Abstract
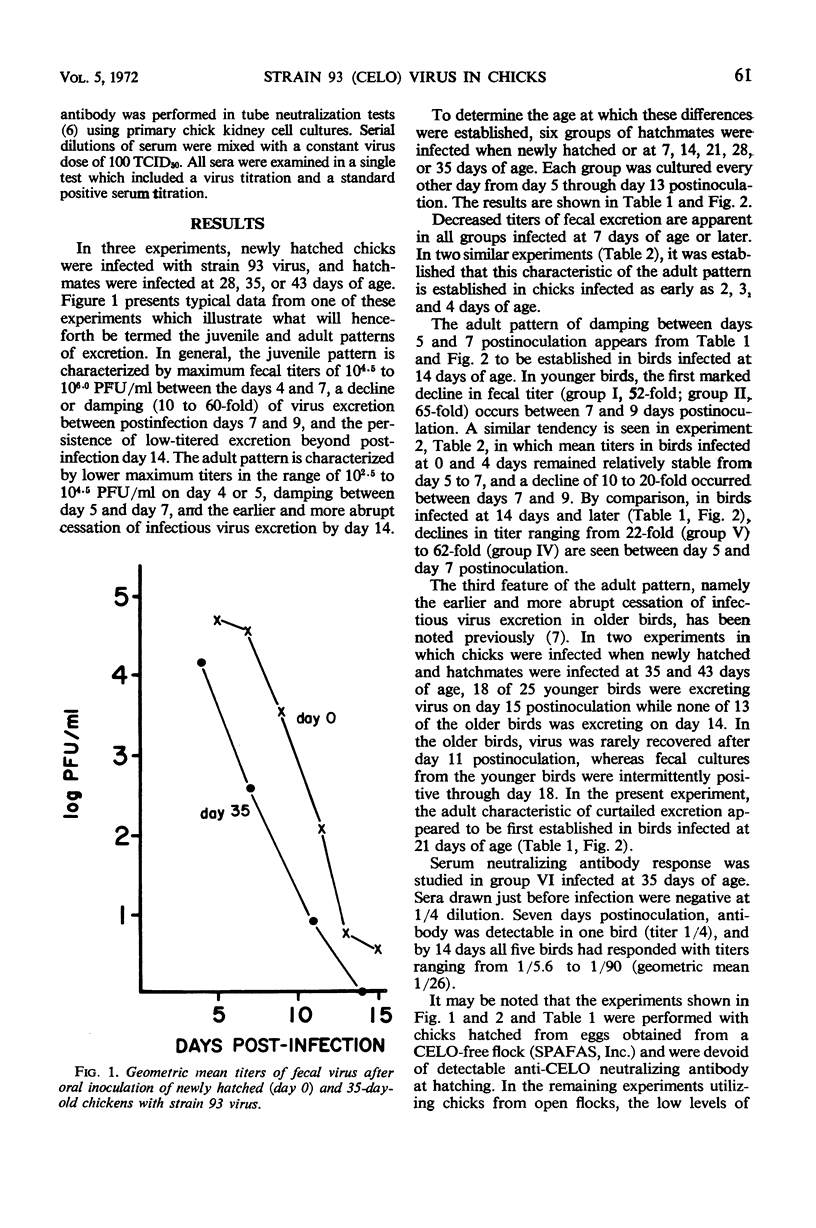
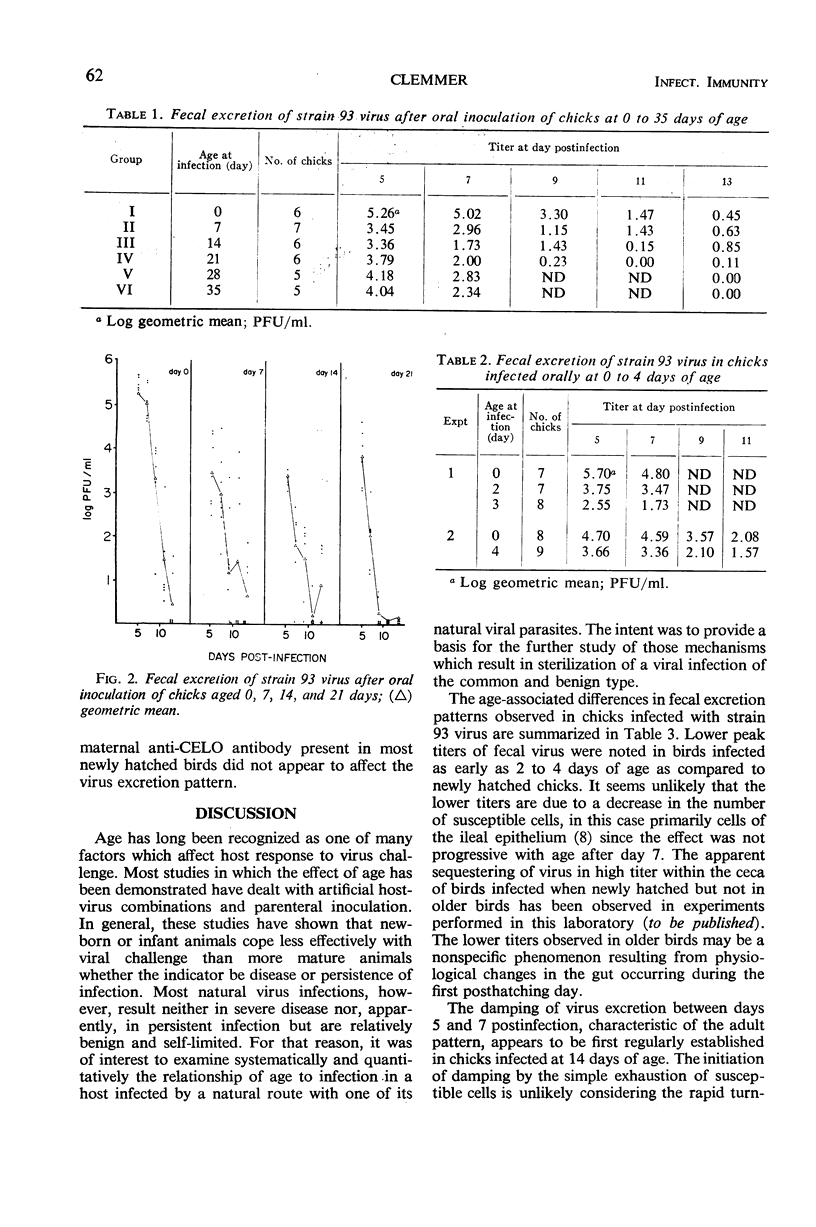
Oral incoulation of chickens with strain 93 (chick embryo lethal orphan) virus produced a subclinical infection of the gastrointestinal tract. The pattern of fecal virus excretion in birds infected at 4 or more weeks of age (adult pattern) differed from that in chicks infected when newly hatched (juvenile pattern). By comparison with the juvenile pattern, the adult pattern was characterized by lower peak titers of fecal virus, earlier decline in virus titer, and shorter duration of excretion. Quantitative studies on hatchmates infected at various ages showed that these characteristics are established sequentially with age: lower peak titers were observed in birds infected at 2 to 4 days of age; early decline in titer was first observed in birds infected at 14 days of age; and curtailed excretion was observed in birds infected at 21 days of age. Possible mechanisms operative in the autosterilization process are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borden E. C., Murphy F. A., Nathanson N., Monath T. P. Effect of antilymphocyte serium on tacaribe virus infection in infant mice. Infect Immun. 1971 Mar;3(3):466–471. doi: 10.1128/iai.3.3.466-471.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke C. N., Luginbuhl R. E., Williams L. F. Avian adeno-like viruses--characterization and comparison of seven isolates. Avian Dis. 1968 Aug;12(3):483–505. [PubMed] [Google Scholar]
- CLEMMER D. I. CHARACTERIZATION OF AGENTS ISOLATED FROM MARKET CHICKENS IN A QUEST FOR ENTERIC VIRUSES. J Infect Dis. 1964 Dec;114:386–400. doi: 10.1093/infdis/114.5.386. [DOI] [PubMed] [Google Scholar]
- CLEMMER D. L. EXPERIMENTAL ENTERIC INFECTION OF CHICKENS WITH AN AVIAN ADENO-VIRUS (STRAIN 93). Proc Soc Exp Biol Med. 1965 Apr;118:943–948. doi: 10.3181/00379727-118-30013. [DOI] [PubMed] [Google Scholar]
- Clarke R. On the constancy of the number of villi in the duodenum of the post-embryonic domestic fowl. J Embryol Exp Morphol. 1967 Feb;17(1):131–138. [PubMed] [Google Scholar]
- Clemmer D. I., Ichinose H. The cellular site of virus replication in the intestine of chicks infected with an avian adenovirus. Arch Gesamte Virusforsch. 1968;25(3):277–287. doi: 10.1007/BF01556556. [DOI] [PubMed] [Google Scholar]
- Cook J. K. Incidence of chick embryo lethal orphan virus antibody in the fowl (Gallus domesticus) in Britain. Res Vet Sci. 1970 Jul;11(4):343–348. [PubMed] [Google Scholar]
- Fox J. P., Brandt C. D., Wassermann F. E., Hall C. E., Spigland I., Kogon A., Elveback L. R. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969 Jan;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- HEINEBERG H., GOLD E., ROBBINS F. C. DIFFERENCES IN INTERFERON CONTENT IN TISSUES OF MICE OF VARIOUS AGES INFECTED WITH COXSACKIE B1 VIRUS. Proc Soc Exp Biol Med. 1964 Apr;115:947–953. doi: 10.3181/00379727-115-29086. [DOI] [PubMed] [Google Scholar]
- Imondi A. R., Bird F. H. The turnover of intestinal epithelium in the chick. Poult Sci. 1966 Jan;45(1):142–147. doi: 10.3382/ps.0450142. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justines G., Johnson K. M. Immune tolerance in Calomys callosus infected with Machupo virus. Nature. 1969 Jun 14;222(5198):1090–1091. doi: 10.1038/2221090a0. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S., MELNICK J. L. Oral administration of Coxsackie viruses to newborn and adult mice. Proc Soc Exp Biol Med. 1951 Feb;76(2):312–315. doi: 10.3181/00379727-76-18475. [DOI] [PubMed] [Google Scholar]
- Keller R., Dwyer J. E. Neutralization of poliovirus by IgA coproantibodies. J Immunol. 1968 Aug;101(2):192–202. [PubMed] [Google Scholar]
- Kincade P. W., Cooper M. D. Development and distribution of immunoglobulin-containing cells in the chicken. An immunofluorescent analysis using purified antibodies to mu, gamma and light chains. J Immunol. 1971 Feb;106(2):371–382. [PubMed] [Google Scholar]
- Mettler N. E., Casals J. Susceptibility of mice aged 0-14 days to infection with Junin virus. Proc Soc Exp Biol Med. 1970 Sep;134(4):1051–1054. doi: 10.3181/00379727-134-34942. [DOI] [PubMed] [Google Scholar]
- Ogra P. L. Distribution of echovirus antibody in serum, nasopharynx, rectum, and spinal fluid after natural infection with echovirus type 6. Infect Immun. 1970 Aug;2(2):150–155. doi: 10.1128/iai.2.2.150-155.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARPLESS G. R. GAL virus. Ann N Y Acad Sci. 1962 Nov 30;101:515–519. doi: 10.1111/j.1749-6632.1962.tb18892.x. [DOI] [PubMed] [Google Scholar]
- SIEGERT R., ENDERS-RUCKLE G., OEHME J., WALLER H. D. DER EINFLUSS DER PRAEVAKZINALEN IMMUNITAET AUF DIE VIRUSAUSSCHEIDUNG UND ANTIKOERPERPRODUKTION NACH POLIO-SCHLUCKIMPFUNG MIT TYP I (SABIN) Dtsch Med Wochenschr. 1963 Aug 16;88:1586–1594. doi: 10.1055/s-0028-1112269. [DOI] [PubMed] [Google Scholar]


