Abstract
MAP kinase modules propagate diverse extracellular signals to downstream effectors. The two dual phosphorylation reactions catalyzed by the modules are thought to control the switch behavior of the pathway. Here we review recent approaches to understand these pathways through signal-to-response studies in cells and in vitro. These data are reconciled with physical models as well as predictions made on mathematical and theoretical grounds. Biochemical analysis has shown recently that the dual phosphorylation reactions catalyzed by MAP kinase modules are sequential at both levels of the cascade. The observed order of phosphorylation events suggests an excursion from the Ser/Thr kinase activity of the MAP3K into Tyr kinase activity of the central dual specificity MAP2K. How the order of events might be encoded in the structures and interactions is discussed. The ordered mechanism confirms predictions that reactions should be sequential to generate the steep signal-to-response curves and delayed responses observed in cells.
Main Text
Phosphorylation-mediated cellular responses to hormones and stress can appear strongly sigmoid, and are capable of inducing irreversible changes (1,2). The kinases that create these switchlike responses, the MAP kinases and their activators, are well known. Nevertheless, the molecular phosphorylation and binding events that induce sigmoid behaviors have remained poorly understood. The MAP kinases and their activating enzymes were discovered by purifying the kinases responsible for growth-factor stimulated phosphorylation (3,4). Studies of ribosomal subunit S6 phosphorylation led to the cloning of the MAPK ERK2 (5,6). The cloning of the MAP2K MEK1 followed, which allowed for the purification and analysis of MAP kinase modules at a biochemical level (7,8). MAP kinase modules have proved to be the common signaling pathway underlying processes such as cell differentiation, proliferation, and stress responses (9).
The switches have been replicated numerous times, and function in diverse contexts, suggestive of significant driving forces for their retention and replication (10). The core of MAP kinase modules is comprised of a MAPK, a MAP2K, and a MAP3K. These enzymes catalyze two double phosphorylation reactions (11). These reactions were recognized by Ferrell et al. (12) as a possible source for the sigmoid behavior of the cascades. There are four well-studied cascades, each of which has the same dual-dual phosphorylation chemistry. The cascades are named for the MAPK activated: ERK, p38, JNK, and ERK5 (also known as BMK) (10,13). MAP3Ks phosphorylate two activation loop Ser/Thr residues on MAP2Ks (14). MAP2Ks, in turn, phosphorylate a Tyr and then a Ser/Thr residue on MAPKs, also in the activation loop, making MAP2Ks the only known kinase to run both Ser/Thr and Tyr kinase activity on a substrate protein (Fig. 1) (11,15). Recent analysis suggests a role for the dual specificity of the MAP2K in setting up an order to the phosphorylation reactions (16).
Figure 1.
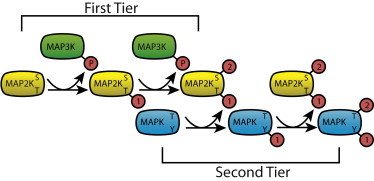
A schematic of the MAPK cascade. (Green) MAP3K; (yellow) MAP2K; and (cyan) MAPK. (Red spheres) Phosphorylation, with the number indicating order. Activation loop residues that are phosphorylated are shown in the block. To see this figure in color, go online.
MAP kinase modules have been studied from a variety of perspectives, including signal-to-output studies in cells, biochemical assays in vitro, and using purely mathematical approaches to understanding possible kinetic mechanisms and their implications. Signal-to-output studies in cells (17,18), as well as potential mathematical models (12,19–21), have been reviewed. Here we review more recent data and attempt to integrate conclusions from diverse approaches.
Cellular Outputs
We discuss signal-to-response measurements and recent efforts to quantitate chemical transformations and molecular interactions of cascade components in vivo. These data serve as a target for our molecular understanding of the cascade.
Sigmoid and graded responses
Oocyte maturation exhibits a sigmoid response to hormones (22). Huang and Ferrell (12) proposed that the multiple phosphorylation events catalyzed on MAPK module components might be responsible and probed the activation of the MAPK ERK2 by the MAP3K Mos in Xenopus oocyte lysates, revealing a sigmoid response they attributed to the two double phosphorylation events of the MAP kinase cascade. Subsequent studies demonstrated a very strong all-or-nothing response in Xenopus oocyte maturation, where a graded progesterone input resulted in purely on-or-off ERK2 activation. The apparent Hill coefficient for ERK2 activation was 35 or more, which they attribute in part to the MAPK cascade, and part due to positive feedback via protein synthesis (23). These same authors also showed that similar strong responses occurred in the activation of the kinase JNK in response to stress signals in several different cell lines (24). More recently, O’Shaughnessy et al. (25) expressed a complete MAP kinase module with an estrogen receptor-Raf fusion protein in yeast to determine whether the cascade would be able to function divorced from other potential interaction partners. On stimulation with estrogen, signal-to-response curves similar to those observed by Huang and Ferrell (12) were shown. They also demonstrated that the sigmoidicity was robust, and maintained even when challenged with cascade modulators, such as phosphatases and MEK inhibitors.
MAP kinase cascades also demonstrate graded responses to activation. Stimulation of ERK1/2 by EGF (epidermal growth factor) and PMA (phorbol myristate acetate) in individual human fibroblasts and HeLa cells demonstrated graded responses in the formation of phosphorylated ERK1/2 (26). These results were recapitulated in Swiss 3T3 cells stimulated by platelet-derived growth factor (27). In this study, ERK1/2 activation was demonstrated to be graded despite a more switchlike response in the downstream transcription factor c-fos. These data are suggestive that other factors may be required in addition to the cascade to generate sigmoidicity in some systems.
Quantifying cellular responses
Various attempts have been made to determine in vivo MAPK cascade kinetic parameters. Time- resolved EGF activation of MAP kinases measured in cells was fit to models. Yamada et al. (28), Sasagawa et al. (29), and Schoeberl et al. (30) each collected EGF-ERK2 response data, and built similar kinetic models based on the previously determined pathway structure. However, their models had low data/parameter, and thus were poorly constrained. Fujioka et al. (31) made significant improvements by directly measuring the persistence of protein-protein interactions, and rates of phosphorylation, among MAP kinase module components in HeLa cells. They used FRET to observe interactions between both ERK and MEK proteins, and Ras-Raf and MEK. The FRET analysis, in combination with tracking phosphorylation rates, allowed approximate kcat and Km values to be determined for the phosphorylation of ERK1/2 by MEK. The authors compared their values to those derived by the model approaches listed above. Their kcat and Km assignments tended to fall in the middle of the two-order-of-magnitude range of the other studies: Vmax values for both levels of the cascade were ∼10/min, and Km values were ∼0.1 μM. In these studies, modeling of the core MAP kinase reactions kept single and double phosphorylation reactions separate, but did not distinguish between the amino-acid targets. These results are compared below with in vitro measurements.
In Vitro Biochemical Characterization of Phosphorylation Order
Molecular characterization of MAP kinase module chemistry has produced information on the order of the phosphorylation reactions; whether the reactions are processive or nonprocessive; the role of individual phosphorylation events in the activity and activation of MAPKs; and the nature and specificity of protein-protein interactions.
Order and processivity of phosphorylation of MAPKs
The order of phosphorylation of the MAPK ERK2 was demonstrated in vitro (32–34). The reaction was found to be ordered, with phosphotyrosine appearing first, then phosphothreonine. Burack and Sturgill (35) also showed that the phosphorylation of ERK2 was nonprocessive, because an increase in the concentration of ERK2 in the presence of active MEK2 suppressed the appearance of the final product ERK2/T∗Y∗, where T and Y are the two phosphorylation sites on the activation loop, and the asterisk (∗) denotes phosphorylation. Aoki et al. (36) also addressed the order of phosphorylation by measuring the appearance of ERK2/TY∗ and ERK2/T∗Y∗ in EGF-stimulated HeLa cells. Time-resolved studies revealed significant processivity because ERK/TY∗ formed at the same time as ERK/T∗Y∗. No ERK2/T∗Y was observed, indicative of an order to the phosphorylation events, i.e., ERK2/TY∗ first. The authors found the reaction to be nonprocessive in vitro, but followed the same order. Analysis of the MAPK p38 in vitro revealed the same order of events—phosphorylation through a phosphotyrosine intermediate and nonprocessivity at this level of the cascade (16).
Phosphorylation requirements for the activity of MAPKs
Given that MAPK/TY∗ appears first raises the question of whether tyrosine phosphorylation is required for MAPK activity, or if it is instead a stepping-stone, required for the second phosphorylation event. To test the activity of the preferred intermediate MAPK/TY∗, as well as the nonpreferred intermediate MAPK/T∗Y, YY. Zhang et al. (37) and Zhou et al. (38) took the approach of making monophosphorylated forms through the action of Tyr specific or Ser/Thr specific phosphatases on ERK2 and p38 MAP kinases. Both ERK2/T∗Y and p38/T∗Y have 10–20-fold lower activity than the fully phosphorylated form. The results were different in the assays of the tyrosine monophosphorylated forms. p38/TY∗ was 100-fold less active than p38/T∗Y, although in ERK2, ERK2/TY∗ is only approximately fourfold less active than ERK2/T∗Y. These data suggest Thr phosphorylation is most important to the activity. The Thr is homologously positioned in the activation loop to the primary activating phosphorylation site in many Ser/Thr protein kinases (Fig. 2). This suggests that Tyr phosphorylation is a stepping-stone to full kinase activity.
Figure 2.
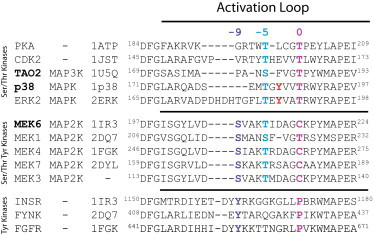
Sequence alignment of activation loop sequences of MAP kinases. The sequences of activation loops of protein kinases can be aligned with respect to conserved sequences at either end and the DFG box and the APE sequence. Zero is defined as the position of the substrate binding residue—a threonine in Ser/Thr kinases. The tyrosines (red) in p38 MAPK and ERK MAPK do not align with any of the phosphorylation sites in either Ser/Thr kinases or Tyr kinases. The threonine (pink) of p38 and ERK2 aligns with the primary activating phosphorylation in other Ser/Thr kinases. The two phosphorylation sites on MAP2Ks align with the primary activating phosphorylation sites in both Ser/Thr (blue) and Tyr (purple) kinases. (Boldface) Kinases in the p38 module. The Protein Data Bank (PDB) files are indicated. To see this figure in color, go online.
Similar conclusions were reached by Bell et al. (39) in studies of the yeast MAPK HOG1. The isolated active mutants of HOG1 were autophosphorylated on Thr in cells. Apparently, Tyr phosphorylation was not required for activity. However, the tyrosine residue was required for stress activation of the HOG1 pathway (40). These data further suggest that the Tyr phosphorylation is a stepping-stone to the phosphorylation of Thr and activation of the MAPK. The mutations that activated HOG1, presumably by an autophophorylation mechanism, were introduced into p38 MAP kinase giving similar results (41).
Order, processivity, and phosphorylation requirements for MAP2Ks
Resing et al. (42) observed an order in phosphorylation at the MAP2K level. The MAP3K v-Mos was used to phosphorylate the MAP2K MEK1. MEK1/SS∗ (MEK1/S218 S222∗) formation was preferential to MEK1/S∗S MEK1/S∗A had higher apparent kinase activity than MEK1/AS∗. Further, less MEK1/S∗A was formed than MEK1/S∗S∗, suggestive that phosphorylation of S222 accelerates S218 phosphorylation. These data argue that S222 may be a stepping-stone to S218 phosphorylation. Humphreys et al. (16) found that the phosphorylation of the MAP2K MEK6 by the MAP3K ASK1 occurred in a similar order, T211 phosphorylation preceding S207. However, unlike the behavior of MEK1, the studies of MEK6 showed that the intermediate in MEK6 phosphorylation, MEK6/ST∗, had activity in the phosphorylation of p38 (unpublished data).
Phosphatase reactions are ordered
The phosphorylation of MAP kinases is assumed to be reversible through the action of phosphatases, and these phosphatases may also work by ordered mechanisms. The dephosphorylation of ERK2 by the dedicated phosphatase MKP3 goes through the intermediate ERK2/T∗Y, with chemistry on the phosphotyrosine occurring first (43,44). Thus, apparently, the phosphorylation/dephosphorylation of MAPKs has aspects of a cyclic mechanism outlined by Salazar and Höfer (45), where the first residue to be phosphorylated is also the first to be dephosphorylated. Inasmuch as ERK2/T∗Y or p38/T∗Y is partially active, the tyrosine-first dephosphorylation maintains the activity until the dephosphorylation is complete (37,38,46). Despite the importance of phosphatases to signal propagation through the pathway, their biochemical characterization has lagged behind the kinases.
Structural Model for Specificity and Reaction Order
The reactions in MAP kinase modules occur in a precise order. Addressing the question of how this order is established at a molecular level, for both tiers of the cascade, leads to a hypothesis concerning the mechanism of the dual specificity of MAP2Ks and the role of the dual specificity in the switch: a forced excursion into Tyr chemistry from Ser/Thr chemistry creates additional thresholds for activation.
Structural basis for reaction order at the two tiers of the cascade
What molecular interactions determine the order of phosphorylation reactions? The structural basis of the phosphorylation order is best understood for the reactions at the first tier of the cascade. We addressed the question of why MEK6/ST∗ forms first in the phosphorylation of MEK6 by the MAP3K TAO2 (47). The structure of phosphorylated and active TAO2 has been determined, and is very similar to protein kinase A (47,48). Based on this structure, it was possible to model the interaction of TAO2 with the activation loop of MEK6. This model suggested that the aspartic acid and isoleucine of the MEK6 activation loop sequence DSVAKTI might be involved in substrate binding. In the model, the isoleucine of the MEK6 activation loop is in the P+1 specificity pocket of the MAP3K when threonine is in the active site (Fig. 3). The modeling also suggested that the aspartic acid should be near a cluster of positive charges in the MAP3K when threonine is in the active site. Mutants of the aspartic acid and the isoleucine both reduced activity of the MAP3K TAO2 toward MEK6. Mutation of the TAO2 lysine and arginine residues, predicted to interact with the aspartic acid, also reduced activity of TAO2 on MEK6 (47). At the second tier, the molecular mechanisms dictating Tyr-first phosphorylation are not known, but may involve a tighter binding interaction for tyrosine in the active site. No structure with the substrate in the active site of the MAP2K is available (49–51).
Figure 3.
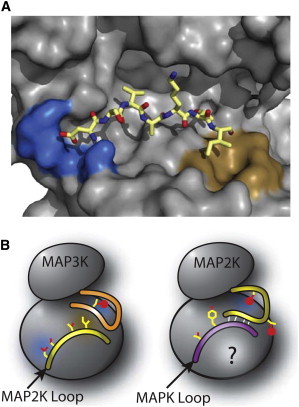
Structural basis for reaction order. (A) Model of MAP2K activation loop (yellow) docking in the MAP3K (gray) based on peptide docking on protein kinase A (PDB:1ATP). (Blue) A positively charged patch that interacts with the exposed aspartic acid; (brown) a hydrophobic patch that interacts with the isoleucine. (B) Cartoon representation of peptide docking models for MAP3Ks and MAP2Ks. (Blue regions) Areas of positive surface charge; (brown areas) exposed hydrophobic regions. (Red spheres) Phosphorylation sites. (Orange) Activation loop of the MAP3K; (yellow) activation loop of the MAP2K; (purple) activation loop of the MAPK. The MAP2K cartoon represents the structure and interactions during the first step of MAPK phosphorylation (on Tyr). (White lines) Predicted hydrogen bonds between the activation loops. To see this figure in color, go online.
Do structural rearrangements of the activation loop of MAP2Ks support Tyr versus Thr phosphorylation?
It appears likely that the activation loop of MAP2Ks rearranges to support the two different chemistries of Tyr versus Thr phosphorylation. Both Tyr kinases and Ser/Thr kinases use activation loop phosphorylation to build the active structure. The primary activating phosphorylation sites are differently positioned in the activation loop and promote different structures by binding to homologous positively charged patches on the surface of the kinase (Fig. 3) (48,52). It is interesting that the two phosphorylation sites of MAP2Ks align with the primary activating phosphorylation sites in Tyr and Ser/Thr kinases (Fig. 2). This idea suggests that the Ser phosphorylation of the MAP2K drives the formation of a conformation supporting Tyr-kinase activity. The Ser is phosphorylated second in the activation of MAP2Ks. This suggests further that the intermediate in MAP2K activation, MEK6/ST∗, should not have Tyr-kinase activity. Studies of MEK1 support this model as noted above (42).
The excursion model
The chemical logic is apparent: the overall MAP kinase module starts out with a Ser/Thr protein kinase, the MAP3K, and ends with a Ser/Thr kinase, the MAPK (Fig. 4). However, a forced excursion into tyrosine kinase chemistry takes place. At the first tier, a kinase capable of tyrosine phosphorylation chemistry is built: the phosphorylated MAP2K. Then, at the second tier, the MAPK is phosphorylated, first on Tyr, then on Thr. Tyr phosphorylation is a stepping stone to Thr phosphorylation, which activates the MAPK.
Figure 4.
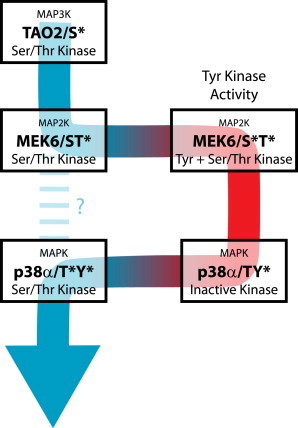
Excursion model. The MAP kinase modules start out with a Ser/Thr kinase, the MAP3K, and end with a Ser/Thr kinase, the MAPK. However, the phosphorylation of the MAPK requires an excursion into Tyr kinase activity that precedes the activating phosphorylation of the threonine of the MAPK. The phosphorylation of the MAP2K generates a catalytically competent Tyr kinase. To see this figure in color, go online.
Protein-Protein Interactions in MAPK Modules
The strengths of docking interactions and docking-induced conformational changes are discussed.
Protein-protein interactions mediated by docking
MAP kinase module components bind each other via docking motifs. MAP kinases have a major docking site, named the “domain for common docking”, which engages substrates, MAP2Ks, and phosphatases, at a site removed from the active site (53–57). The strength of the domain for common-docking interactions has been measured for ERK2 and p38, giving a range from 70 nM to 1 μM for cognate substrates (43,58). Competition assays reveal binding constants in the 10 nM to 100 μM range (56). Further, competition between substrates and products has been measured for MAP kinase phosphatase MKP3 activity toward ERK2, showing a Ki of 0.08 μM (43).
Docking-induced conformational changes
In addition to affecting specificity by straightforward differences in affinities, docking likely contributes to pathway specificity because binding events can lead to conformational changes and kcat effects. The best evidence for this comes from the observation that ERK2 activates its own phosphatase, MKP3 (43). The likely mechanism is that docking between ERK2 and the kinase docking domain (KBD) of MKP3 induces conformational changes that relieve autoinhibition of the phosphatase. Conformational changes may occur in the kinase as well. The structure of the MAP kinase p38 bound to the KBD of MKP5 reveals changes in p38 as well as the KBD of MKP5 (58). Observed conformational changes include disordering of the p38 activation loop. We have hypothesized that these changes may be necessary for the activation loop to bind to the active site of the phosphatase (59). Similar activation loop conformational changes have been observed in each structural study of MAP kinase docking interactions, whether the MAP kinase be p38, ERK2, or JNK, or the docking peptide is derived from a phosphatase, a substrate, or an activating MAP2K (60–63). Thus, docking-induced conformational changes may be contributing to pathway specificity in contexts besides the kinase-phosphatase interaction. Unfortunately, it is normally difficult to measure the contribution of conformational changes to the kinetics. However, the MAP kinase-phosphatase interaction is intriguing because the conformational effect can be measured using the small molecule phosphatase substrate p-nitrophenyl phosphate (38).
Mathematical Models for Generating Switchlike Response in Kinase Cascades
What is the role of the order of phosphorylation events in switch behavior? How do protein-protein interactions contribute to switching? Mathematical modeling has addressed these questions.
Zero order effects and multisite phosphorylation
Our understanding of the potential of protein modification to induce all-or-nothing responses originates from Goldbeter and Koshland (64). They used the Michaelis-Menten equation to show that highly sigmoid responses are available from only a single, reversible protein modification. This phenomenon, “zero-order ultrasensitivity”, is possible when both the forward and reverse enzymes, such as kinases and phosphatases, are at or near saturation (thus zero-order, rate proportional to enzyme only) (65). As the active kinase concentration increases, there is no phosphorylated substrate accumulation until the kinase activity supersedes that of the phosphatase, at which point the substrate phosphorylation increases sharply. The output will be dominated by the enzyme with the highest activity. The importance of long-lived protein-protein interactions is implicit to zero-order ultrasensitivity when the substrate is a protein (because the system must approach saturation). The same authors further showed that addition of multiple cascade tiers enhances the degree of ultrasensitivity, and expands the regime of enzyme and substrate concentrations under which ultrasensitivity is available (65). Huang and Ferrell (12) applied these ideas to analysis of the MAP kinase cascade, showing that the same assumptions should lead to a sigmoid response. They show further that the multiple kinase reactions, two at each tier of the cascade, should be nonprocessive to have maximal sigmoid responses.
Ordered phosphorylation is best for a signaling switch
Salazar and Höfer (45) addressed how the order of phosphorylation in multisite phosphorylation reactions affects output. They show analytically that between ordered and random models, ordered reactions generate the greatest sigmoid behavior. This is understood from Fig. 5, A and B: when multiple phosphorylation sites are present, if the reaction occurs in a precise order, the concentration of substrate does not decrease very much as the kinase/phosphatase activity ratio increases. In contrast, in the random mechanism, the available kinase substrate drops with kinase/phosphatase activity ratio, and radically increases the available phosphatase substrate, thus limiting the ability to reach the fully phosphorylated form. The authors made the prediction that kinase cascades that include multisite phosphorylation species are likely to operate by a sequential mechanism.
Figure 5.
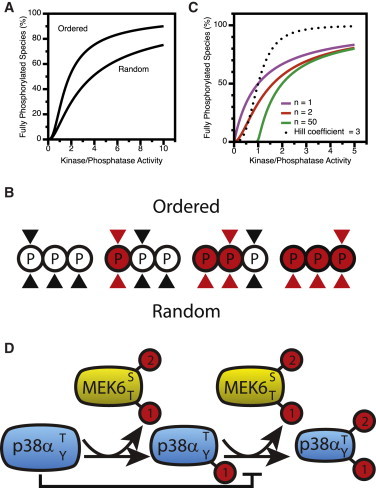
Multisite phosphorylation. (A) The sequence of phosphorylation and dephosphorylation events impacts the steady-state concentration of fully phosphorylated product based on Eqs. 6 and 9 from Salazar and Höfer (45). (B) An example of random versus ordered phosphorylation in a substrate with three phosphorylation sites. (Open circles) Unphosphorylated sites; (red circles) phosphorylated. (Black triangles) Potential placement of the phosphorylation site in the kinase active site; (red triangles) indicate potential placement of the phosphorylation site in the phosphatase active site. Ordered reactions exhibit a stronger switchlike steady-state response to stimulation because increasing overall phosphorylation does not decrease available sites or increase phosphatase sites as quickly. (C) The impact of multiple phosphorylation sites on the percentage of fully phosphorylated substrate as a function of kinase/phosphatase ratio as based on Eq. 9 in Gunawardena (68). The Hill function is shown for comparison: % fully phosphorylated kinase = (K/P)3/1 + (K/P)3, where K is the activity of the kinase, and P the activity of the phosphatase. Higher numbers of phosphorylation sites do not increase sigmoidicity. (D) Positive feedback is inherent to each MAP kinase tier by merit of its architecture. For each tier of the cascade, the rate of the second reaction is inhibited by the amount of unphosphorylated substrate. As the reaction progresses, the amount of unphosphorylated substrate decreases, relieving inhibition and driving positive feedback. To see this figure in color, go online.
How protein-protein interactions contribute to switch behavior
Markevich et al. (66) discussed how protein-protein interactions lead to ultrasensitivity in a multiple phosphorylation reaction of a single protein substrate. Ultrasensitivity can occur when the second reaction is inhibited by the initial substrate (Fig. 5 D). As the reaction progresses, the concentration of the initial substrate is reduced, relieving the inhibition and resulting in positive feedback. If this positive feedback is sufficiently strong, bistability may arise, where two steady substrate phosphorylation states are available for a single set of concentrations of the substrate, kinase, and phosphatase. In addition, competition for a MAP2K within the MAPK module has been proposed as a further source of ultrasensitivity and bistability (67).
Do additional phosphorylation sites improve sigmoidicity?
Gunawardena (68) addressed the problem of how signal-to-response behaviors are affected by the number of phosphorylation events. Assuming ordered phosphorylation, equivalent rate constants, and that the phosphatase can operate on the intermediate species, he showed that increasing numbers of phosphorylation sites increase thresholding behavior, but do not improve sigmoidicity (Fig. 5 C). As the number of phosphorylation sites increases, the number of nonmaximally phosphorylated states also increases. The increasing number of states decreases the amount of maximally phosphorylated substrate for any given kinase/phosphatase ratio. This is most obvious at low levels of relative kinase activity, where it becomes increasingly improbable to fully phosphorylate any single protein. The Hill equation is given in the legend of Fig. 5 C, and graphed for comparison. It does not capture the behavior of multisite phosphorylation. To generate a more sigmoid response, the forward rate must increase as the reaction progresses: for example, if the second reaction is faster than the first. It is interesting that a relatively small number of phosphorylation sites are present in MAP kinase cascades, as well as other switching cascades and modules, consistent with these predictions (69–71).
In Vitro Time-Resolved Studies in the Activation of MAPK modules
Recent in vitro time-resolved studies are beginning to bridge the gap between signal-to-output in cells and kinetic models offered for multistage phosphorylation. We carried out in vitro time-resolved, phosphorylation state kinetic studies on the p38 MAP kinase cascade (16). In addition to tracking the order of events at the second tier of the cascade, the phosphorylation of p38, we also determined the order of events at the first tier of the cascade, the phosphorylation of MEK6. These data were fit to time-courses, allowing us to estimate kinetic parameters and identify kinetically relevant elemental reactions.
Reactions are ordered at both levels of the cascade
The mass spectrometry data published in Humphreys et al. (16) is reproduced in Fig. 6. As mentioned above, the data show that the reactions are ordered at both tiers of the cascade. In the phosphorylation of the MAPK p38, tyrosine is phosphorylated first, and threonine second, in the activation loop sequence T∗GY∗. In the phosphorylation of the MAP2K MEK6, threonine is phosphorylated first, serine second, in the activation loop sequence DS∗VAKT∗I. It is interesting that Salazar and Höfer (45) predicted that the reactions should be sequential (ordered), inasmuch as sequential reactions would generate the most sigmoid response.
Figure 6.
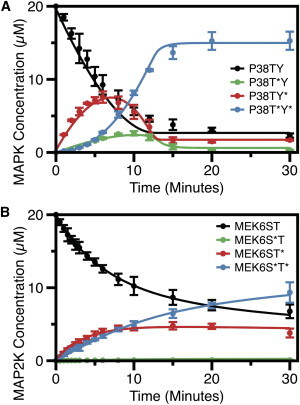
Time courses of MAPK cascade phosphorylation reactions in Humphreys et al. (16). (A) The time-course for phosphorylation of p38 MAPK by MEK6 was generated from mass-spectrometric data and then fit to a set of elemental differential equations in the software DYNAFIT (BioKin, Watertown, MA; http://www.biokin.com/dynafit/) (73). A Monte Carlo method is then employed to estimate errors associated with individual parameters and eliminate those that are poorly constrained. (B) The time course of the phosphorylation of MEK6 by ASK1, as above. Data reprinted with permission (16). To see this figure in color, go online.
The two levels of the cascade showed disparate behavior with respect to processivity (Fig. 7). The phosphorylation of p38 MAPK followed the expected nonprocessive mechanism (12,33). In contrast, the MAP3K ASK1 phosphorylation of MEK6 is largely processive, with the appearance of both the final product and the intermediate occurring simultaneously. The elemental reaction constants were estimated using the data from Fig. 6, A and B. Curve fitting confirmed that the reactions are sequential: all of the flux at the first tier goes through MEK6/ST∗; at the second tier, most of the flux is through p38/TY∗. The model building also recapitulated the clear difference in processivity between the two reactions.
Figure 7.
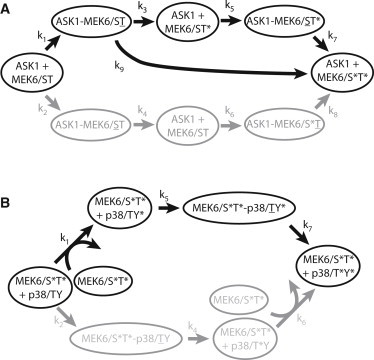
(A and B) Kinetic reaction schemes of the two tiers of the p38 MAPK module. Kinetically significant reactions and intermediates are displayed. (Shaded lines) Disfavored reaction pathways. (Dashes) Complexes; (pluses) reactants. (Underlined letters) Residues in the active site of the phosphorylating kinase in the complex. (Asterisks) Phosphorylation. Constants are shown in Table 1.
Protein-protein interactions
The importance of protein-protein interactions could be assessed from the curve-fitting. In the case of the phosphorylation of MEK6 by ASK1, fitting suggested that both the ASK1-MEK6/ST complex and the ASK1-MEK6/ST∗ complex are persistent. In comparison, in the phosphorylation of p38 by MEK6, apparently only the MEK6-p38/TY∗ complex had a significant lifetime. The duration of these complexes leads to Km values <10−2, which are low enough given cellular concentrations of MAPK components to induce zero-order ultrasensitivity (31,64). This suggests that the p38 cascade may not require additional components or processes, such as scaffolds or additional sources of feedback, to generate switchlike responses.
Conclusions
In this review, we have discussed recent advances in the knowledge of the chemistry and kinetics of MAPK modules, and then show how these data meet the predictions made by mathematical approaches.
Detailed time-course analyses of the reactions of the MAP kinase module are revealing the chemical logic and connecting cellular data with theoretical concepts about pathway kinetics. Reactions through MAPK modules occur sequentially: the MAPK modules start with Ser/Thr chemistry, and end with a Ser/Thr chemistry, but take a forced excursion into Tyr chemistry that creates extra barriers to the activation of MAP kinases. The ability of MAP2Ks to carry out two different reactions at the same active site may involve conformational changes to recognize Tyr versus Thr. The structures of MAP2Ks captured in action at these two chemical steps have yet to be determined. Nevertheless, the chemical interpretation of an excursion into tyrosine kinase chemistry explains how recognition events can set up a precise order of reactions, and the relationship between MAP2Ks and dedicated Ser/Thr and Tyr kinases. The observed precise order of reactions advances the original hypotheses of Ferrell (17) that the multiple phosphorylation reactions have the potential to induce the sigmoid responses exhibited by MAPK modules.
It is also very interesting that several modeling efforts captured features of MAPK modules in advance of their experimental explication. Salazar and Höfer (20) predicted that the reactions should be sequential; sequential (ordered) reactions offer an increase in sigmoid, or switchlike, behavior over random reactions. Further, the MAPK modules involve only a few phosphotransfer reactions, as predicted by Gunawardena (68). The kinetic measurements also revealed persistence of complexes between the MAP3K and the MAP2K, and the MAP2K and the MAPK (Table 1, Fig. 7) (16). Persistent enzyme-substrate complexes can contribute to zero-order ultrasensitivity as suggested by Goldbeter and Koshland (65), and demonstrated by Kim and Ferrell (72). These long-lived complexes could also indicate that the MAP kinase cascades are capable of bistability without invoking any additional sources of positive feedback (66). However, such behaviors have not yet been demonstrated in vivo or in vitro. All of these complex phenomena also require the action of phosphatases, the study of which has lagged behind that of the kinases in the pathway. Whether or not phosphatase activity also operates via an ordered or random mechanism will have significant impacts on how signal propagates (45). Future experimental work is likely to be focused on reconstitution of complete MAP kinase modules together with phosphatases to further probe the effects of competition.
Table 1.
Parameters from Humphreys et al. (16) compared with previous studies
| Author | Reaction | k1a | k−1b | k2a | k3b | k4b | k5a | k6a | k7b | k8b | k9b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Humphreys et al. (16) | ASK1 + MEK6 | 2 | c | 0.044 | 11 | 0.72 | 2.5 | 4.7 | 5.3 | 0.011 | 4.6 |
| Fujioka et al. (31) | Ras/Raf + MEK | 39 | 3.9 | — | 10.8 | — | — | — | — | — | — |
| Schoeberl et al. (30) | Ras/Raf + MEK | 660 | 1.08 | — | 174 | — | — | — | — | — | — |
| Sasagawa et al. (29) | Ras/Raf + MEK | 936 | 120 | — | 30 | — | — | — | — | — | — |
| Bhalla et al. (74) | Ras/Raf + MEK | 198 | 24 | — | 6.4 | — | — | — | — | — | — |
| Yamada et al. (28) | Ras/Raf + MEK | 552 | 54 | — | 102 | — | — | — | — | — | — |
| Humphreys et al. (16) | MEK6 + p38α | 154 | c | 31 | c | 15 | 101 | 44 | 6.6 | c | c |
| Fujioka et al. (31) | MEK + ERK | 52.8 | 5.28 | — | 13.2 | — | — | — | — | — | — |
| Schoeberl et al. (30) | MEK + ERK | 66 | 1.98 | — | 342 | — | — | — | — | — | — |
| Sasagawa et al. (29) | MEK + ERK | 960 | 36 | — | 9 | — | — | — | — | — | — |
| Bhalla et al. (74) | MEK + ERK | 978 | 36 | — | 9 | — | — | — | — | — | — |
| Yamada et al. (28) | MEK + ERK | 19.1 | 54 | — | 6 | — | — | — | — | — | — |
Parameters were derived from fits of experimental MS/MS progress curves of single MAPK cascade tiers. In previous studies, k1 and k−1 represent formation and dissolution of the enzyme-substrate complex, where k3 is the turnover. Steps not accounted for by the model are indicated by long dashes.
μM−1 min−1.
Min−1.
Values were either too fast or slow to be captured by the model.
Acknowledgments
We thank Elliott Ross, Natalie Ahn, and Melanie Cobb for discussions.
This work was supported in part by the Welch Foundation, grant No. I1128, and the National Institutes of Health, grant No. DK46993 to E.J.G.
References
- 1.Malaisse W.J. Insulin secretion: multifactorial regulation for a single process of release. The Minkowski award lecture delivered on September 7, 1972 before the European Association for the Study of Diabetes at Madrid, Spain. Diabetologia. 1973;9:167–173. doi: 10.1007/BF01219778. [DOI] [PubMed] [Google Scholar]
- 2.Janicot M., Lane M.D. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 1989;86:2642–2646. doi: 10.1073/pnas.86.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray L.B., Sturgill T.W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc. Natl. Acad. Sci. USA. 1987;84:1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blenis J., Spivack J.G., Erikson R.L. Phorbol ester, serum, and Rous sarcoma virus transforming gene product induce similar phosphorylations of ribosomal protein S6. Proc. Natl. Acad. Sci. USA. 1984;81:6408–6412. doi: 10.1073/pnas.81.20.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulton T.G., Yancopoulos G.D., Cobb M.H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249:64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 6.Seger R., Seger D., Krebs E.G. Human T-cell mitogen-activated protein kinase kinases are related to yeast signal transduction kinases. J. Biol. Chem. 1992;267:25628–25631. [PubMed] [Google Scholar]
- 7.Seger R., Ahn N.G., Krebs E.G. Purification and characterization of mitogen-activated protein kinase activator(s) from epidermal growth factor-stimulated A431 cells. J. Biol. Chem. 1992;267:14373–14381. [PubMed] [Google Scholar]
- 8.Zheng C.F., Guan K.L. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J. Biol. Chem. 1993;268:11435–11439. [PubMed] [Google Scholar]
- 9.Chen Z., Gibson T.B., Cobb M.H. MAP kinases. Chem. Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 10.Keshet Y., Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol. Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- 11.Ahn N.G., Seger R., Krebs E.G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J. Biol. Chem. 1991;266:4220–4227. [PubMed] [Google Scholar]
- 12.Huang C.Y., Ferrell J.E., Jr. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raman M., Chen W., Cobb M.H. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 14.Mansour S.J., Matten W.T., Ahn N.G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 15.Lochhead P.A., Sibbet G., Cleghon V. Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell. 2005;121:925–936. doi: 10.1016/j.cell.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Humphreys J.M., Piala A.T., Goldsmith E.J. Precisely ordered phosphorylation reactions in the p38 mitogen-activated protein (MAP) kinase cascade. J. Biol. Chem. 2013;288:23322–23330. doi: 10.1074/jbc.M113.462101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrell J.E., Jr. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem. Sci. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 18.Futran A.S., Link A.J., Shvartsman S.Y. ERK as a model for systems biology of enzyme kinetics in cells. Curr. Biol. 2013;23:R972–R979. doi: 10.1016/j.cub.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shvartsman S.Y., Coppey M., Berezhkovskii A.M. MAPK signaling in equations and embryos. Fly (Austin) 2009;3:62–67. doi: 10.4161/fly.3.1.7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salazar C., Höfer T. Multisite protein phosphorylation—from molecular mechanisms to kinetic models. FEBS J. 2009;276:3177–3198. doi: 10.1111/j.1742-4658.2009.07027.x. [DOI] [PubMed] [Google Scholar]
- 21.Blüthgen N., Legewie S. Robustness of signal transduction pathways. Cell. Mol. Life Sci. 2013;70:2259–2269. doi: 10.1007/s00018-012-1162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dekel N., Sherizly I. Induction of maturation in rat follicle-enclosed oocyte by forskolin. FEBS Lett. 1983;151:153–155. doi: 10.1016/0014-5793(83)80362-7. [DOI] [PubMed] [Google Scholar]
- 23.Ferrell J.E., Jr., Machleder E.M. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 24.Bagowski C.P., Besser J., Ferrell J.E., Jr. The JNK cascade as a biochemical switch in mammalian cells: ultrasensitive and all-or-none responses. Curr. Biol. 2003;13:315–320. doi: 10.1016/s0960-9822(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 25.O’Shaughnessy E.C., Palani S., Sarkar C.A. Tunable signal processing in synthetic MAP kinase cascades. Cell. 2011;144:119–131. doi: 10.1016/j.cell.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehurst A., Cobb M.H., White M.A. Stimulus-coupled spatial restriction of extracellular signal-regulated kinase 1/2 activity contributes to the specificity of signal-response pathways. Mol. Cell. Biol. 2004;24:10145–10150. doi: 10.1128/MCB.24.23.10145-10150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKeigan J.P., Murphy L.O., Blenis J. Graded mitogen-activated protein kinase activity precedes switch-like c-Fos induction in mammalian cells. Mol. Cell. Biol. 2005;25:4676–4682. doi: 10.1128/MCB.25.11.4676-4682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada S., Taketomi T., Yoshimura A. Model analysis of difference between EGF pathway and FGF pathway. Biochem. Biophys. Res. Commun. 2004;314:1113–1120. doi: 10.1016/j.bbrc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Sasagawa S., Ozaki Y., Kuroda S. Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nat. Cell Biol. 2005;7:365–373. doi: 10.1038/ncb1233. [DOI] [PubMed] [Google Scholar]
- 30.Schoeberl B., Eichler-Jonsson C., Müller G. Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat. Biotechnol. 2002;20:370–375. doi: 10.1038/nbt0402-370. [DOI] [PubMed] [Google Scholar]
- 31.Fujioka A., Terai K., Matsuda M. Dynamics of the Ras/ERK MAPK cascade as monitored by fluorescent probes. J. Biol. Chem. 2006;281:8917–8926. doi: 10.1074/jbc.M509344200. [DOI] [PubMed] [Google Scholar]
- 32.Haystead T.A., Dent P., Sturgill T.W. Ordered phosphorylation of p42mapk by MAP kinase kinase. FEBS Lett. 1992;306:17–22. doi: 10.1016/0014-5793(92)80828-5. [DOI] [PubMed] [Google Scholar]
- 33.Ferrell J.E., Jr., Bhatt R.R. Mechanistic studies of the dual phosphorylation of mitogen-activated protein kinase. J. Biol. Chem. 1997;272:19008–19016. doi: 10.1074/jbc.272.30.19008. [DOI] [PubMed] [Google Scholar]
- 34.Robbins D.J., Zhen E., Cobb M.H. Regulation and properties of extracellular signal-regulated protein kinases 1, 2, and 3. J. Am. Soc. Nephrol. 1993;4:1104–1110. doi: 10.1681/ASN.V451104. [DOI] [PubMed] [Google Scholar]
- 35.Burack W.R., Sturgill T.W. The activating dual phosphorylation of MAPK by MEK is nonprocessive. Biochemistry. 1997;36:5929–5933. doi: 10.1021/bi970535d. [DOI] [PubMed] [Google Scholar]
- 36.Aoki K., Yamada M., Matsuda M. Processive phosphorylation of ERK MAP kinase in mammalian cells. Proc. Natl. Acad. Sci. USA. 2011;108:12675–12680. doi: 10.1073/pnas.1104030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y.Y., Mei Z.Q., Wang Z.X. Enzymatic activity and substrate specificity of mitogen-activated protein kinase p38α in different phosphorylation states. J. Biol. Chem. 2008;283:26591–26601. doi: 10.1074/jbc.M801703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou B., Wang Z.X., Zhang Z.Y. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J. Biol. Chem. 2002;277:31818–31825. doi: 10.1074/jbc.M203969200. [DOI] [PubMed] [Google Scholar]
- 39.Bell M., Capone R., Engelberg D. Isolation of hyperactive mutants of the MAPK p38/Hog1 that are independent of MAPK kinase activation. J. Biol. Chem. 2001;276:25351–25358. doi: 10.1074/jbc.M101818200. [DOI] [PubMed] [Google Scholar]
- 40.Bell M., Engelberg D. Phosphorylation of Tyr-176 of the yeast MAPK Hog1/p38 is not vital for Hog1 biological activity. J. Biol. Chem. 2003;278:14603–14606. doi: 10.1074/jbc.C300006200. [DOI] [PubMed] [Google Scholar]
- 41.Avitzour M., Diskin R., Livnah O. Intrinsically active variants of all human p38 isoforms. FEBS J. 2007;274:963–975. doi: 10.1111/j.1742-4658.2007.05644.x. [DOI] [PubMed] [Google Scholar]
- 42.Resing K.A., Mansour S.J., Ahn N.G. Determination of v-Mos-catalyzed phosphorylation sites and autophosphorylation sites on MAP kinase kinase by ESI/MS. Biochemistry. 1995;34:2610–2620. doi: 10.1021/bi00008a027. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y., Zhang Z.Y. The mechanism of dephosphorylation of extracellular signal-regulated kinase 2 by mitogen-activated protein kinase phosphatase 3. J. Biol. Chem. 2001;276:32382–32391. doi: 10.1074/jbc.M103369200. [DOI] [PubMed] [Google Scholar]
- 44.Farooq A., Zhou M.M. Structure and regulation of MAPK phosphatases. Cell. Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Salazar C., Höfer T. Versatile regulation of multisite protein phosphorylation by the order of phosphate processing and protein-protein interactions. FEBS J. 2007;274:1046–1061. doi: 10.1111/j.1742-4658.2007.05653.x. [DOI] [PubMed] [Google Scholar]
- 46.Sohaskey M.L., Ferrell J.E., Jr. Distinct, constitutively active MAPK phosphatases function in Xenopus oocytes: implications for p42 MAPK regulation in vivo. Mol. Biol. Cell. 1999;10:3729–3743. doi: 10.1091/mbc.10.11.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou T., Raman M., Goldsmith E.J. Crystal structure of the TAO2 kinase domain: activation and specificity of a Ste20p MAP3K. Structure. 2004;12:1891–1900. doi: 10.1016/j.str.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 48.Knighton D.R., Zheng J.H., Sowadski J.M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- 49.Ohren J.F., Chen H., Hasemann C.A. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 2004;11:1192–1197. doi: 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- 50.Min X., Akella R., Goldsmith E.J. The structure of the MAP2K MEK6 reveals an autoinhibitory dimer. Structure. 2009;17:96–104. doi: 10.1016/j.str.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto T., Kinoshita T., Tada T. Crystal structures of MKK4 kinase domain reveal that substrate peptide binds to an allosteric site and induces an auto-inhibition state. Biochem. Biophys. Res. Commun. 2010;400:369–373. doi: 10.1016/j.bbrc.2010.08.071. [DOI] [PubMed] [Google Scholar]
- 52.Hubbard S.R. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharrocks A.D., Yang S.H., Galanis A. Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci. 2000;25:448–453. doi: 10.1016/s0968-0004(00)01627-3. [DOI] [PubMed] [Google Scholar]
- 54.Kallunki T., Su B., Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 55.Tanoue T., Nishida E. Molecular recognitions in the MAP kinase cascades. Cell. Signal. 2003;15:455–462. doi: 10.1016/s0898-6568(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 56.Bardwell A.J., Frankson E., Bardwell L. Selectivity of docking sites in MAPK kinases. J. Biol. Chem. 2009;284:13165–13173. doi: 10.1074/jbc.M900080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bardwell L. Mechanisms of MAPK signaling specificity. Biochem. Soc. Trans. 2006;34:837–841. doi: 10.1042/BST0340837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y.Y., Wu J.W., Wang Z.X. A distinct interaction mode revealed by the crystal structure of p38α with the MAPK binding domain of MKP5. Sci. Signal. 2011;4:ra88. doi: 10.1126/scisignal.2002241. [DOI] [PubMed] [Google Scholar]
- 59.Goldsmith E.J. Three-dimensional docking in the MAPK p38α. Sci. Signal. 2011;4:pe47. doi: 10.1126/scisignal.2002697. [DOI] [PubMed] [Google Scholar]
- 60.Zhou T., Sun L., Goldsmith E.J. Docking interactions induce exposure of activation loop in the MAP kinase ERK2. Structure. 2006;14:1011–1019. doi: 10.1016/j.str.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Akella R., Min X., Goldsmith E.J. The third conformation of p38α MAP kinase observed in phosphorylated p38α and in solution. Structure. 2010;18:1571–1578. doi: 10.1016/j.str.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Chang C.I., Xu B.E., Goldsmith E.J. Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol. Cell. 2002;9:1241–1249. doi: 10.1016/s1097-2765(02)00525-7. [DOI] [PubMed] [Google Scholar]
- 63.Heo Y.S., Kim S.K., Yang C.H. Structural basis for the selective inhibition of JNK1 by the scaffolding protein JIP1 and SP600125. EMBO J. 2004;23:2185–2195. doi: 10.1038/sj.emboj.7600212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldbeter A., Koshland D.E., Jr. An amplified sensitivity arising from covalent modification in biological systems. Proc. Natl. Acad. Sci. USA. 1981;78:6840–6844. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldbeter A., Koshland D.E., Jr. Ultrasensitivity in biochemical systems controlled by covalent modification. Interplay between zero-order and multistep effects. J. Biol. Chem. 1984;259:14441–14447. [PubMed] [Google Scholar]
- 66.Markevich N.I., Hoek J.B., Kholodenko B.N. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J. Cell Biol. 2004;164:353–359. doi: 10.1083/jcb.200308060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Legewie S., Schoeberl B., Herzel H. Competing docking interactions can bring about bistability in the MAPK cascade. Biophys. J. 2007;93:2279–2288. doi: 10.1529/biophysj.107.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunawardena J. Multisite protein phosphorylation makes a good threshold but can be a poor switch. Proc. Natl. Acad. Sci. USA. 2005;102:14617–14622. doi: 10.1073/pnas.0507322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richardson C., Alessi D.R. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signaling pathway. J. Cell Sci. 2008;121:3293–3304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- 70.Trunnell N.B., Poon A.C., Ferrell J.E., Jr. Ultrasensitivity in the regulation of Cdc25C by Cdk1. Mol. Cell. 2011;41:263–274. doi: 10.1016/j.molcel.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dajani R., Fraser E., Pearl L.H. Crystal structure of glycogen synthase kinase 3 β: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 72.Kim S.Y., Ferrell J.E., Jr. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell. 2007;128:1133–1145. doi: 10.1016/j.cell.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 73.Kuzmic P. DYNAFIT—a software package for enzymology. Methods Enzymol. 2009;467:247–280. doi: 10.1016/S0076-6879(09)67010-5. [DOI] [PubMed] [Google Scholar]
- 74.Bhalla U.S. Sigaling in small subcellular voumes II. Stochastic and diffusion effects on synaptic network properties. Biophys. J. 2004;87:745–753. doi: 10.1529/biophysj.104.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]


