Abstract
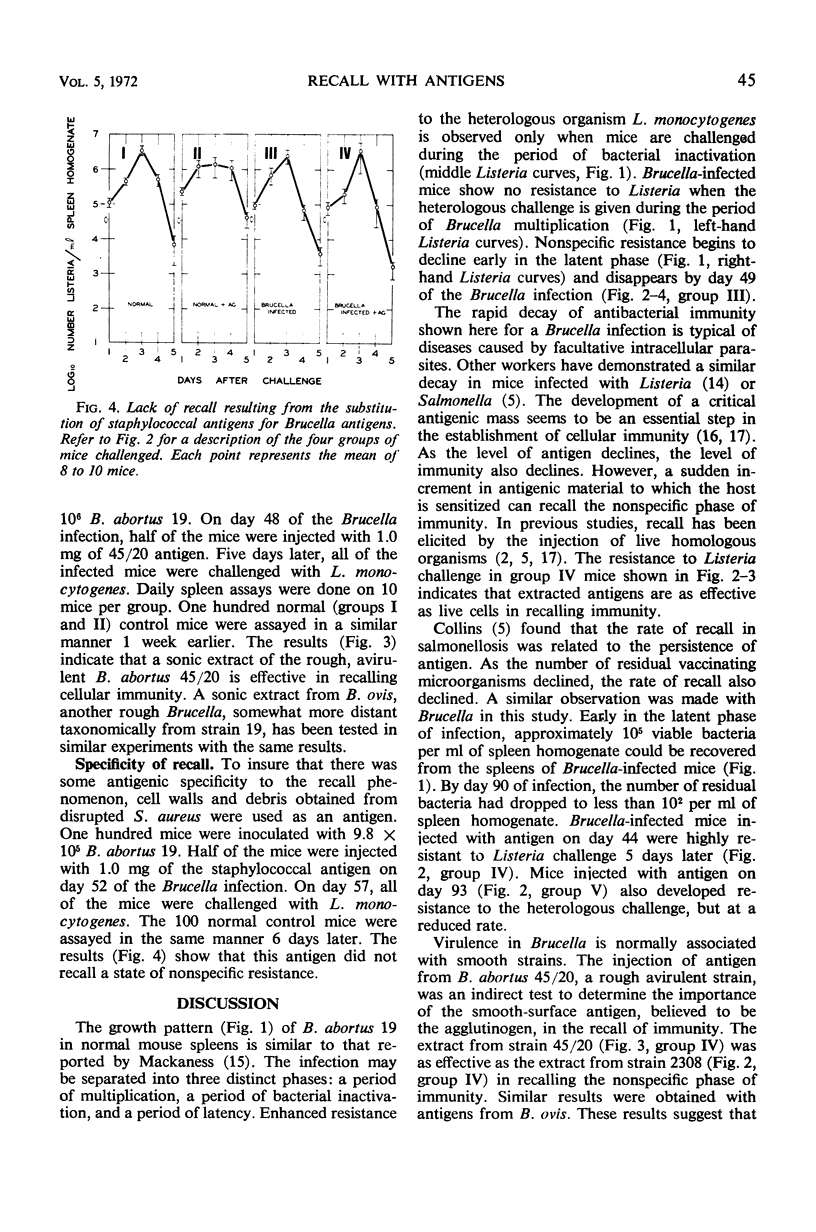
Mice infected with Brucella abortus 19 were challenged intravenously with Listeria monocytogenes. Spleen assays to determine the number of viable Listeria cells present revealed that these mice were highly resistant to Listeria when challenged on day 17 of the Brucella infection. Resistance was absent in mice challenged on the 5th day and was declining in mice challenged on the 33rd day. Resistance could not be detected by day 49 of the Brucella infection but could be recalled by the injection of antigens from smooth B. abortus 2308. Thus, extracted antigens appeared to be as effective in recall as the live cells used in earlier studies. Similar injections of extracts from rough B. abortus 45/20, or from B. ovis REO 198, were also effective in recalling resistance; this suggests that the smooth surface agglutinogen may be relatively unimportant in recall.
Full text
PDF

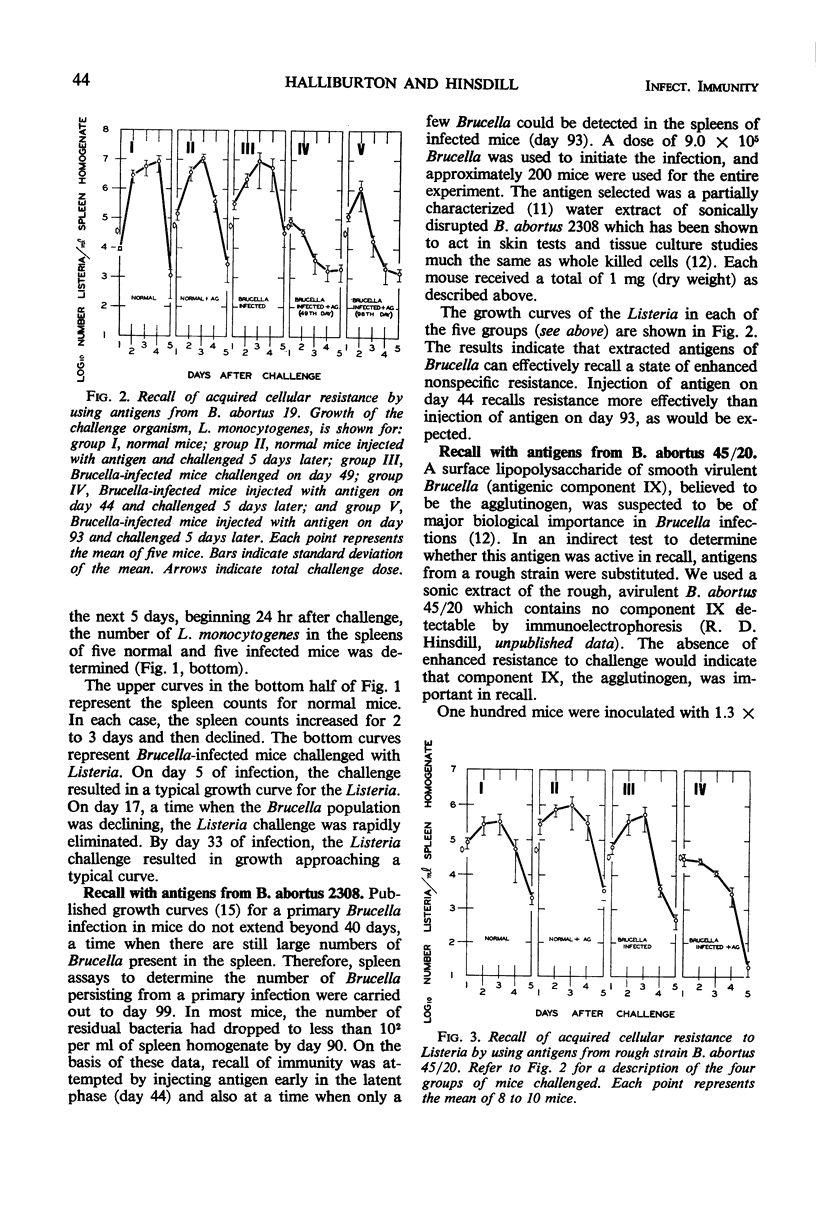


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger F. M., Fukui G. M., Ludwig B. J., Rosselet J. P. Increased host resistance to infection elicited by lipopolysaccharides from Brucella abortus. Proc Soc Exp Biol Med. 1969 Sep;131(4):1376–1381. doi: 10.3181/00379727-131-34111. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Cross-protection against Salmonella enteritidis infection in mice. J Bacteriol. 1968 Apr;95(4):1343–1349. doi: 10.1128/jb.95.4.1343-1349.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Recall of immunity in mice vaccinated with Salmonella enteritidis or Salmonella typhimurium. J Bacteriol. 1968 Jun;95(6):2014–2021. doi: 10.1128/jb.95.6.2014-2021.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Wilson J. B. Antigenic relationship of the gram-negative organism causing canine abortion to smooth and rough brucellae. J Bacteriol. 1968 Feb;95(2):618–624. doi: 10.1128/jb.95.2.618-624.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano V. J., Hinsdill R. D. Characterization of a Staphylococcus aureus bacteriocin. J Bacteriol. 1970 Oct;104(1):117–125. doi: 10.1128/jb.104.1.117-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G. Resistance to infection with Salmonella paratyphi C in mice parasitized with a relatively avirulent strain of Salmonella typhimurium. Nature. 1961 Jul 1;191:87–88. doi: 10.1038/191087a0. [DOI] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. II. Toxicity for macrophages in culture. J Infect Dis. 1968 Jun;118(3):307–316. doi: 10.1093/infdis/118.3.307. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B. The relationship of delayed hypersensitivity to acquired cellular resistance. Br Med Bull. 1967 Jan;23(1):52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- Ralston D. J., Elberg S. S. Sensitization and recall of anti-Brucella immunity in Guinea pig macrophages by attenuated and virulent Brucella. Infect Immun. 1971 Feb;3(2):200–208. doi: 10.1128/iai.3.2.200-208.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Resistance to virus challenge in mice infected with protozoa or bacteria. Proc Soc Exp Biol Med. 1969 Sep;131(4):1184–1188. doi: 10.3181/00379727-131-34066. [DOI] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968 Apr 5;160(3823):72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- Ruskin J., Rengton J. S. Role for the macrophage in acquired immunity to phylogenetically unrelated intracellular organisms. Antimicrob Agents Chemother (Bethesda) 1968;8:474–477. doi: 10.1128/AAC.8.4.474. [DOI] [PubMed] [Google Scholar]
- Senterfitt V. C., Shands J. W. Salmonellosis in Mice Infected with Mycobacterium bovis BCG II. Resistance to Infection. Infect Immun. 1970 Jun;1(6):583–586. doi: 10.1128/iai.1.6.583-586.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE P. G., WILSON J. B. Differentiation of smooth and nonsmooth colonies of Brucellae. J Bacteriol. 1951 Feb;61(2):239–240. doi: 10.1128/jb.61.2.239-240.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]



