA, Schrijver B, et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2 dominated phenotype. Diabetes. 2012;61:401–8. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55:2583–92. doi: 10.1007/s00125-012-2607-0. [DOI] [PubMed] [Google Scholar]