Abstract
AIM: To investigate the effects of extracts from Rhazya stricta (R. stricta) and Zingiber officinale (Z. officinale) on human colorectal cancer cells.
METHODS: Human colorectal cancer cells (HCT116) were subjected to increasing doses of crude alkaloid extracts from R. stricta (CAERS) and crude flavonoid extracts from Z. officinale (CFEZO). Cells were then harvested after 24, 48 or 72 h and cell viability was examined by trypan blue exclusion dye test; clonogenicity and soft agar colony-forming assays were also carried out. Nuclear stain (Hoechst 33342), acridine orange/ethidium bromide double staining, agarose gel electrophoresis and comet assays were performed to assess pro-apoptotic potentiality of the extracts. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR), using gene-specific primers and Western blot analyses were performed to assess the impact of CAERS and CFEZO on the expression levels of key regulatory proteins in HCT116 cells.
RESULTS: Treatment with a combination of CAERS and CFEZO synergistically suppressed the proliferation, colony formation and anchorage-independent growth of HCT116 cells. Calculated IC50, after 24, 48 and 72 h, were 70, 90 and 130 μg/mL for CAERS, 65, 85 and 120 μg/mL for CFEZO and 20, 25 and 45 μg/mL for both agents, respectively. CAERS- and CFEZO-treated cells exhibited morphologic and biochemical features of apoptotic cell death. The induction of apoptosis was associated with the release of mitochondrial cytochrome c, an increase in the Bax/Bcl-2 ratio, activation of caspases 3 and 9 and cleavage of poly ADP-ribose polymerase. CAERS and CFEZO treatments downregulated expression levels of anti-apoptotic proteins including Bcl-2, Bcl-X, Mcl-1, survivin and XIAP, and upregulated expression levels of proapoptotic proteins such as Bad and Noxa. CAERS and CFEZO treatments elevated expression levels of the oncosuppressor proteins, p53, p21 and p27, and reduced levels of the oncoproteins, cyclin D1, cyclin/cyclin-dependent kinase-4 and c-Myc.
CONCLUSION: These data suggest that a combination of CAERS and CFEZO is a promising treatment for the prevention of colon cancer.
Keywords: Apoptosis, Colorectal cancer, Oncogenes, Phytochemicals, Tumor suppressor proteins
Core tip: Many medicinal plants represent a cornerstone for chemoprevention of colorectal cancer (CRC). Nonetheless, monotherapy with these agents has been unsatisfactory; therefore attention has turned towards therapies combining these plant-based extracts. In this study, we found that a combination of crude alkaloid and flavonoid extracts prepared from the medicinal herbs Rhazya stricta and Zingiber officinale, respectively, acted synergistically to suppress proliferation, induce apoptosis and modulate expression levels of cell cycle regulatory proteins in CRC cells. These results provide a foundation and rationale for exploiting these extracts for the prevention of CRC.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer deaths worldwide[1]. Although chemotherapy plays an important role in the comprehensive treatment of CRC, an increasing incidence has been reported, with more than 40% of the cases diagnosed in advanced stages[2,3]. Therefore, there is an urgent need to find chemopreventive agents having high specificity for CRC tumor cells.
Available evidence shows that chemopreventive agents inhibit colon tumor growth by enhancing apoptosis[4]. Consistent with this notion, almost all human colon cancers involve the acquisition of specific genetic aberrations that inhibit apoptosis[5,6]. The process of apoptosis occurs via intracellular cysteine proteases called caspases[7,8]. These caspases are activated by two pathways: the death receptor (extrinsic) and mitochondrial (intrinsic) pathways[7,8]. The mitochondrial pathway initiates apoptosis in most physiologic and pathologic situations and is triggered by a variety of apoptotic stimuli. However, both pathways eventually converge on caspase-3 activation, which triggers downstream caspases that cleave proteins essential for cell viability, resulting in morphologic hallmarks of apoptosis[9]. These hallmarks include condensation of the cytoplasm and chromatin, nuclear breakdown, and cell shrinkage and fragmentation into membrane-bound apoptotic bodies that are rapidly phagocytosed by surrounding cells.
Recently, considerable attention has been focused on dietary and medicinal phytochemicals as a rich reservoir for discovery of novel anticancer drugs[10]. However, most human tumors are highly heterogenous as a result of multiple genetic abnormalities[11]. Therefore, relying on a single dietary agent to target a distinct molecular target for therapeutic purposes might not be sufficient to elicit the desired outcome. Furthermore, dietary agents have relatively low potencies compared with pharmacologic compounds[12]. In this regard, it might be possible to attain synergistic or additive preventive effects and improve therapeutic indices by combining dietary agents[13]. Indeed, data from human and animal studies indicate that combinations of dietary agents are more effective than a single agent for chemoprevention of CRC[13-17].
Rhazya stricta Decne (R. stricta, harmal), a member of the Apocynaceae family, is an important medicinal species used in folkloric medicine to cure various diseases in South Asia and the Middle East[18,19]. Extracts of R. stricta leaves have been prescribed for the treatment of various disorders including diabetes, sore throat, helminthiasis, inflammatory conditions and rheumatism[18,19]. In addition, we previously reported that an aqueous extract of R. stricta inhibited cell proliferation and induced apoptotic cell death in the breast cancer cell lines, MCF-7 and MDA MB-231[20]. The herb is particularly rich in alkaloids, over 100 of which have been isolated, characterized and identified from its roots, stems and leaves[18]. The fact that R. stricta is an alkaloid-rich herb deserves attention since alkaloids are among the most important phytochemicals known to display antiproliferative and antimetastatic effects on various types of cancers both in vitro and in vivo[21]. For example, some alkaloids, such as camptothecin, and vinca alkaloids, including vincristine and vinblastine[22], have already been successfully developed into anticancer drugs. In our earlier study, we found that a crude alkaloid extract from R. stricta inhibited cell growth and sensitized human lung cancer cells (A549) to cisplatin through induction of apoptosis[23]. Finally, a recent study by El Gendy and and his associates demonstrated that the strongly basic alkaloid fraction in R. stricta induced activity of the chemopreventative enzyme, Nqo1[24]. The authors concluded that this activity could be a novel mechanism for the traditional use of R. stricta’s alkaloid as an antitumor agent[24].
Zingiber officinale Rosc (Z. officinale, ginger), a member of the Zingiberaceae family, is a medicinal herb used for treatment of various illnesses, including gastrointestinal ailments, arthritis, rheumatism, pain, muscle discomfort, cardiovascular diseases, and metabolic disorders[25]. Some compounds present in the herb possess strong anti-inflammatory and antioxidative properties and exert substantial antimutagenic and anticarcinogenic activities[25-27]. Recent work has shown that the bioactive molecules of Z. officinale are 6-gingerol, flavonoids and phenolic acids[28]. In particular, 6-gingerols, 6-shogaols and related compounds have been shown to inhibit growth and induce apoptosis in human colorectal carcinoma cells[25]. Thus, research has focused on these as the anticancer bioactive compounds and ignored the flavonoids in Z. officinale. Flavonoids are the polyphenolic phytochemicals that possess a wide range of pharmacologic properties, such as antimicrobial, antiviral, anti-inflammatory, anti-allergic, analgesic, antioxidant and hepatoprotective activities[29]. In addition, many flavonoids mediate various biologic activities targeted by anticancer agents, such as apoptosis and cell cycle arrest, and demonstrate antiproliferative, anti-angiogenic and antioxidative effects[30,31]. Furthermore, Z. officinale supplementation is chemopreventive during the initiation of colon cancer in Wistar rats[32] and in colon cancer cell lines[33]. However, further studies are required to investigate the impact of flavonoids in Z. officinale on colon cancer cells.
Thus far, basic reports of combining R. stricta and Z. officinale are lacking and not credible enough to allow a general recommendation for using both herbs as effective agents for chemoprevention of CRC. The current study was carried out to assess the combined effect of R. stricta’s alkaloids and Z. officinale’s flavonoids on treatment of CRC. We hypothesized that because of their substantially different biochemical activities, a combinational approach would allow targeting of multiple molecular and cellular pathways involved in the process of CRC carcinogenesis.
MATERIALS AND METHODS
Preparation of crude extracts
For preparation of crude flavonoid extract from Z. officinale (CFEZO), a rhizome of the herb was purchased from a local market and powdered. Extraction was performed by cold percolation with 70% (2 L) ethanol for 72 h at room temperature and filtered. The process was repeated twice and the combined filtrates were concentrated in a vacuum evaporator. The residue was suspended in 250 mL of hot water (60 °C), filtered and defatted by using petroleum ether (250 mL × 3). The aqueous portion was then separated, collected and fractionated with N-butanol-saturated water (250 mL × 3). The aqueous portion was discarded and the N-butanol portion was then separated, collected before being fractionated with 1% KOH. The KOH portion was then fractionated with dilute HCl (2%) and N-butanol-saturated water. The dilute HCl portion was discarded. The N-butanol portion was then separated, collected and dried. Before use, the stock was further diluted in dimethyl sulfoxide (DMSO) to give the final indicated concentrations.
A crude alkaloid extract of R. stricta (CAERS) was prepared as previously described[23], with some modifications. Briefly, air-dried leaves of R. stricta (350 g) were soaked in 80% methanol (1 L) at ambient temperature for seven days, after which the methanolic extract was evaporated in a rotatory evaporator and the remaining residue was suspended in water and filtered. The aqueous extract was then acidified with 10% glacial acetic acid and extracted with chloroform. The chloroform was discarded, and the aqueous solution was alkalinized using NaOH and the pH was adjusted to 11. The alkaline aqueous layer was extracted with chloroform to yield a chloroform fraction enriched in strongly basic alkaloids. The chloroform was then evaporated to obtain a crude extract. Before use, the stock was further diluted in DMSO to give the final indicated concentrations.
Phytochemical examination to confirm the presence of alkaloids in CAERS was carried out using Dragendorff’s and Mayer’s tests. Briefly, 5 mL of CAERS was stirred with 5 mL of 1% aqueous HCl before being filtered and aliquoted into two test tubes. The presence of alkaloid materials was indicated by an orange precipitate with the addition of Dragendorff’s reagent (solution of potassium bismuth iodide), and the formation of a buff-colored precipitate when Mayer’s reagent (potassium mercuric iodide) was added[34].
Phytochemical examination to confirm the presence of flavonoids in CFEZO was carried out using a sodium hydroxide test. Five mL of the prepared CFEZO was dissolved in water, filtered and mixed with 2 mL of 10% sodium hydroxide. The presence of flavonoids was indicated by the initial formation of a yellow color, which turned colorless after the addition of dilute hydrochloric acid[34].
Cell culture
The human CRC cell line HCT116 was obtained from the King Fahd Center for Medical Research, King Abdulaziz University, Saudi Arabia. The cells were grown in DMEM (Promega, Madison, WI, USA) containing 10% FBS (Promega) and 1% penicillin-streptomycin antibiotics (Promega) in tissue culture flasks under a humidifying atmosphere containing 5% CO2 and 95% air at 37 °C. The cells were subcultured every 3-4 d.
Cell viability, clonogenic and soft agar colony-forming assays
Cell viability and the effects of CAERS and/or CFEZO on the growth of HCT116 cells were assessed with the trypan blue dye exclusion assays. Briefly, HCT116 cells were seeded onto 24-well plates (5 × 104 cells/well) and grown overnight. The cells were then treated with indicated concentrations of the extracts and incubated for 24, 48 or 72 h. At the end of each incubation, the floating and adherent cells were collected (with care being taken that none of the floating cells were lost during washes) and pelleted by centrifugation (700 g, 5 min). The cells were re-suspended in 25 μL of PBS, mixed with 5 μL of 0.4% trypan blue solution and counted using a hemocytometer under an inverted microscope. Cell growth rates were determined by counting the number of viable cells in each CAERS- and/or CFEZO-treated wells and expressed as a percentage of the total number of viable cells in the control well (no treatment). The effects of CAERS- and/or CFEZO on growth inhibition were assessed as percent cell viability, where non-treated cells were considered 100% viable. For these studies, all experiments were repeated three or more times.
For clonogenic assays, approximately 500 cells were seeded into six-well plates. After 24 h, media were changed and cells were treated with indicated concentrations of CAERS and/or CFEZO. The cells were grown for 15 d to form colonies. Then, plates were washed with PBS, fixed with -20 °C methanol and stained with 0.1% Coomassie Brilliant Blue. The colonies that had > 50 cells/colony were counted and expressed as percent control.
For soft agar colony-forming assays, HCT116 cells were seeded at 5000 cells per well in 0.35% top agarose (Promega) with a base of 0.7% agarose supplemented with complete medium. Cultures were treated with indicated concentrations of CAERS and/or CFEZO and incubated in a humidified incubator at 37 °C for 3 wk. Cells were then stained with 0.5 mL of 0.0005% crystal violet, and colonies were counted visually. All experiments were performed in triplicate with two independent experiments.
Assessment of cell morphologic changes and electron microscopy
Cells (2 × 104) were plated on coverslips, allowed to attach overnight, and exposed to indicated concentrations of CAERS and/or CFEZO for 48 h. After incubation, cells were harvested and centrifuged for 5 min at room temperature. Then, the supernatant was decanted and pellets were dried and fixed with 2.5% glutaraldehyde and 2% formaldehyde in 0.1 M sodium cacodylate buffer (pH 7.2). Fixed samples were then washed in the same buffer and postfixed in 1% osmium tetroxide. The cells were dehydrated in a graded ethanol series and propylene oxide. The resin infiltration was performed with a 1:1 mixture of propylene oxide and epon for 5 h, followed by 100% epon for another 5 h. Next, the material was embedded, followed by 48 h of polymerization. Thin sections were produced using an ultramicrotome, (LEICA EM UC6; Leica Microsystems GmbH, Wetzler, Germany) and stained with Toluidine Blue.
For scanning electron microscopy studies, cells grown on coverslips were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) with 2% sucrose at room temperature for 20 min. After postfixation with 1% OsO4 in 0.1 mM cacodylate buffer (pH 7.3) at room temperature for 30 min, cells were dehydrated through graded ethanol concentrations, critical point-dried in CO2 (CPD 030; Bal-Tec of Leica Microsystems) and gold coated by sputtering (SCD 040 Balzers device). The samples were then examined with a Cambridge Stereoscan 360 scanning electron microscope (Cambridge Instruments, Cambridge, UK).
Apoptotic assay
The nuclear morphologic changes associated with apoptosis were analyzed using Hoechst 33342 as well as acridine orange (AO)/ethidium bromide (EB) (Sigma-Aldrich, St. Louis, MO, USA) staining[35]. Briefly, cells (2 × 104) were plated on coverslips, allowed to attach overnight, and exposed to indicated concentrations of CAERS and/or CFEZO for 48 h. The cells were washed with PBS and fixed for 15 min at room temperature. Fixed cells were again washed with PBS, and stained with Hoechst 33342 or AO/EB for 15 min at room temperature. The cells were washed twice more with PBS and analyzed via a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
DNA fragmentation assay
DNA gel electrophoresis was used to determine the presence of internucleosomal DNA cleavage as described previously[23]. Briefly, HCT116 cells (3 × 106 cells/100 mm dish) treated with indicated concentrations of CAERS and/or CFEZO for 24 h were collected, washed in PBS and centrifuged at 12500 g for 5 min. Cell pellets were then lysed in 600 μL lysis buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA and 0.5% Triton X-100], kept on ice for 30 min, and centrifuged at 12500 g for 20 min. The supernatant with DNA fragments was transferred into 1.5 mL tubes and incubated with 2 μL RNase A (20 mg/mL) for 1 h at 37 °C, and then with 2 μL proteinase K (20 mg/mL) at -20 °C overnight. After centrifugation at 12500 g for 20 min, the sediment was dissolved in 30 μL TE buffer [10 mM Tris (pH 7.4), 1 mM EDTA (pH 8.0)] and the concentration of DNA was determined spectrophotometerically. DNA was resolved by electrophoresis (80-100 V) on a 1.5% agarose gel stained with ethidium bromide, and visualized with a UV trans-illuminator (Bio-Rad, Hercules, CA, USA).
Single-cell gel electrophoresis (comet assay)
CAERS-/CFEZO-induced DNA damage was determined using the comet assay. Cells were treated with indicated concentrations of CAERS and/or CFEZO for 24 h in complete medium, harvested, resuspended in ice-cold PBS and processed under a dimmed light as described earlier[36]. Prepared comet slides were viewed and nuclei images were visualized and captured at 400× magnification with an Axioplan 2 fluorescence microscope (Zeiss) equipped with a CCD camera (Optronics, Goleta, CA, USA). Hundreds of cells were scored to calculate the overall percentage of comet tail-positive cells.
RNA extraction and qRT-PCR
Cells were seeded (2 × 105/well) onto 6-well plates and treated with indicated concentrations of CAERS and/or CFEZO for 24 h. After this period, floating and adherent cells were carefully collected and pelleted by centrifugation (700 g, 5 min). RNA extraction and reverse transcriptase-PCR were performed as previously described[23]. Briefly, total RNA was extracted using the SV Total RNA Isolation System (Promega) before being reverse transcribed and amplified with the GoTaq 1-Step RT-qPCR System (Promega) according to the manufacturer’s instructions. PCR was initiated with a reverse transcription step at 37 °C for 15 min followed by a reverse transcription inactivation/hot-start activation step at 95 °C for 10 min. Then, the PCR conditions were optimized for each gene and terminated within the linear portion of the amplification. Briefly, PCR conditions included an initial denaturation step at 95 °C for 5 min followed by 28 cycles of: 95 °C for 30 s, annealing at primer pair-optimized temperatures, and 72 °C for 1 min. The primer sequences used to detect Bcl-2, Bcl-xL, Mcl-1, survivin, Bad, Noxa, X-linked inhibitor of apoptosis protein (XIAP) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were described earlier[37]. Amplification products were separated by electrophoresis on 1% agarose gels and visualized with EB (0.5 μg/mL) staining.
Preparation of mitochondrial and cytosolic extracts
Mitochondrial and cytosolic extracts were obtained as described previously[23]. Briefly, cells were seeded (2 × 105/well) onto 6-well plates and treated with the indicated concentrations of CAERS and/or CFEZO for 24 h. After this incubation, the cells were collected by centrifugation, washed twice with cold PBS, resuspended in 500 μL of ice-cold cytosol extraction buffer [20 mM HEPES (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA] containing a protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride, 1% aprotinin, 1 mM leupeptin and 1 μg/mL pepstatin A). After incubating for 30 min on ice, the cells were homogenized in the same buffer using a dounce homogenizer (30 strokes) and centrifuged (1000 g, 10 min at 4 °C). The supernatant was collected and centrifuged again (14000 g, 30 min) to collect the mitochondria-rich (pellet) and cytosolic (supernatant) fractions. The supernatant was used as cytosolic lysate while the pellet was suspended in lysis buffer [137 mM NaCl, 20 mM Tris (pH 7.9), 10 mM NaF, 5 mM EDTA, 1 mM EGTA, 10% (v/v) glycerol, 1% Triton X-100] supplemented with a protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem of Merck KGaA, Darmstadt, Germany) before being centrifuged to obtain the mitochondrial lysate. Protein concentrations were determined with a BCA protein assay kit (Pierce of Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instructions.
Western blot analysis
Cells were seeded (2 × 105/well) onto 6-well plates and treated with the indicated concentrations of CAERS and/or CFEZO for 24 h. Then, protein lysates were prepared using cold lysis buffer [0.05 mM Tris-HCl, 0.15 mM NaCl, 1 M EGTA, 1 M EDTA, 20 mM NaF, 100 mM Na3VO4, 0.5% NP40, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (pH 7.4)] with a protease inhibitor cocktail. The lysates were cleared by centrifugation, and the supernatants were aliquoted and stored at -80 °C. The protein concentrations were measured using the BCA protein assay as described above.
Western immunoblotting was performed as described elsewhere[23]. Briefly, aliquots of the lysates were boiled for 5 min in SDS-PAGE sample buffer containing 5% β-mercaptoethanol and loaded into 10% gels for SDS-PAGE at 110 V for 2 h. Proteins were transferred to polyvinylidene fluoride membranes and incubated with primary antibody: anti-caspase 3 (C9598), anti-caspase 9 (C7729), anti-Bcl-2 (SAB45000053), anti-Bax (B3428), anti-cytochrome c (C9616), anti-p53 C-terminal (SAB4503001), anti-β-actin (1A5441), anti-p21 (SAB4500065), anti-p27 (SAB4500067) (Sigma-Aldrich); anti-cyclin D1 C-terminal (M-20) (sc-718), anti-CDK-4 (sc-260) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-c-Myc C-terminal (C-19) (sc-788) (Santa Cruz Biotechnology Inc., Dallas, TX, USA); anti-poly ADP-ribose polymerase (PARP) (E4224; Spring BioScience, Pleasanton, CA, USA). The PVDF membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies: anti-rabbit IgG (A4914), anti-mouse IgG (A9044), anti-sheep IgG (A3415) (Sigma). Proteins were visualized using an enhanced chemiluminescence detection kit (Amersham of GE Healthcare, Buckinghamshire, UK). In all experiments, the membranes were stripped with stripping buffer [62.5 mM Tris (pH 6.7), 2% SDS and 90 mM β-mercaptoethanol] and reprobed with anti-β-actin as a control for protein loading.
Statistical analyses
All experiments were carried out using three replicates in three independent experiments. The results are presented as mean ± SD for continuous variables. Differences between samples were analyzed with by one-way analysis of variance (ANOVA). The level of statistical significance was set at P ≤ 0.05.
RESULTS
CAERS and CFEZO act synergistically to inhibit cell proliferation and colony formation in HCT116 cells
Although CRC carcinogenesis is a multistage process involving numerous molecular events, enhanced cell proliferation and ablation of apoptosis are early underlying events[6]. Therefore, to study whether CAERS or CFEZO might inhibit proliferation of CRC cells, we examined the sensitivity of HCT116 cells to various doses of both extracts. Cells were treated with increasing concentrations of CAERS or CFEZO (0, 50, 75, 100 and 125 μg/mL) for 24, 48 or 72 h. The trypan blue exclusion dye assay showed that incubation with either extract efficiently inhibited cell viability in dose- and time-dependent manners (Figure 1A). The IC50 concentrations after 24, 48 and 72 h of incubation were 70, 90 and 130 μg/mL, respectively, for CAERS and 65, 85 and 120 μg/mL for CFEZO. Cells were also treated for 24, 48 and 72 h with increasing combined doses of CAERS and CFEZO (0, 7.5, 12.5, 20 and 25 μg/mL each). The IC50 values for the combined were lower (20, 25 and 45 μg/mL for 24, 48 and 72 h, respectively), indicating a synergistic effect of the two extracts. Although the IC50 for the 20 μg/mL doses of CAERS and CFEZO was lower than for the 12.5 μg/mL doses, an unacceptable level of cytotoxicity (necrotic cells) was found using Wright staining (data not shown). Therefore, the 12.5 μg/mL doses were chosen for subsequent experiments with combination treatments, and a 100 μg/mL dose was chosen for single treatments.
Figure 1.
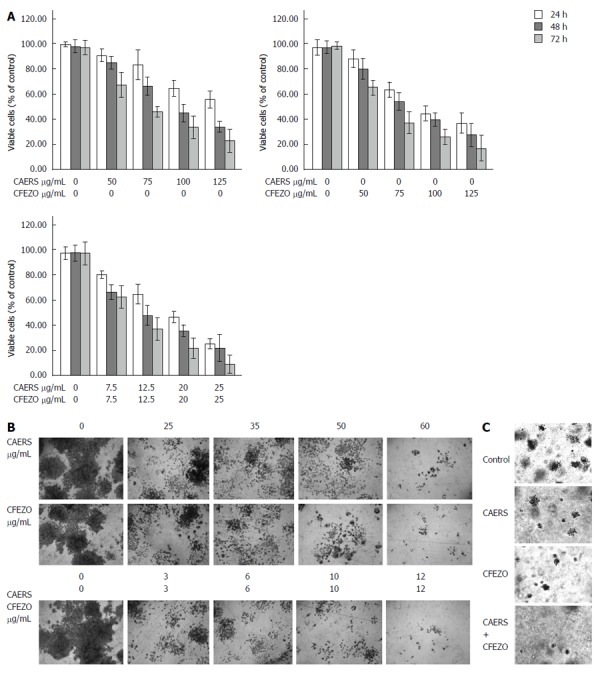
Combination of crude alkaloid and flavonoids acts synergistically to inhibit cell proliferation and colony formation in HCT116 cells. A: Cell viability of HCT116 cells (5 × 104 cells/well) treated with the indicated concentrations of crude alkaloid extract of R. stricta (CAERS) and/or crude flavonoid extract of Z. officinale (CFEZO) for 24 h (white bars), 48 h (gray bars) and 72 h (light grey bars) were assess by trypan blue dye exclusion assay. The experiments were repeated five times in triplicates, and values are reported as the mean ± SD; B: For colony formation, HCT116 cells treated with the indicated concentrations of CAERS and/or CFEZO and counted under a dissection microscope and the experiment was repeated three times (magnification: × 6); C: Crystal violet staining of HCT116 cells grown in soft agarose assays to assess anchorage-independent growth. HCT116 cells were treated with CAERS (25 μg/mL), CFEZO (25 μg/mL), or a combination (3 μg/mL) for 2 wk (magnification: × 6).
Having established growth-inhibiting potentials of CAERS and CFEZO, we next determined their effects on colony formation (clonogenicity assay) in HCT116 cells. The clonogenicity assay provides an indirect estimation of the tendency of tumor cells to undergo neoplastic transformation. HCT116 cells were plated onto multiple-well tissue culture dishes with and without addition of increasing doses of CAERS and/or CFEZO. Control and treated cells were maintained in culture for an additional 14 d to allow formation of colonies. Figure 1B shows that single and combination treatments reduced the number and size of growing colonies.
To further validate effects of CAERS and CFEZO on HCT116 colony formation, we carried out agar colony-forming assays. This assay is used to measure the ability of cells to grow in soft agar in an anchorage-independent manner, which is considered the most stringent assay for detecting malignant transformation of cells. Whereas the growth of untreated HCT116 cells in soft agar was robust, treatments with CAERS and/or CFEZO inhibited colony growth (Figure 1C).
CAERS and CFEZO induce morphologic features of apoptosis
It is generally believed that the induction of apoptosis is the primary cytotoxic mechanism of phytochemicals[4]. To determine whether CAERS and CFEZO inhibited cell growth by inducing apoptosis, HCT116 cells were treated with increasing concentrations of the extracts alone or in combination for 48 h, and the frequency of apoptotic cell death was assessed by light microscopy. As seen under an inverted phase microscope, untreated HCT116 cells grew well to form a confluent monolayer with a homogenous morphology containing lightly and evenly stained nuclei (Figure 2A). In contrast, morphologic changes characteristic of apoptosis appeared with extract treatment, and were most apparent with the combination of CAERS and CFEZO. Pairwise comparisons demonstrated that control HCT116 cells appeared adherent with a normal epithelial morphology, whereas CAERS- and CFEZO-treated cells much more readily detached and exhibited rounded-up, balloon-like shapes. These observations were confirmed by electron microscopy of toluidine blue-stained semithin sections (Figure 2B). Figure 2C presents scanning electron microscope images showing the global form, integrated surface and abundant microvilli of control cells, compared to the wrinkled and smaller cells with irregular outline, broken surfaces, and loss of microvilli with CAERS and/or CFEZO treatments.
Figure 2.
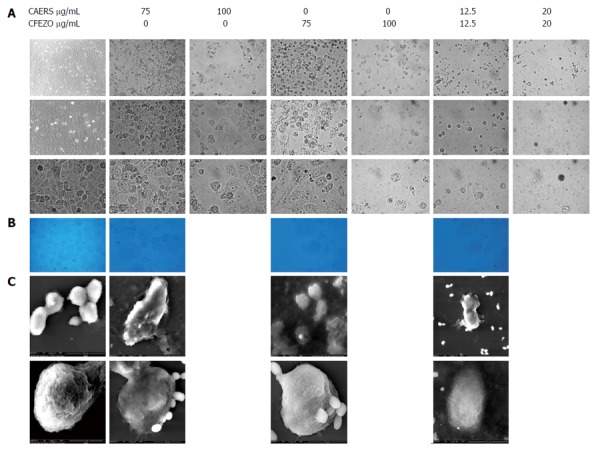
Photomicrographs showing crude alkaloid and/or flavonoid treatments induced morphologic features of apoptosis in HCT116 cells. Cell were treated for 48 h with indicated concentrations of crude alkaloid extract of R. stricta (CAERS) and/or crude flavonoid extract of Z. officinale (CFEZO) and observed with A: Light microscopy (first row: magnification × 20; second row: magnification × 40; third row: magnification × 63); B: Electron microscopy of toluidine blue-stained semithin sections (magnification: × 100); and C: Scanning electron microscopy (magnification: × 25026). Note deformed morphology of treated cells, blebbing of cellular membrane, granular surface and fragmentation of cells into apoptotic bodies.
CAERS and CFEZO induce DNA damage
A hallmark of apoptotic cell death is the shrinkage and fragmentation of both cells and their nuclei[9]. As depicted in Figure 3A (top two rows), Hoechst staining revealed the occurrence of nuclear condensation, DNA fragmentation, and perinuclear apoptotic bodies in HCT116 cells treated with CAERS and CFEZO, but not in control cells. To further confirm these findings, cells were stained with AO, which permeates all cells and the nuclei become green, and EB, which is only taken up when cytoplasmic membrane integrity is lost, and their nuclei are stained red. The results in Figure 3A (last row) demonstrate that untreated cells displayed bright green nuclei, whereas cells treated with CAERS and CFEZO appeared red.
Figure 3.
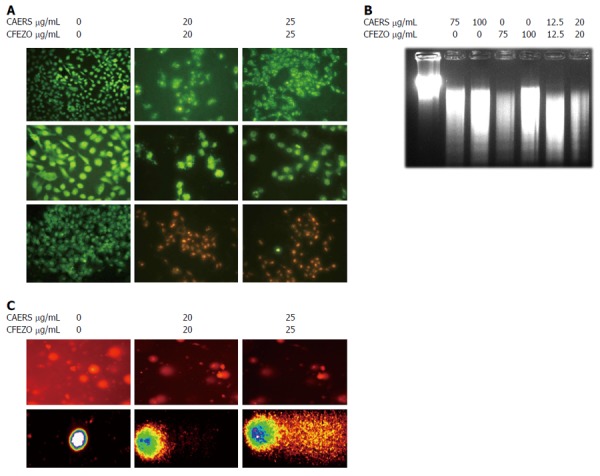
Combination of crude alkaloid and flavonoid induce biochemical features of apoptosis. HCT116 cells were treated for 24 h with indicated concentrations of crude alkaloid extract of R. stricta (CAERS) and/or crude flavonoid extract of Z. officinale (CFEZO) and assayed for existence of apoptotic cell death. A: Fluorescent images from Hoechst 33342 staining (first and second rows) or acridine orange/ethidium bromide (third row) (arrows indicate nuclear condensation, DNA fragmentation, and perinuclear apoptotic bodies) (magnification: first row: × 20; second and third rows: × 40); B: Agarose gel showing DNA fragmentation in HCT116 cells; C: Comet assay showing formation of DNA tail in CAERS and CFEZO-treated HCT116 cells. Nuclei with damaged DNA have the appearance of a Comet with a bright head and a tail, whereas nuclei with undamaged DNA appear round with no tail.
A characteristic feature of apoptosis is fragmentation of DNA to yield DNA ladders[9]. To examine whether CAERS or CFEZO might induce such fragmentation in HCT116 cells, genomic DNA was extracted and separated by agarose gel electrophoresis. Figure 3B shows the presence of DNA ladders in samples from cells treated with CAERS and/or CFEZO. To substantiate these findings, a comet assay was performed, which is a sensitive method for monitoring single strand DNA breaks at the single cell level and is used as a biomarker of apoptosis[38,39]. As shown in Figure 3C, treatment of HCT116 cells with CAERS and CFEZO for 48 h resulted in an increased comet length, indicative of marked DNA damage. Taken together, these data suggest that CAERS and induce apoptosis in HCT116 cells.
CAERS and CFEZO downregulate anti-apoptotic proteins and activate caspase cascades
Apoptosis is tightly regulated by the Bcl-2 family of proteins (including Bax), and involves release of cytochrome c from mitochondria and activation of caspases[7]. To examine these processes, HCT116 cells were treated with CAERS and CFEZO, and the levels of cytochrome c were measured in cytosolic and mitochondrial fractions. The data in Figure 4A reveal that treatments with CAERS and CFEZO reduced the levels of cytochrome c in the mitochondrial fraction, and increased the levels in the cytosolic fraction. To verify the purity and uniform loading of the fractions, blots were re-probed with an anti-cytochrome oxidase IV antibody. Moreover, treatment with CAERS and CFEZO increased the levels of cysteine proteases caspase-3 and -9. The increased activity of caspase-3 was verified by the reduced level of the nuclear DNA repair enzyme PARP.
Figure 4.
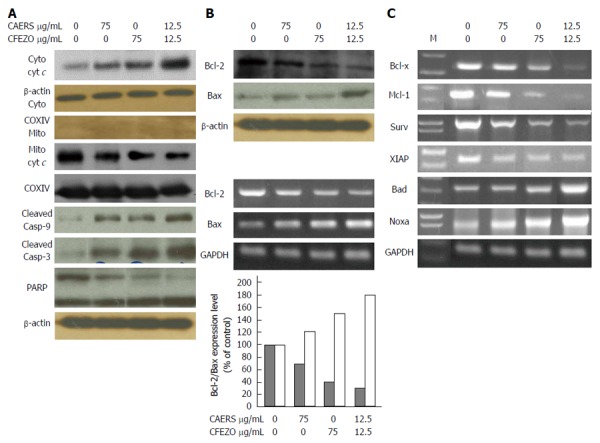
Crude alkaloid and flavonoid treatments trigger mitochondrial-dependent apoptotic pathway. HCT116 cells were treated with indicated concentrations of crude alkaloid extract of R. stricta (CAERS) and/or crude flavonoid extract of Z. officinale (CFEZO) for 24 h. A: Western blots for apopototic proteins in cytosolic and mitochondrial fractions; B: Western blotting (top panel) and qRT-PCR analysis for Bcl-2 and Bax. The histogram depicts the Bcl-2 (dark bars) and Bax (white bars) mRNA ratio measured by using densitometric analysis; C: Ethidium bromide staining of antiapoptotic and proapoptotic gene products obtained from qRT-PCR. M, DNA ladder.
Next, we examined the effect of the CAERS and CFEZO treatments on expression levels of Bcl-2 (anti-apoptotic) and Bax (proapoptotic). As shown in Figure 4B, treatments with CAERS and CFEZO increased expression of Bax and concomitantly reduced expression of Bcl-2 as observed by Western blotting (top panels) and by qRT-PCR (bottom panels). qRT-PCR analysis also revealed that treatments with CAERS and CFEZO resulted in downregulation of the proapoptotic genes, Bcl-xL, Mcl-1, survivin and XIAP, but upregulation of Bad and Noxa (Figure 4C).
CAERS and CFEZO modulated expression levels of molecules involved in cell survival and proliferation
To further examine the effects of CAERS and CFEZO on HCT116 cells, we monitored the expression level of p53, the tumor suppressor protein that can initiate either cell cycle arrest and DNA repair or apoptosis[40]. Expression of p53 protein was increased in cells treated with CAERS and CFEZO (Figure 5). Furthermore, treatments reduced the expression of cyclin D1 and cyclin/cyclin-dependent kinase (Cdk)-4, proteins that regulate the G1/S phase transitions of the cell cycle. These were accompanied by an increase in the expression of p21, but not p27, both of which regulates the Cdk-4/cyclin complex. In addition, treatments decreased the expression of c-Myc, another cell cycle control oncoprotein whose increased expression is associated with early stages of colon carcinogenesis in both human and rodents[41,42]. These findings indicate that treatment with CAERS and CFEZO modulates several molecular and cellular targets involved in CRC carcinogenesis.
Figure 5.
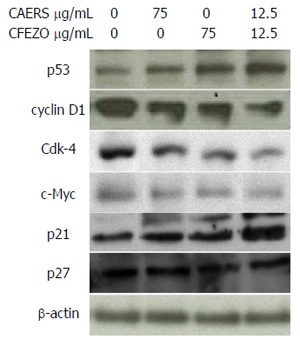
Crude alkaloid and flavonoid treatments modulate the expression of cell cycle-regulating proteins. Western blot results for cell cycle-regulating proteins in HCT116 cells treated with indicated concentrations of crude alkaloid extract of R. stricta (CAERS) and/or crude flavonoid extract of Z. officinale (CFEZO) for 24 h.
DISCUSSION
Many studies suggest the utility of natural compounds as chemopreventive agents against CRC[43]. The present study is part of a large-scale approach to develop novel strategies for treatment CRC cancer using combinations of phytochemicals. The rationale for using a combination of phytochemicals is to increase chemopreventive efficacy and to neutralize the adverse effects and toxicities of specific individual chemicals. Many earlier studies documented that dietary/natural agents can work in a synergistic or additive manner to inhibit CRC carcinogenesis. However, the challenge is to pinpoint an effective combination, with chemopreventive agents working through complementary mechanisms to produce an additive or synergistic chemopreventive effect. We previously reported that ethanol/aqueous extract of R. stricta (and ethanol/aqueous of Z. officinale) independently suppressed proliferation and induced apoptosis in human breast cancer cell lines[20,44]. In light of these earlier studies, we carried out the current work to evaluate the beneficial effect of a combination of Z. officinale and R. stricta in an in vitro model of CRC.
The results of this study demonstrate that alkaloids and flavonoids extracted from these herbs inhibited cell proliferation and colony formation in a dose- and time-dependent manner. More importantly, the results reveal that a combination of low doses of CAERS and CFEZO (that were insufficient to suppress growth individually) suppressed HCT116 proliferation and colony formation and induced apoptosis more effectively than high-dose individual treatments. This was also apparent from IC50 values of cell viability assays, where the IC50 value for the combined treatment of CAERS and CFEZO was lower than the IC50 values for single treatments. These results indicate that there was a synergistic effect for the combined treatment and raises a possibility that the chemical entities found in the CAERS and CFEZO formula represent an effective combination for neutralizing the adverse effects and toxicities of individual alkaloids and flavonoids.
The synergistic effect of CAERS and CFEZO was supported by observations from the soft agar colony formation assay. This assay was developed to measure the ability of tumor cells to grow and form foci in a manner unrestricted by growth contact inhibition, as is characteristically found in normal, untransformed cells[45]. In this assay cells are seeded in a soft agar media matrix where they are unable to attach to an underlying substrate, which is an essential perquisite for cell growth. If cells are able to proliferate they will grow and form colonies. The data generated from this assay ascertain that only control, but not treated, HCT116 cells were able to grow in soft agar. This suggests that CAERS and CFEZO treatments inhibited the anchorage-independent growth potentiality of HCT116 cells. Therefore, in the present study, we have identified a novel combination of two phytochemical agents, CAERS and CFEZO, which exhibit a synergistic mode of action to target growth of HCT116 CRC cells. Thereby, these agents could be promising candidates for designing new remedies against CRC. Accumulating evidence demonstrates that interactions between cancer cells and extracellular substrates are essential for cell growth and survival, and disruption of this connection has deleterious effects[46]. The observation that CAERS- and CFEZO-treated cells more readily detached suggests that treatment caused an interruption of HCT116 cell-substrate adhesions, which might contribute to the inhibition of cell proliferation.
A growing list of studies demonstrate that deregulated apoptotic pathways play a central role in developing CRC[5,6]. The onset of apoptosis is characterized by shrinkage of the cell and condensation of the nuclear chromatin into sharply delineated masses, followed by karyorrhexis and eventual cleavage of the cells into apoptotic bodies[9]. These characteristics were observed in HCT116 cells treated with CAERS and CFEZO. In addition, scanning electron microscope images of cells treated with CAERS and CFEZO showed signs typical of apoptosis, such membrane blebbing and shrinkage. Furthermore, nuclear condensation, DNA fragmentation, and perinuclear apoptotic bodies were observed in CAERS- and CFEZO-treated cells stained with Hoechst or AO/EB, a well-accepted technique for quantitation of apoptosis[47], and by DNA laddering. DNA damage was also confirmed by a comet assay, which is more sensitive than DNA ladder assay in detecting DNA damage and distinguishes apoptosis from necrosis[48]. Untreated cells did not exhibit a comet tail, however, we could observe the appearance of a tail after CAERS and CFEZO treatments, indicating that there is DNA damage. Since cells can undergo apoptosis in response to DNA damage that is beyond repair, therefore, all these findings suggest that CAERS and CFEZO treatments might trigger events leading to DNA damage and initiation of apoptotic cascades, which may contribute, at least in part, to reduction of HCT116 cell viability.
It is generally accepted that most anticancer agents either directly induce DNA damage or indirectly induce secondary stress-responsive signaling pathways to trigger apoptosis by activation of the intrinsic (mitochondrial) apoptotic pathway[4]. The key element in the intrinsic pathway is the release of cytochrome c from the mitochondria to the cytosol, which, together with Apaf-1, activates caspase-9. Caspase-9 cleaves procaspase-3, releasing the active 17 kDa fragment from the 32 kDa inactive precursor. Caspase-3 is a prevalent cysteine protease ultimately responsible for the majority of apoptotic processes, and mediates the cleavage or degradation of several important substrates. Once activated, caspase-3 proteolytically cleaves the 116 kDa PARP protein into an 85 kDa fragment, which is a nuclear enzyme that is involved in DNA repair in response to various stresses[49] and considered to be a biochemical characteristic of apoptosis[9]. Our findings fit very well with this scenario, since Western blot analysis indicated that treatment of HCT116 cells with CAERS and CFEZO induced the release of cytochrome c from the mitochondria into the cytosol. Additionally, we found increases in caspases 9 and 3 and cleavage of PARP. Intact PARP can help cells to maintain their viability, but cleavage of PARP facilitates cellular disassembly and serves as a marker of cells undergoing apoptosis. Therefore, cleavage of PARP might be the key for the ultimate apoptotic death of HCT116 cells induced by CAERS and CFEZO treatments. Collectively, these results support the hypothesis that the mitochondrial pathway is involved in apoptosis induced by CAERS and CFEZO in HCT116 cells.
The balance between proapoptotic and anti-apoptotic proteins within apoptotic pathways controls programmed cell death or survival50]. The pro-survival Bcl-2 and its helpers compete with Bax and other proapoptosis proteins to regulate the release of proteins and cytochrome c from mitochondria, which in turn activate “initiator” caspases including caspase-9[50]. In the current study, we observed that CAERS and CFEZO treatments caused upregulation of Bax with concomitant downregulation of Bcl-2, in essence, leading to an increase in the Bax/Bcl-2 ratio, which tips the balance towards apoptotic death. This is a noteworthy finding, knowing that Bax expression obviously decreases in both the initiating and developmental stages in human CRC tissue[6] and CRC exhibits intrinsic apoptosis resistance related, in part, to overexpression of Bcl-2 protein[6]. Moreover, earlier studies have related a poor prognosis for colon cancer patients with low Bax and high Bcl-2[50,51]. Furthermore, CAERS and CFEZO treatments led to decreased expression of other critical anti-apoptotic proteins, including Bcl-xL, Mcl-1, survivin and XIAP. Overexpression of Bcl-xL has been found to suppress the activity of the proapoptotic molecules, Bax and Bak, which contributes to CRC progression[5,6] and a poor prognosis for colon cancer patients[52]. Furthermore, the NIH Developmental Therapeutics Program revealed that Bcl-xL may play a unique role in the general resistance of cancer cells to cytotoxic agents, as a variety of cancer cell lines demonstrating resistance to 70000 cytotoxic agents are characterized by high Bcl-xL expression[53]. Like Bcl-xL, overexpression of Mcl-1 contributes to the resistance of neoplastic cells towards apoptosis in multiple tumors, including CRC[54]. In addition, studies of molecular targeted therapies showed that the Bcl-2 family-targeting compounds, such as BH3 mimetics and ABT-737, bind with high affinity to anti-apoptotic proteins Bcl-2, Bcl-xL and Bcl-w, but not to Mcl-1[55]. Furthermore, none of the BH3 mimetics under current development are potent and specific Mcl-1 antagonists[56]. Hence, a molecular ABT-737 therapy would be ineffective in cells expressing significant amounts of Mcl-1 such as CRC cells[54]. On the other hand, it has been demonstrated that only Noxa, but not other BH3-only family members, finely tunes cell death decisions by targeting the Mcl-1 for proteasomal degradation[56] and induction of Noxa sensitized human CRC cells over-expressing Mcl-1 to ABT-737[57]. Since CAERS and CFEZO treatments downregulated expression of Mcl-1 (but upregulated expression of Noxa), these treatments might be a practical remedy to promote apoptotic death or to sensitize CRC cells to molecular-targeted agents, such as ABT-737. Survivin and XIAP proteins are members of the inhibitors of apoptosis protein family, which block apoptosis by inhibiting activity of caspase-3. Furthermore, the targeted inhibition of XIAP or survivin genes has been shown to directly sensitize cancer cells to apoptosis induced by various conventional chemotherapeutic drugs[58]. In particular, survivin has been found to confer radio/chemotherapy resistance to CRC and other neoplastic cells[59]. The data herein demonstrate that expression levels of the survivin and XIAP were downregulated with CAERS and CFEZO treatments. Thus, the ability of CAERS and CFEZO treatments to down-regulate expression levels of the anti-apoptotic proteins, Bcl-xL, Mcl-1, survivin and XIAP, play a role, at least in part, to the increase in apoptosis observed in the HCT116 cells.
The p53 protein can act like a transcription factor and upregulate the transcription of several genes implicated in the control of cell proliferation or apoptosis[60]. Many of the proapoptotic Bcl-2 family members, including PUMA, Bax, Noxa, Bik, and Bid, are transcriptional targets of p53[60]. p53 is important in CRC, as it is a key molecular target for contemporary therapeutic agents. For instance, the cornerstone of the current systemic therapy of metastatic colorectal cancers is the antimetabolite 5-FU[61]. However, the ability of 5-FU to induce apoptosis is dependent on p53, which is lost or inactivated in at least 85% of human CRC[61,62]. Therefore, searching for chemotherapeutic agents that restore normal expression of p53 are of major importance. In this study, we found that treatment of HCT116 cells with a combination of CAERS and CFEZO resulted in a significant induction of p53 protein, which was associated with the concomitant induction of its downstream transcriptional targets, key anti-apoptotic molecules, Bax, Bad and Noxa[60]. Thus, these findings suggest that the induction of p53 is responsible, at least in part, for the combination of CAERS and CFEZO treatment-induced apoptosis in HCT116 cells.
Disruption of the normal regulation of cell-cycle progression and division are important events in the development of cancer[45]. In all eukaryotic organisms, the transition from the G1 phase to the S phase of the cell cycle is controlled by sequential activation of cyclin/Cdk complexes[63]. Excessive cyclin D1 activates Cdk-4/cyclin D1 complex and initiates events that facilitate cell cycle progression and transition through the restriction point in the G1 phase[63]. In addition to cyclin/Cdk complexes, cell cycle progression is tightly regulated by other hub proteins, such as c-Myc[64]. Ample studies have highlighted the roles of cyclin D1, Cdk-4 and c-Myc in the evolution and progression of CRC. For example, the cyclin D1 gene is frequently overexpressed in human colon cancer, and antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells[65]. Meanwhile, some compounds derived from natural sources have been found to block the cell cycle progression and inhibited proliferation of human colon cancer cells through targeting the Cdk-4 pathway[66-68]. c-Myc expression is a notable biomarker for CRC, as increased expression is an early event of colon carcinogenesis[41,42], and inactivation of c-Myc leads to a sustained regression of a neoplastic phenotypes and increase in apoptosis in a mouse model of colon carcinogenesis[69,70]. Related to this, pharmaceuticals that target structural features of the c-Myc promoter, and suppress expression of c-Myc, are being developed as potential CRC chemotherapies[71]. The data herein demonstrate that CAERS and CFEZO downregulate expression of Cdk-4, cyclin D1 and c-Myc, and upregulate expression of p21, a transcriptional target of p53 that inhibits Cdk-4/cyclin D1 complexes, thereby, inhibiting cell cycle progression. Importantly, reduced p21 protein level has been found to play an important role in progression of colon cancer[72]. Thus, these results bring to mind that CAERS and CFEZO treatments may cause cell cycle arrest at the G0 to G1 stages of the cell cycle by modifying expression of regulator proteins, including c-Myc, Cdk-4 and cyclin D1. Together, these findings suggest the multi-targeting effects of CAERS and CFEZO treatments with mechanistic insight, and support its translational potential in CRC chemoprevention.
In conclusion, the results show that CAERS and CFEZO act synergistically to control the growth and to trigger apoptosis of human CRC cells by modulating the expression of cell cycle regulators and apoptotic proteins. Although further in vivo evaluations of the potential of CAERS and CFEZO treatments as antitumorigenic agents are clearly warranted, this study indicates that alkaloid and flavonoid extracts of R. stricta and Z. officinale, respectively, comprise active components or potential leads that could be useful in CRC treatment. Further studies on the isolation and characterization of the active chemical constituents of CAERS and CFEZO are currently being carried out in our laboratories.
ACKNOWLEDGMENTS
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant No (1431/130/159). The authors, therefore, acknowledge with thanks DRS technical and financial support.
COMMENTS
Background
Colon cancer is one of the major causes of cancer mortality worldwide. Rhazya stricta (R. stricta) and Zingiber officinale (Z. officinale) are medicinal herbs traditionally used in folkloric medicine that have antioxidant, anticarcinogenic, and free radical scavenging properties.
Research frontiers
Crude alkaloid and flavonoid extracts prepared from R. stricta and Z. officinale, respectively, worked synergistically to suppress proliferation and colony formation, as well as induce morphologic and biochemical features of apoptosis, in human colon cancer cell cells.
Innovations and breakthroughs
Previous studies indicated that R. stricta and Z. officinale inhibited growth of several cancer cell lines. This is the first study investigating the effects of crude alkaloids and flavonoid extracts prepared from these herbs on growth of colon cancer cells, and elucidating the molecular mechanisms underlying growth inhibition.
Peer review
The authors investigated the effects of extracts derived from medicinal herbs, R. stricta and Z. officinale on growth and apoptosis in human colon cancer cells. The results showed that a combination of crude alkaloid and flavonoid extracts prepared from these medicinal herbs synergistically inhibited proliferation, colony formation and anchorage-independent growth of the cancer cell line HCT116 via apoptosis. The study is of interest and the authors provided many data that may be the basis for forthcoming research in colon cancer biology and therapy.
Footnotes
Supported by Funding from the Deanship of Scientific Research, King Abdulaziz University, No. 1431/130/159
P- Reviewer: Teresa Valenti M, Wu S S- Editor: Ding Y L- Editor: AmEditor E- Editor: Wang CH
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chau I, Cunningham D. Treatment in advanced colorectal cancer: what, when and how? Br J Cancer. 2009;100:1704–1719. doi: 10.1038/sj.bjc.6605061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasile L, Olaru A, Munteanu M, Pleşea IE, Surlin V, Tudoraşcu C. Prognosis of colorectal cancer: clinical, pathological and therapeutic correlation. Rom J Morphol Embryol. 2012;53:383–391. [PubMed] [Google Scholar]
- 4.Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 5.Huerta S, Goulet EJ, Livingston EH. Colon cancer and apoptosis. Am J Surg. 2006;191:517–526. doi: 10.1016/j.amjsurg.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Yang SY, Sales KM, Fuller B, Seifalian AM, Winslet MC. Apoptosis and colorectal cancer: implications for therapy. Trends Mol Med. 2009;15:225–233. doi: 10.1016/j.molmed.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien MA, Kirby R. Apoptosis: a review of pro-apoptotic and antiapoptotic pathways and dysregulation in disease. J Vet EmergCrit Care. 2008;18:572–585. [Google Scholar]
- 9.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Van Kuiken ME, Iyer LH, Harikumar KB, Sung B. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 2009;234:825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci USA. 2003;100:776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin AR, Wang D, Zhang H, Peng S, Shin HJ, Brandes JC, Tighiouart M, Khuri FR, Chen ZG, Shin DM. Enhanced anti-tumor activity by the combination of the natural compounds (-)-epigallocatechin-3-gallate and luteolin: potential role of p53. J Biol Chem. 2010;285:34557–34565. doi: 10.1074/jbc.M110.141135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani CM. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–1028. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan BA, Narayanan NK, Desai D, Pittman B, Reddy BS. Effects of a combination of docosahexaenoic acid and 1,4-phenylene bis(methylene) selenocyanate on cyclooxygenase 2, inducible nitric oxide synthase and beta-catenin pathways in colon cancer cells. Carcinogenesis. 2004;25:2443–2449. doi: 10.1093/carcin/bgh252. [DOI] [PubMed] [Google Scholar]
- 15.Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerner EW, Meyskens FL. Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009;15:758–761. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y, McIntosh GH, Le Leu RK, Nyskohus LS, Woodman RJ, Young GP. Combination of selenium and green tea improves the efficacy of chemoprevention in a rat colorectal cancer model by modulating genetic and epigenetic biomarkers. PLoS One. 2013;8:e64362. doi: 10.1371/journal.pone.0064362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilani SA, Kikuchi A, Shinwari ZK, Khattak ZI, Watanabe KN. Phytochemical, pharmacological and ethnobotanical studies of Rhazya stricta Decne. Phytother Res. 2007;21:301–307. doi: 10.1002/ptr.2064. [DOI] [PubMed] [Google Scholar]
- 19.Marwat SK, Rehman F, Usman K, Shah SS, Anwar N, Ullah I. A review of phytochemistry, bioactivities and ethnomedicinal uses of RhazyastrictaDecsne (Apocynaceae) Afr J Microbiol Res. 2012;6:1629–1641. [Google Scholar]
- 20.Baeshen NA, Elkady AI, Abuzinadah OS, Mutwakil MH. Potential anticancer activity of the medicinal herb, Rhazyastricta, against human breast cancer. Afr J Biotechnol. 2012;11:8960–8972. [Google Scholar]
- 21.Lu JJ, Bao JL, Chen XP, Huang M, Wang YT. Alkaloids isolated from natural herbs as the anticancer agents. Evid Based Complement Alternat Med. 2012;2012:485042. doi: 10.1155/2012/485042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Shao Y, Hu L, Zhang X, Chen Y, Tong L, Li C, Shen X, Ding J. BM6, a new semi-synthetic vinca alkaloid, exhibits its potent in vivo anti-tumor activities via its high binding affinity for tubulin and improved pharmacokinetic profiles. Cancer Biol Ther. 2007;6:787–794. doi: 10.4161/cbt.6.5.4006. [DOI] [PubMed] [Google Scholar]
- 23.Elkady AI. Crude alkaloid extract of Rhazya stricta inhibits cell growth and sensitizes human lung cancer cells to cisplatin through induction of apoptosis. Genet Mol Biol. 2013;36:12–21. doi: 10.1590/S1415-47572013005000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Gendy MA, Ali BH, Michail K, Siraki AG, El-Kadi AO. Induction of quinone oxidoreductase 1 enzyme by Rhazya stricta through Nrf2-dependent mechanism. J Ethnopharmacol. 2012;144:416–424. doi: 10.1016/j.jep.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Baliga MS, Haniadka R, Pereira MM, D’Souza JJ, Pallaty PL, Bhat HP, Popuri S. Update on the chemopreventive effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr. 2011;51:499–523. doi: 10.1080/10408391003698669. [DOI] [PubMed] [Google Scholar]
- 26.Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 28.Ghasemzadeh A, Jaafar HZ, Rahmat A. Identification and concentration of some flavonoid components in Malaysian young ginger (Zingiber officinale Roscoe) varieties by a high performance liquid chromatography method. Molecules. 2010;15:6231–6243. doi: 10.3390/molecules15096231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kale A, Gawande S, Kotwal S. Cancer phytotherapeutics: role for flavonoids at the cellular level. Phytother Res. 2008;22:567–577. doi: 10.1002/ptr.2283. [DOI] [PubMed] [Google Scholar]
- 30.Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: A versatile source of anticancer drugs. Pharmacogn Rev. 2011;5:1–12. doi: 10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18:427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin Chim Acta. 2005;358:60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Abdullah S, Zainal-Abidin SA, Murad NA, Makpol S, NgahWZ , Yusof YA. Ginger extract (Zingiberofficinale) triggers apoptosis and G0/G1 cells arrest in HCT 116 and HT 29 colon cancer cell lines. Afr J Biochem Res. 2010;4:134–142. [Google Scholar]
- 34.Trease GE, Evans WC. Pharmcognosy.16th ed. London: Saunder Elsevier; 2002. pp. 135–147. [Google Scholar]
- 35.Roche Applied Science. Apoptosis, Cytotoxicity and Cell Proliferation. 2008; 4: 49. Available from: http://www.roche-applied-science.com/wcsstore/.../05242134001_05.08.pdf.
- 36.Elkady AI. Crude extract of Nigella sativa inhibits proliferation and induces apoptosis in human cervical carcinoma HeLa cells. Afr J Biotechnol. 2012;11:12710–12720. [Google Scholar]
- 37.El-Kady A, Sun Y, Li YX, Liao DJ. Cyclin D1 inhibits whereas c-Myc enhances the cytotoxicity of cisplatin in mouse pancreatic cancer cells via regulation of several members of the NF-κB and Bcl-2 families. J Carcinog. 2011;10:24. doi: 10.4103/1477-3163.90437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins AR. Comet assay principles, applications, and limitations. Methods Mol Biol. 2002;203:163–177. doi: 10.1385/1-59259-179-5:163. [DOI] [PubMed] [Google Scholar]
- 39.Chakraborty S, Kundu T, Dey S, Bhattacharya RK, Siddiqi M, Roy M. Tea-induced apoptosis in human leukemia K562 cells as assessed by comet formation. Asian Pac J Cancer Prev. 2006;7:201–207. [PubMed] [Google Scholar]
- 40.Laptenko O, Prives C. Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 2006;13:951–961. doi: 10.1038/sj.cdd.4401916. [DOI] [PubMed] [Google Scholar]
- 41.Sato H, Tsuchiya A, Abe R. Correlation between c-myc protein expression and phases of the cell cycle in human colorectal carcinomas. Fukushima J Med Sci. 1995;41:111–123. [PubMed] [Google Scholar]
- 42.Yander G, Halsey H, Kenna M, Augenlicht LH. Amplification and elevated expression of c-myc in a chemically induced mouse colon tumor. Cancer Res. 1985;45:4433–4438. [PubMed] [Google Scholar]
- 43.Pan MH, Lai CS, Wu JC, Ho CT. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res. 2011;55:32–45. doi: 10.1002/mnfr.201000412. [DOI] [PubMed] [Google Scholar]
- 44.Elkady AI, Abuzinadah OA, Baeshen NA, Rahmy TR. Differential control of growth, apoptotic activity, and gene expression in human breast cancer cells by extracts derived from medicinal herbs Zingiber officinale. J Biomed Biotechnol. 2012;2012:614356. doi: 10.1155/2012/614356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 47.Gorman A, McCarthy J, Finucane D, Reville W, Cotter T. Morphological assessment of apoptosis in Techniques in Apoptosis. A user’s guide. New York: Portland Press; 1996. pp. 1–20. [Google Scholar]
- 48.Yasuhara S, Zhu Y, Matsui T, Tipirneni N, Yasuhara Y, Kaneki M, Rosenzweig A, Martyn JA. Comparison of comet assay, electron microscopy, and flow cytometry for detection of apoptosis. J Histochem Cytochem. 2003;51:873–885. doi: 10.1177/002215540305100703. [DOI] [PubMed] [Google Scholar]
- 49.Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–328. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- 50.Ogura E, Senzaki H, Yamamoto D, Yoshida R, Takada H, Hioki K, Tsubura A. Prognostic significance of Bcl-2, Bcl-xL/S, Bax and Bak expressions in colorectal carcinomas. Oncol Rep. 2002;6:365–369. doi: 10.3892/or.6.2.365. [DOI] [PubMed] [Google Scholar]
- 51.Sturm I, Köhne CH, Wolff G, Petrowsky H, Hillebrand T, Hauptmann S, Lorenz M, Dörken B, Daniel PT. Analysis of the p53/BAX pathway in colorectal cancer: low BAX is a negative prognostic factor in patients with resected liver metastases. J Clin Oncol. 1999;17:1364–1374. doi: 10.1200/JCO.1999.17.5.1364. [DOI] [PubMed] [Google Scholar]
- 52.Maurer CA, Friess H, Bühler SS, Wahl BR, Graber H, Zimmermann A, Büchler MW. Apoptosis inhibiting factor Bcl-xL might be the crucial member of the Bcl-2 gene family in colorectal cancer. Dig Dis Sci. 1998;43:2641–2648. doi: 10.1023/a:1026695025990. [DOI] [PubMed] [Google Scholar]
- 53.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 54.Okumura K, Huang S, Sinicrope FA. Induction of Noxa sensitizes human colorectal cancer cells expressing Mcl-1 to the small-molecule Bcl-2/Bcl-xL inhibitor, ABT-737. Clin Cancer Res. 2008;14:8132–8142. doi: 10.1158/1078-0432.CCR-08-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ. 2008;15:977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azmi AS, Wang Z, Philip PA, Mohammad RM, Sarkar FH. Emerging Bcl-2 inhibitors for the treatment of cancer. Expert Opin Emerg Drugs. 2011;16:59–70. doi: 10.1517/14728214.2010.515210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ploner C, Kofler R, Villunger A. Noxa: at the tip of the balance between life and death. Oncogene. 2008;27 Suppl 1:S84–S92. doi: 10.1038/onc.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 59.Rödel C, Haas J, Groth A, Grabenbauer GG, Sauer R, Rödel F. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: survivin as a radioresistance factor. Int J Radiat Oncol Biol Phys. 2003;55:1341–1347. doi: 10.1016/s0360-3016(02)04618-7. [DOI] [PubMed] [Google Scholar]
- 60.Brady CA, Attardi LD. p53 at a glance. J Cell Sci. 2010;123:2527–2532. doi: 10.1242/jcs.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med. 2001;7:1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker SJ, Preisinger AC, Jessup JM, Paraskeva C, Markowitz S, Willson JK, Hamilton S, Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990;50:7717–7722. [PubMed] [Google Scholar]
- 63.Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–684. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 64.Liao DJ, Thakur A, Wu J, Biliran H, Sarkar FH. Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit Rev Oncog. 2007;13:93–158. doi: 10.1615/critrevoncog.v13.i2.10. [DOI] [PubMed] [Google Scholar]
- 65.Arber N, Doki Y, Han EK, Sgambato A, Zhou P, Kim NH, Delohery T, Klein MG, Holt PR, Weinstein IB. Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 1997;57:1569–1574. [PubMed] [Google Scholar]
- 66.Karim BO, Rhee KJ, Liu G, Zheng D, Huso DL. Chemoprevention utility of silibinin and Cdk4 pathway inhibition in Apc(-/+) mice. BMC Cancer. 2013;13:157. doi: 10.1186/1471-2407-13-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shan BE, Wang MX, Li RQ. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Invest. 2009;27:604–612. doi: 10.1080/07357900802337191. [DOI] [PubMed] [Google Scholar]
- 68.Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res. 2007;143:58–65. doi: 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 69.Ignatenko NA, Holubec H, Besselsen DG, Blohm-Mangone KA, Padilla-Torres JL, Nagle RB, de Alboránç IM, Guillen-R JM, Gerner EW. Role of c-Myc in intestinal tumorigenesis of the ApcMin/+ mouse. Cancer Biol Ther. 2006;5:1658–1664. doi: 10.4161/cbt.5.12.3376. [DOI] [PubMed] [Google Scholar]
- 70.Jain M, Arvanitis C, Chu K, Dewey W, Leonhardt E, Trinh M, Sundberg CD, Bishop JM, Felsher DW. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 71.Gerner EW, Ignatenko NA, Lance P, Hurley LH. A comprehensive strategy to combat colon cancer targeting the adenomatous polyposis coli tumor suppressor gene. Ann N Y Acad Sci. 2005;1059:97–105. doi: 10.1196/annals.1339.033. [DOI] [PubMed] [Google Scholar]
- 72.Bukholm IK, Nesland JM. Protein expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb in human colon carcinomas. Virchows Arch. 2000;436:224–228. doi: 10.1007/s004280050034. [DOI] [PubMed] [Google Scholar]


