Abstract
AIM: To systematically evaluate the association between the miR-146a rs2910164 polymorphism and susceptibility to gastric cancer.
METHODS: A comprehensive electronic search was conducted for articles published up until January 27, 2014 in Medline (PubMed), Excerpta Medica Database (Embase), the Cochrane Library and Google Scholar. Only case-control studies published in English that evaluated the association between the miR-146a rs2910164 polymorphism and susceptibility to gastric cancer were included. Furthermore, only studies with sufficient data allowing for calculation of odds ratio (OR) and corresponding 95% confidence interval (CI) were included. These values were used in the quantitative synthesis to assess the strength of the association between the miR-146a rs2910164 polymorphism and risk of gastric cancer.
RESULTS: The database search identified 1002 eligible studies, of which seven (comprising 4112 cases and 5811 controls) were included for the meta-analysis. The results indicate that miR-146a rs2910164 polymorphism is more likely to be associated with gastric cancer risk. In the overall analysis, a significantly increased cancer risk was found in the heterozygote (GG vs GC) comparison (OR = 1.14, 95%CI: 1.03-1.27; P = 0.01 for pooled OR). In the ethnicity subgroup analysis, a similar result was found among Caucasians (OR = 1.36, 95%CI: 1.01-1.85; P = 0.04 for pooled OR). In the stratified analysis by quality of studies, a significantly increased cancer risk was found in the heterozygote comparison among high quality studies (OR = 1.12, 95%CI: 1.01-1.26; P = 0.04 for pooled OR). When stratified on the basis of sample size, a significantly increased cancer risk was found among small sample size subgroups for the allelic (G vs C: OR = 1.16, 95%CI: 1.03-1.30; P = 0.01), homozygote (GG vs CC: OR = 1.33, 95%CI: 1.03-1.73; P = 0.03) and recessive model (GG vs GC + CC: OR = 0.05, 95%CI: 0.00-0.10; P = 0.03) comparisons.
CONCLUSION: The miR-146a rs2910164 polymorphism is associated with increased gastric cancer risk, particularly evident in high quality studies with small sample sized Caucasian populations.
Keywords: Gastric cancer, MiR-146a, Polymorphism, Risk, Meta-analysis
Core tip: Recent attention has been focused on the role of miR-146a gene variants in the etiology of several cancers. An increasing number of studies have suggested that the single nucleotide polymorphism rs2910164 in miR-146a is associated with gastric cancer risk. However, previous meta-analyses have failed to find an association. To better understand this association, an up-to-date comprehensive meta-analysis was conducted, which indicates that the miR-146a rs2910164 polymorphism is indeed more likely to be associated with gastric cancer risk.
INTRODUCTION
Gastric cancer is the fourth most common cancer worldwide and the second leading cause of cancer death, with an estimated 989600 new cases and 738000 deaths in 2008, of which more than two-thirds occurred in developing countries[1-3]. Gastric cancer is a multi-factorial disease caused by various risk factors, including genetic pre-disposition, environment, and viral/bacterial infections. As gastric cancer-related deaths can be minimized by early identification and better risk factor control[4], the identification of new markers for classifying high-risk populations and strategies for early detection and preventive care is urgently needed.
MicroRNAs (miRNAs) represent a class of evolutionarily conserved, endogenous, single-stranded, non-coding RNA molecules of ~20 nucleotides that regulate gene expression by degrading mRNAs or suppressing translation. miRNAs have been implicated in a wide range of physiologic and pathologic processes, including development, cell differentiation, proliferation, apoptosis and carcinogenesis[5,6]. Accumulating evidence indicates that the expression of roughly 10%-30% of all human genes is regulated by miRNAs[7]. More than half of the known miRNAs are located in cancer-associated genomic regions, and miRNAs are thought to contribute to oncogenesis because they can function either as tumor suppressors or oncogenes[8]. Analyses in human epithelial malignancies have shown that cancers can be distinguished and classified by distinct tumor-specific miRNA signatures[9]. Some of the key dysregulated miRNAs could serve as molecular biomarkers, leading to improved diagnosis and monitoring of cancer treatment response[10-12].
Single nucleotide polymorphisms (SNPs) are a type of common genetic variation associated with population diversity, disease susceptibility, drug metabolism and genome evolution[13]. SNPs may affect the expression and function of miRNAs, which could therefore contribute to the susceptibility to cancer occurrence and development[14-17]. Although there are a variety of studies indicating that a G/C polymorphism in the gene encoding miRNA-146a could be a risk factor for gastric cancer[18-24], the variability in ethnicity and geographic location, along with the limited sample size in these studies, renders this finding inconclusive and controversial. Moreover, several systematic reviews investigating the association failed to achieve a comprehensive conclusion, though not all eligible studies were included[25-29]. To address this issue, an updated meta-analysis was performed to investigate the association between the miRNA-146a G/C polymorphism and gastric cancer susceptibility.
MATERIALS AND METHODS
Publication search
A comprehensive electronic search was performed to identify articles published up until January 27, 2014 in Medline (PubMed), Excerpta Medica Database (Embase), the Cochrane Library and Google Scholar using the following search terms: “miR-146a” or “rs2910164” and “gastric cancer” or “stomach cancer” or “gastric carcinoma” and “polymorphism” or “SNPs”. All eligible studies published in English were retrieved, and their bibliographies were checked for additional relevant publications. Review articles and bibliographies of other identified relevant studies were searched by hand to identify any additional eligible studies.
Inclusion and exclusion criteria
Studies included in this meta-analysis had to meet all of the following criteria: (1) case-control study evaluating the association between miR-146a rs2910164 polymorphism and susceptibility to gastric cancer; (2) sufficient published data for calculating odds ratios (ORs) with corresponding 95% confidence intervals (CIs); (3) full-text manuscript; and (4) only the most recent or complete study reporting on the same population of patients was included. Exclusion criteria included: (1) reviews, other meta-analyses, comments, letters and editorial articles; (2) not a case-control study; and (3) no usable data reported. The meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[30].
Data extraction
Information regarding the following aspects was independently retrieved from each study by two reviewers (Xie WQ and Wang XF): the first author’s surname, year of publication, country of origin, ethnicity, study design, total number of cases and controls, source of cases and controls, detected sample, genotyping methods, allele and genotype frequencies of cases and controls, and evidence of Hardy-Weinberg equilibrium (HWE) in the controls. In studies including subjects of more than one ethnicity, genotype data was extracted separately for each ethnic group. Data from studies containing more than one case-control group were considered as independent studies. Any discrepancies between the reviewers were resolved through discussion to reach a consensus.
Quality assessment
The quality of selected studies was independently assessed by two authors (Xie WQ and Wang XF) according to a set of predetermined criteria described by Wang et al[25]. Quality scores ranged from 0 to 18, with a higher score indicating better quality. The predetermined criteria encompassed the following five aspects: source of cases and controls, total sample size, source of specimen, evidence of HWE and the condition of case-control matching. Any discrepancies in assessments by the two reviewers were resolved by discussion to achieve a consensus.
Statistical analysis
The HWE for miR-146a in the control group of each study was evaluated using a χ2 test with P < 0.05 indicating a state of disequilibrium[31]. Crude ORs with 95%CIs were used to assess the association between the miRNA gene polymorphism and gastric cancer under six genetic models: the allelic comparison (G vs C), homozygote comparison (GG vs CC), heterozygote comparison (GG vs GC, GC vs CC), recessive model (GG vs GC + CC), and dominant model (GG + GC vs CC). The significance of the pooled ORs was determined by the Z-test with P < 0.05 indicating statistical significance. Subgroup analyses were also conducted by ethnicity (Caucasian and Asian), quality of studies (score < 12 = low quality; score ≥ 12 = high quality) and sample size (total number of controls and cases < 1000 = small; total number > 1000 = large). A χ2-based Q-statistic was used to assess the between-study heterogeneity[32]. If the heterogeneity was significant, indicated by P < 0.05, a random effects model was used to estimate the summary OR and 95%CI; otherwise, a fixed effects model was used[33,34]. The effect of heterogeneity was also examined using the I2 test (range: 0%-100%), which represented the proportion of inter-study variability that could be attributed to heterogeneity rather than to chance[35]. Sensitivity analyses were performed by omitting one single study each time to examine the influence of individual data sets on the pooled ORs. Publication bias was assessed by Begg’s funnel plots and Egger’s linear regression tests, indicated by an asymmetric plot or P < 0.05, respectively[36,37].
RESULTS
Characteristics of eligible studies
A total of 1002 articles were retrieved after the first search in PubMed, Embase, the Cochrane Library and Google Scholar. Selection following the specified criteria eliminated 995 studies, leaving seven case-control studies (Figure 1). The publication years of included articles ranged from 2010 to 2014 (Table 1), with overall sample sizes ranging from 608 to 3581. Two of the studies were conducted in Caucasian populations[18,19] and five studies were conducted in Asian populations[20-24]. The distributions of miR-146a rs2910164 genotype in all studies were in accordance with HWE in the control cohorts. No significant differences were found between cases and controls with respect to gender and age distributions. The modified quality scores of all studies ranged from 9 to 16, with 71% (5/7) of the included studies classified as high quality (≥ 12).
Figure 1.
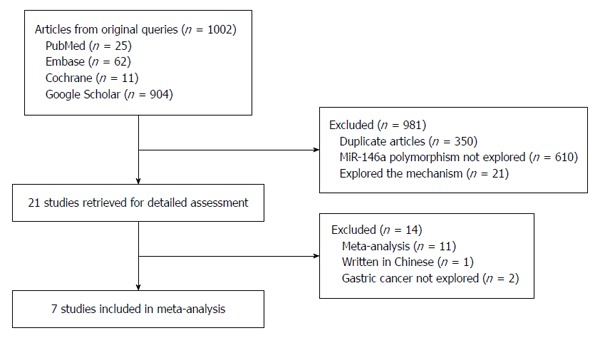
Flow chart of the selection of studies included in the meta-analysis.
Table 1.
Characteristics of all studies included in the meta-analysis
| Study | Year | Country | Ethnicity | Source of controls | Genotyping method | Cases (n) | Control (n) | Quality score | HWE, P value |
| Zeng et al[23] | 2010 | China | Asian | HB | PCR-RFLP | 304 | 304 | 15 | 0.12 |
| Okubo et al[22] | 2010 | China | Asian | HB | PCR-RFLP | 552 | 697 | 15 | 0.28 |
| Hishida et al[21] | 2011 | China | Asian | HB | TaqMan | 583 | 1637 | 15 | 0.74 |
| Zhou et al[24] | 2012 | China | Asian | HB | TaqMan | 1686 | 1895 | 15 | 0.93 |
| Ahn et al[20] | 2012 | China | Asian | PB | PCR-RFLP | 461 | 447 | 16 | 0.36 |
| Dikeakos et al[18] | 2013 | Greek | Caucasian | PB | PCR-RFLP | 163 | 480 | 15 | 0.29 |
| Kupcinskas et al[19] | 2014 | Germany | Caucasian | HB | TaqMan | 363 | 351 | 11 | 0.44 |
HB: Hospital-based; HWE: Hardy-Weinberg equilibrium; PB: Population-based; PCR-RFLP: Polymerase chain reaction-restriction fragment length polymorphism.
Meta-analysis results
Results of the meta-analyses are presented in Figure 2 and Table 2. In the overall analysis, a significantly increased cancer risk was found in the GG vs GC heterozygote comparison (P = 0.01 for pooled OR) (Figure 2C, Table 2). In the subgroup analysis by ethnicity, a similar result was found among Caucasians (P = 0.04 for pooled OR). In the stratified analysis by quality of studies, a significantly increased cancer risk in the same heterozygote comparison was found among high quality studies (P = 0.04 for pooled OR). Stratified analyses on the basis of sample size showed a significantly increased cancer risk among several small sample size subgroups (G vs C, P = 0.01; GG vs CC, P = 0.03; and GG vs GC + CC, P = 0.03). Sensitivity analyses indicated that no individual study significantly altered the pooled ORs (data not shown), demonstrating the robustness of the results.
Figure 2.
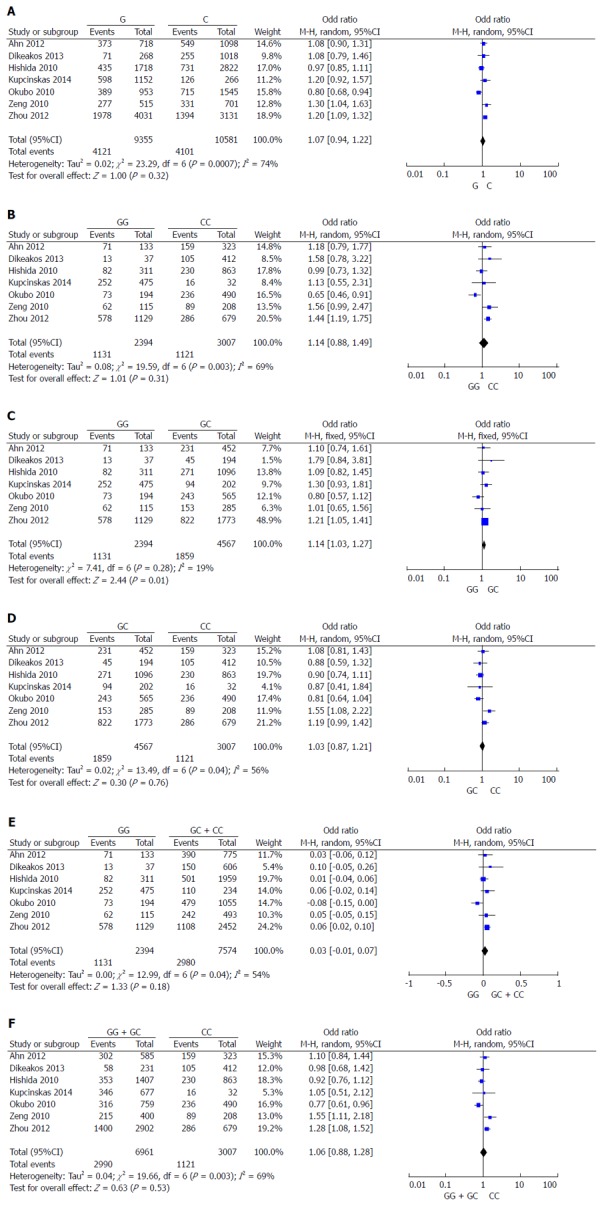
Forest plots of odds ratios for the association of the mir-146a G/C polymorphism with risk of gastric cancer in overall analyses. A: G vs C; B: GG vs. CC; C: GG vs GC; D: GC vs CC; E: GG vs GC + CC; F: GG + GC vs CC.
Table 2.
Pooled results for overall and stratified meta-analyses of the miR-146a polymorphism
| Contrast | Overall or subgroup | Comparisons (n) | OR (95%CI) | P value | I2 |
| G vs C | Overall | 7 | 1.07 (0.94-1.22) | 0.32 | 74% |
| Asian | 5 | 1.05 (0.89-1.24) | 0.54 | 82% | |
| Caucasian | 2 | 0.99 (0.72-1.38) | 0.97 | 0% | |
| High quality | 6 | 1.05 (0.91-1.22) | 0.48 | 78% | |
| Large sample size | 3 | 0.98 (0.78-1.24) | 0.89 | 90% | |
| Small sample size | 4 | 1.16 (1.03-1.30) | 0.01 | 0% | |
| GG vs CC | Overall | 7 | 1.14 (0.88-1.49) | 0.31 | 69% |
| Asian | 5 | 1.11 (0.81-1.51) | 0.51 | 79% | |
| Caucasian | 2 | 1.33 (0.80-2.21) | 0.27 | 0% | |
| High quality | 6 | 1.15 (0.86-1.53) | 0.34 | 74% | |
| Large sample size | 3 | 0.99 (0.63-1.57) | 0.97 | 88% | |
| Small sample size | 4 | 1.33 (1.03-1.73) | 0.03 | 0% | |
| GG vs GC | Overall | 7 | 1.14 (1.03-1.27) | 0.01 | 19% |
| Asian | 5 | 1.11 (0.99-1.25) | 0.06 | 24% | |
| Caucasian | 2 | 1.36 (1.01-1.85) | 0.04 | 0% | |
| High quality | 6 | 1.12 (1.01-1.26) | 0.04 | 26% | |
| Large sample size | 3 | 1.06 (0.84-1.33) | 0.64 | 60% | |
| Small sample size | 4 | 1.19 (0.97-1.47) | 0.10 | 0% | |
| GC vs CC | Overall | 7 | 1.03 (0.87-1.21) | 0.76 | 56% |
| Asian | 5 | 0.88 (0.62-1.25) | 0.48 | 0% | |
| Caucasian | 2 | 1.06 (0.87-1.28) | 0.58 | 68% | |
| High quality | 6 | 1.03 (0.87-1.23) | 0.71 | 62% | |
| Large sample size | 3 | 0.97 (0.77-1.21) | 0.76 | 73% | |
| Small sample size | 4 | 1.12 (0.93-1.36) | 0.22 | 40% | |
| GG vs GC + CC | Overall | 7 | 0.03 (-0.01-0.07) | 0.18 | 54% |
| Asian | 5 | 0.02 (-0.03-0.06) | 0.54 | 66% | |
| Caucasian | 2 | 0.07 (-0.00-0.14) | 0.06 | 0% | |
| High quality | 6 | 0.02 (-0.02-0.07) | 0.36 | 60% | |
| Large sample size | 3 | 0.00 (-0.07-0.08) | 0.94 | 83% | |
| Small sample size | 4 | 0.05 (0.00-0.10) | 0.03 | 0% | |
| GG + GC vs CC | Overall | 7 | 1.06 (0.88-1.28) | 0.53 | 69% |
| Asian | 5 | 1.08 (0.86-1.35) | 0.52 | 29% | |
| Caucasian | 2 | 0.98 (0.72-1.38) | 0.97 | 0% | |
| High quality | 6 | 1.06 (0.87-1.30) | 0.55 | 75% | |
| Large sample size | 3 | 1.18 (0.98-1.40) | 0.07 | 23% | |
| Small sample size | 4 | 0.97 (0.72-1.31) | 0.87 | 86% |
Large sample size: > 1000; Small sample size < 1000.
Evaluation of publication bias
The results of Egger’s linear regression tests are shown in Table 3. The shapes of the funnel plots (not shown) did not identify obvious asymmetry in any of the comparison models, and plot symmetries are evidenced by P values greater than 0.05. Accordingly, no publication bias was evident in the meta-analysis.
Table 3.
Egger’s linear regression tests for funnel plot asymmetries
| Group |
P value |
|||||
| G vs C | GG vs CC | GG vs GC | GC vs CC | GG vs GC + CC | GG + GC vs CC | |
| Overall | 0.712 | 0.742 | 0.902 | 0.823 | 0.961 | 0.689 |
| Asian | 0.519 | 0.452 | 0.181 | 0.764 | 0.975 | 0.266 |
| High quality | 0.607 | 0.767 | 0.830 | 0.944 | 0.964 | 0.672 |
DISCUSSION
It is well known that there is individual cancer susceptibility despite equivalent environmental exposure, likely due to polymorphisms in genes involved in carcinogenesis. Thus, genetic susceptibility to cancer, particularly from SNPs, has been a research focus in the scientific community. Recently, variations of the miR-146a gene have drawn increasing attention in cancer etiologies, and altered expression levels have been observed in inflammatory diseases as well as in cancers[38,39]. Whereas some studies have failed to find an association[18,19], an increasing number of studies have suggested that the rs2910164 SNP in this gene is associated with gastric cancer risk[20-24]. The results of the present meta-analysis confirm that this polymorphism is more likely to be associated with gastric cancer risk. This risk is significant among the individuals with a heterozygous genotype. A distinct variation in the allele frequency of rs2910164 G has been found across different ethnicities, ranging from 0.362 in an Asian population to 0.774 in a Caucasian population[40].
To the best of our knowledge, the present study is the most comprehensive one to date to assess the relationship between the miR-146a rs2910164 polymorphism and gastric cancer risk. Nevertheless, our meta-analysis is not without some of the limitations common to these types of studies. First, relatively large heterogeneity was observed across all the studies involved despite the use of strict criteria for study inclusion and precise data extraction. The overall I2 was high for all comparisons, and although the value was reduced to almost zero in one study after stratifying by subgroup analysis, the value in the other subgroups increased. Therefore, it can be presumed that the heterogeneity partly resulted from differences in ethnicity, study quality and sample size. At the same time, the heterogeneity may also be caused by differences in subject selection. Second, the majority of studies included in this meta-analysis were mainly from Asia. Thus, the inherent genetic and geographic differences require more data from different ethnic group to increase the statistical power. Third, the low sample size in some of the included studies likely influences the statistical power for evaluating the association between the miR-146a rs2910164 polymorphism and gastric cancer risk, especially in subgroup analyses. Fourth, a lack of original data from the reviewed studies limited our further evaluation of potential interactions, considering that gene-to-gene and gene-to-environment interactions might modulate cancer risk. As a result, a more precise analysis stratified by variables such as age, smoking status, alcohol consumption, Helicobacter pylori infection and gastroesophageal reflux could not be performed. Lastly, although the results for publication bias were not statistically significant, publication bias may still exist, because only published studies were included in this meta-analysis.
In conclusion, the meta-analysis presented here indicates that miR-146a rs2910164 polymorphism, the G allele in particular, is more likely to be associated with gastric cancer risk. Further studies based on a homogeneous population of cancer patients and with larger sample sizes are needed to confirm these findings.
COMMENTS
Background
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related mortality worldwide. Many studies have suggested that a G/C polymorphism in the gene encoding miRNA-146a confers susceptibility to gastric cancer. However, the reported associations are inconclusive and controversial.
Research frontiers
Many recent studies and meta-analyses have been performed to understand the association between the miRNA-146a G/C polymorphism and susceptibility to gastric cancer. However, no comprehensive conclusion has been achieved due to insufficient methodologies among these studies.
Innovations and breakthroughs
Based on this meta-analysis, miR-146a rs2910164 polymorphism is associated with gastric cancer risk.
Applications
MiR-146a rs2910164 polymorphism may be directly or indirectly associated with the risk of gastric cancer. An exploration of the mechanism for this association may help to predict gastric cancer risk.
Peer review
Previous studies have demonstrated that the single nucleotide polymorphism rs2910164 in the miR-146a gene is associated with gastric cancer risk. This meta-analysis confirms this association and comprehensively describes the association between this SNP and gastric cancer risk. The results are very interesting and valuable.
Footnotes
Supported by The Natural Science Foundation of Hubei Province, No. 2013CFA076
P- Reviewer: Casadesus D, Lee JI, Wang YH S- Editor: Ding Y L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Noffsinger A, Waxman I. Preinvasive neoplasia in the stomach: diagnosis and treatment. Clin Gastroenterol Hepatol. 2007;5:1018–1023. doi: 10.1016/j.cgh.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.González CA, Sala N, Capellá G. Genetic susceptibility and gastric cancer risk. Int J Cancer. 2002;100:249–260. doi: 10.1002/ijc.10466. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120:21–24. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 9.Hui A, How C, Ito E, Liu FF. Micro-RNAs as diagnostic or prognostic markers in human epithelial malignancies. BMC Cancer. 2011;11:500. doi: 10.1186/1471-2407-11-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu KZ, Zhang K, Li H, Afdhal NH, Albitar M. Circulating microRNAs as biomarkers for hepatocellular carcinoma. J Clin Gastroenterol. 2011;45:355–360. doi: 10.1097/MCG.0b013e3181f18ac2. [DOI] [PubMed] [Google Scholar]
- 11.Yoon SO, Chun SM, Han EH, Choi J, Jang SJ, Koh SA, Hwang S, Yu E. Deregulated expression of microRNA-221 with the potential for prognostic biomarkers in surgically resected hepatocellular carcinoma. Hum Pathol. 2011;42:1391–1400. doi: 10.1016/j.humpath.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shastry BS. SNPs: impact on gene function and phenotype. Methods Mol Biol. 2009;578:3–22. doi: 10.1007/978-1-60327-411-1_1. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Liang J, Wang Z, Tian T, Zhou X, Chen J, Miao R, Wang Y, Wang X, Shen H. Common genetic variants in pre-microRNAs were associated with increased risk of breast cancer in Chinese women. Hum Mutat. 2009;30:79–84. doi: 10.1002/humu.20837. [DOI] [PubMed] [Google Scholar]
- 15.Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L, Zeng Y, Miao R, Jin G, Ma H, et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008;118:2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slaby O, Bienertova-Vasku J, Svoboda M, Vyzula R. Genetic polymorphisms and microRNAs: new direction in molecular epidemiology of solid cancer. J Cell Mol Med. 2012;16:8–21. doi: 10.1111/j.1582-4934.2011.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landau DA, Slack FJ. MicroRNAs in mutagenesis, genomic instability, and DNA repair. Semin Oncol. 2011;38:743–751. doi: 10.1053/j.seminoncol.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dikeakos P, Theodoropoulos G, Rizos S, Tzanakis N, Zografos G, Gazouli M. Association of the miR-146aC& gt; G, miR-149T& gt; C, and miR-196a2T& gt; C polymorphisms with gastric cancer risk and survival in the Greek population. Mol Biol Rep. 2014;41:1075–1080. doi: 10.1007/s11033-013-2953-0. [DOI] [PubMed] [Google Scholar]
- 19.Kupcinskas J, Wex T, Link A, Leja M, Bruzaite I, Steponaitiene R, Juzenas S, Gyvyte U, Ivanauskas A, Ancans G, et al. Gene polymorphisms of micrornas in Helicobacter pylori-induced high risk atrophic gastritis and gastric cancer. PLoS One. 2014;9:e87467. doi: 10.1371/journal.pone.0087467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn DH, Rah H, Choi YK, Jeon YJ, Min KT, Kwack K, Hong SP, Hwang SG, Kim NK. Association of the miR-146aC& gt; G, miR-149T& gt; C, miR-196a2T& gt; C, and miR-499A& gt; G polymorphisms with gastric cancer risk and survival in the Korean population. Mol Carcinog. 2013;52 Suppl 1:E39–E51. doi: 10.1002/mc.21962. [DOI] [PubMed] [Google Scholar]
- 21.Hishida A, Matsuo K, Goto Y, Naito M, Wakai K, Tajima K, Hamajima N. Combined effect of miR-146a rs2910164 G/C polymorphism and Toll-like receptor 4 +3725 G/C polymorphism on the risk of severe gastric atrophy in Japanese. Dig Dis Sci. 2011;56:1131–1137. doi: 10.1007/s10620-010-1376-1. [DOI] [PubMed] [Google Scholar]
- 22.Okubo M, Tahara T, Shibata T, Yamashita H, Nakamura M, Yoshioka D, Yonemura J, Kamiya Y, Ishizuka T, Nakagawa Y, et al. Association study of common genetic variants in pre-microRNAs in patients with ulcerative colitis. J Clin Immunol. 2011;31:69–73. doi: 10.1007/s10875-010-9461-y. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Y, Sun QM, Liu NN, Dong GH, Chen J, Yang L, Wang B. Correlation between pre-miR-146a C/G polymorphism and gastric cancer risk in Chinese population. World J Gastroenterol. 2010;16:3578–3583. doi: 10.3748/wjg.v16.i28.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Zhu H, Luo D, Wang M, Dong X, Hong Y, Lu B, Zhou Y, Zhou J, Zhang Z, et al. A functional polymorphism in Pre-miR-146a is associated with susceptibility to gastric cancer in a Chinese population. DNA Cell Biol. 2012;31:1290–1295. doi: 10.1089/dna.2011.1596. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Sun G, Zou Y, Fan L, Song B. Lack of association of miR-146a rs2910164 polymorphism with gastrointestinal cancers: evidence from 10206 subjects. PLoS One. 2012;7:e39623. doi: 10.1371/journal.pone.0039623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Cao Y, Jiang C, Yang G, Wu J, Ding Y. Lack of association of two common polymorphisms rs2910164 and rs11614913 with susceptibility to hepatocellular carcinoma: a meta-analysis. PLoS One. 2012;7:e40039. doi: 10.1371/journal.pone.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He B, Pan Y, Cho WC, Xu Y, Gu L, Nie Z, Chen L, Song G, Gao T, Li R, et al. The association between four genetic variants in microRNAs (rs11614913, rs2910164, rs3746444, rs2292832) and cancer risk: evidence from published studies. PLoS One. 2012;7:e49032. doi: 10.1371/journal.pone.0049032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin Z, Yan L, Cui Z, Li X, Ren Y, Zhou B. Effects of common polymorphisms rs2910164 in miR-146a and rs3746444 in miR-499 on cancer susceptibility: a meta-analysis. Mol Biol Rep. 2013;40:3003–3013. doi: 10.1007/s11033-012-2372-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Wang Q, Liu H, Shao N, Tan B, Zhang G, Wang K, Jia Y, Ma W, Wang N, et al. The association of miR-146a rs2910164 and miR-196a2 rs11614913 polymorphisms with cancer risk: a meta-analysis of 32 studies. Mutagenesis. 2012;27:779–788. doi: 10.1093/mutage/ges052. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 36.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry MM, Moschos SA, Williams AE, Shepherd NJ, Larner-Svensson HM, Lindsay MA. Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;180:5689–5698. doi: 10.4049/jimmunol.180.8.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reis LO, Pereira TC, Lopes-Cendes I, Ferreira U. MicroRNAs: a new paradigm on molecular urological oncology. Urology. 2010;76:521–527. doi: 10.1016/j.urology.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Tian T, Xu Y, Dai J, Wu J, Shen H, Hu Z. Functional polymorphisms in two pre-microRNAs and cancer risk: a meta-analysis. Int J Mol Epidemiol Genet. 2010;1:358–366. [PMC free article] [PubMed] [Google Scholar]


