Abstract
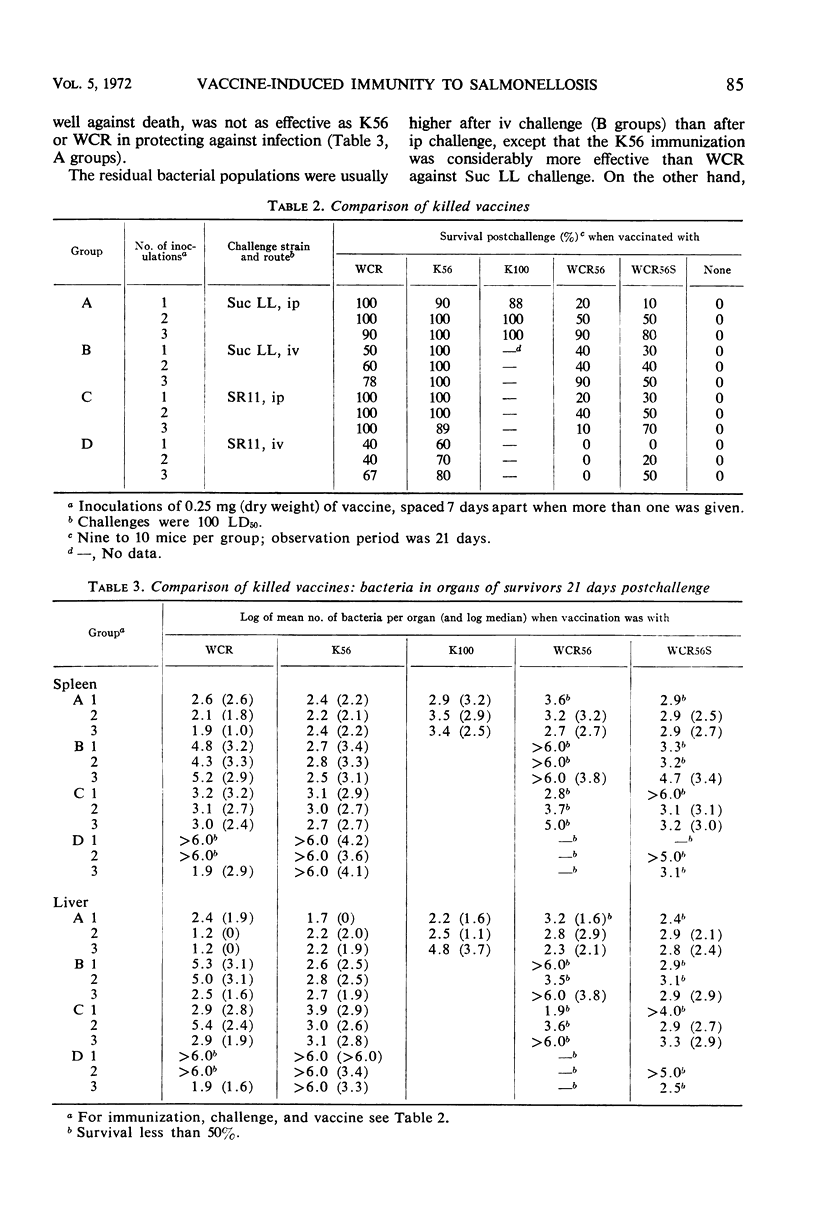
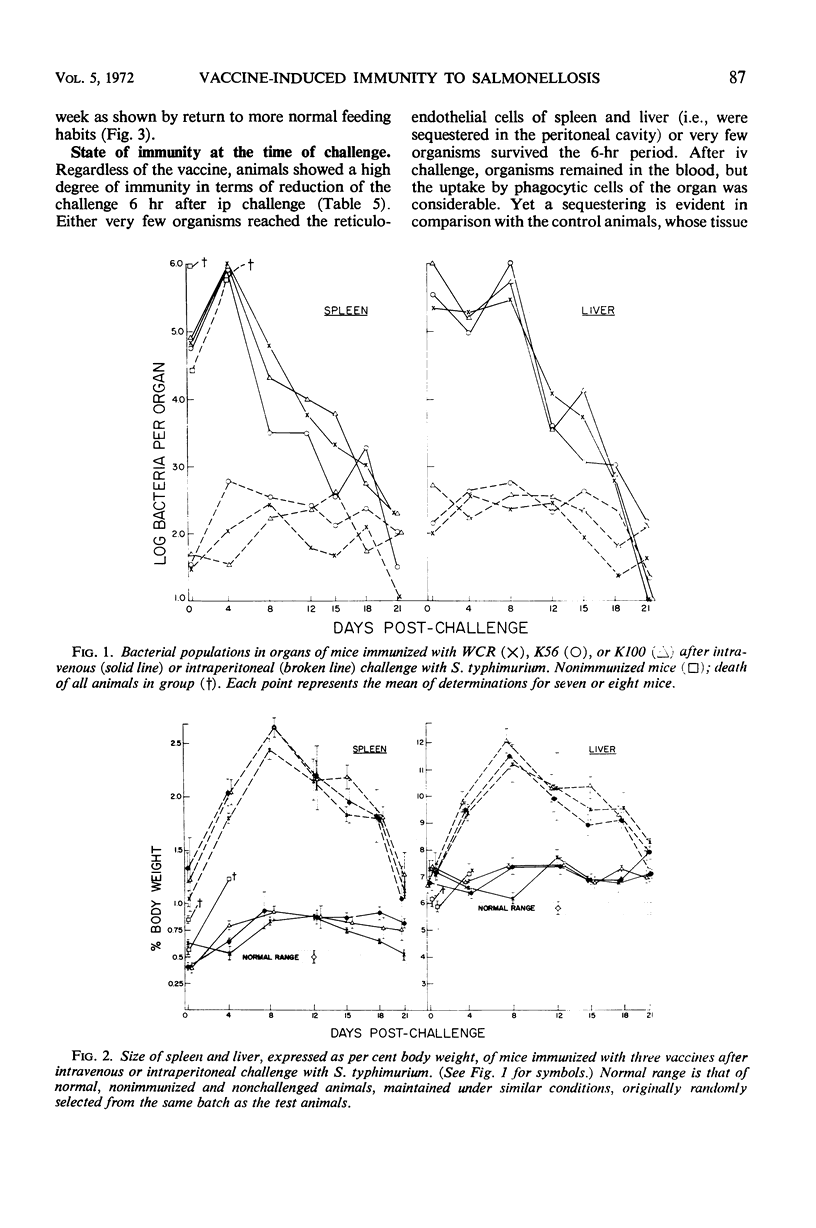
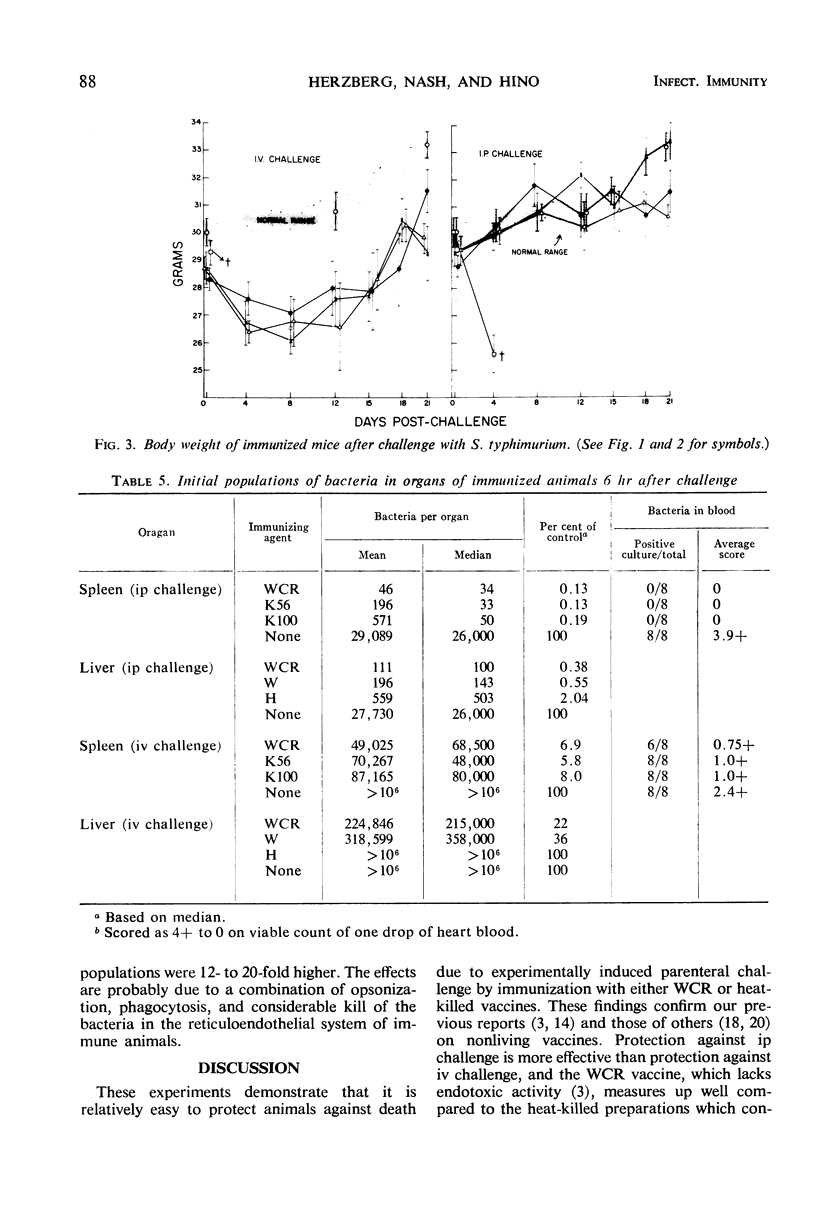
Killed vaccines, deoxycholate-extracted or heated, were shown to induce an effective degree of immunity which protected against death (100%), prevented extensive multiplication, and left the mice with low residual salmonella populations in spleen and liver after intravenous (iv) or intraperitoneal (ip) challenge with virulent Salmonella typhimurium. Protection was most effective against the ip challenge route and less effective against the iv route. A study of the kinetics of the population of bacteria in the spleens and livers of immunized animals showed that after ip challenge there was an initial reduction of 99% at 6 hr after challenge, maintenance of levels of less than 103 bacteria per organ, and a final population of 102 to 103 per organ at 21 days. With iv challenge, after an initial reduction of 90% at 6 hr, growth ensued to levels above 106 bacteria per organ until 8 days, followed by a steady decline yielding residual populations of 103 to 104 in some cases. Organ hypertrophy correlated with bacterial population. Morbidity was prevented (as measured by gain in body weight) by immunization against ip challenge but not against iv challenge. Killed vaccines protected by their ability to induce an immune state which reduced the initial challenge population, prevented extensive multiplication, yet allowed “cellular immunity” to develop due to response to the living challenge infection itself. The consequence was a low-level carrier state similar to that induced by recovery from sublethal virulent infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aserkoff B., Schroeder S. A., Brachman P. S. Salmonellosis in the United States--a five-year review. Am J Epidemiol. 1970 Jul;92(1):13–24. doi: 10.1093/oxfordjournals.aje.a121175. [DOI] [PubMed] [Google Scholar]
- Ashcroft M. T., Singh B., Nicholson C. C., Ritchie J. M., Sorryan E., Williams F. A seven-year field trial of two typhoid vaccines in Guyana. Lancet. 1967 Nov 18;2(7525):1056–1059. doi: 10.1016/s0140-6736(67)90335-2. [DOI] [PubMed] [Google Scholar]
- Badakhsh F. F., Herzberg M. Deoxycholate-treated, nontoxic, whole-cell vaccine protective against experimental salmonellosis of mice. J Bacteriol. 1969 Nov;100(2):738–744. doi: 10.1128/jb.100.2.738-744.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CVJETANOVIC B., UEMURA K. THE PRESENT STATUS OF FIELD AND LABORATORY STUDIES OF TYPHOID AND PARATYPHOID VACCINES WITH SPECIAL REFERENCE TO STUDIES SPONSORED BY WORLD HEALTH ORGANIZATION. Bull World Health Organ. 1965;32:29–36. [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Cross-protection against Salmonella enteritidis infection in mice. J Bacteriol. 1968 Apr;95(4):1343–1349. doi: 10.1128/jb.95.4.1343-1349.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in mice preimmunized with living or ethyl alcohol-killed vaccines. J Bacteriol. 1969 Feb;97(2):676–683. doi: 10.1128/jb.97.2.676-683.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen J. W., Stadler J. Genetic characteristics influencing vaccine-conferred immunity. J Infect Dis. 1967 Apr;117(2):129–150. doi: 10.1093/infdis/117.2.129. [DOI] [PubMed] [Google Scholar]
- Gowen J. W., Stadler J. Specificity of vaccine-conferred resistance to Salmonella typhimurium in mice. J Infect Dis. 1967 Apr;117(2):151–161. doi: 10.1093/infdis/117.2.151. [DOI] [PubMed] [Google Scholar]
- Gröschel D., Paas C. M., Rosenberg B. S. Inherited resistance and mouse typhoid. I. Some factors which affect the survival of infected mice. J Reticuloendothel Soc. 1970 Apr;7(4):484–499. [PubMed] [Google Scholar]
- HERZBERG M., JAWAD M. J., PRATT D. CORRELATION OF SUCCINATE METABOLISM AND VIRULENCE IN SALMONELLA TYPHIMURIUM. J Bacteriol. 1965 Jan;89:185–192. doi: 10.1128/jb.89.1.185-192.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Antibody response and protection induced by immunization with smooth and rough strains in experimental salmonellosis. J Bacteriol. 1968 Feb;95(2):406–417. doi: 10.1128/jb.95.2.406-417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornellas E. P., Roantree R. J., Steward J. P. The specificity and importance of humoral antibody in the protection of mice against intraperitoneal challenge with complement-sensitive and complement-resistant Salmonella. J Infect Dis. 1970 Feb;121(2):113–123. doi: 10.1093/infdis/121.2.113. [DOI] [PubMed] [Google Scholar]
- Previte J. J. Immunogenicity of irradiated Salmonella typhimurium cells and endotoxin. J Bacteriol. 1968 Jun;95(6):2165–2170. doi: 10.1128/jb.95.6.2165-2170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEWE E. Effects of modified host metabolism and altered defense mechanisms on survival time and pathogen counts in tissues and total body of mice infected intravenously with Salmonella typhimurium. J Infect Dis. 1958 May-Jun;102(3):275–293. doi: 10.1093/infdis/102.3.275. [DOI] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Experimental salmonellosis: differential passive transfer of immunity with serum and cells obtained from ribosomal and ribonucleic acid-immunized mice. J Reticuloendothel Soc. 1971 May;9(5):491–502. [PubMed] [Google Scholar]


