Abstract
The multidrug resistance protein 1 (MRP1) encoded by ABCC1 was originally discovered as a cause of multidrug resistance in tumor cells. However, it is now clear that MRP1 serves a broader role than simply mediating the ATP-dependent efflux of drugs from cells. The antioxidant GSH and the pro-inflammatory cysteinyl leukotriene C4 have been identified as key physiological organic anions effluxed by MRP1, and an ever growing body of evidence indicates that additional lipid-derived mediators are also substrates of this transporter. As such, MRP1 is a multitasking transporter that likely influences the etiology and progression of a host of human diseases.
Keywords: ABC Transporter, Inflammation, Leukotriene, Lipid Signaling, Lipid Transport, Lysophospholipid, Multidrug Transporter, Oxidative Stress, MRP1, Organic Anion Transport
Introduction
The ATP-dependent transport of solutes across membranes against a concentration gradient is primarily mediated by members of a superfamily of proteins known as the ATP-binding cassette (ABC)2 transporters. The evolutionary importance of these polytopic membrane proteins is evident from their presence in all eukaryotic species as well as in bacteria and archaea (1). In humans, the 48 ABC transporters are classified into seven subfamilies (A through G) according to their relative degrees of sequence homology. Subfamily ABCC is composed of 12 proteins, at least nine of which collectively mediate the ATP-dependent transmembrane efflux of multiple anticancer drugs and other xenobiotics, their metabolites, and an array of bioactive OAs, including multiple key signaling molecules (2).
The first of the nine drug-transporting human ABCC or MRPs, MRP1, was cloned in 1992 (3, 4). Human MRP1 is encoded by the ABCC1 gene on chromosome 16p13.1, which is amplified at least 100-fold in the multidrug-resistant lung cancer cell line from which the mRNA was first isolated. Since 1992, MRPs, or MRP-like proteins, have been identified in all eukaryotes including plants, sea urchins, and yeast, but unlike some other ABC subfamilies, ABCC proteins have not yet been found in bacteria or archaea (5–7).
In the past two decades, the relevance of MRP1 in human health and disease has been firmly established, and it continues to be of considerable preclinical and clinical interest, largely because of the diversity of drugs (xenobiotics) and physiological molecules that are effluxed by this transporter. Solutes effluxed by MRP1 include hydrophobic natural product antineoplastic agents (e.g. vincristine, doxorubicin), and at present, MRP1 is the only ABCC/MRP-related protein with a widely accepted role in tumor multidrug resistance in clinical oncology. Elevated MRP1 protein and/or mRNA levels have been confirmed in many hematologic and solid tumors, and in some instances, are predictors of poor response to chemotherapy (4, 8–11). The strongest association of MRP1 with unfavorable clinical outcome thus far has been in neuroblastoma (12). Considerable effort has been expended in developing ways to circumvent drug resistance to improve chemotherapy effectiveness in cancer patients, and numerous small molecules and other molecular entities that target MRP1 for this purpose have been described (for recent review, see Ref. 4).
MRP1 also plays a part in the efficacy (and toxicity) of drugs used to treat nonmalignant diseases and transports various antibiotics, opiates, antiviral agents, citalopram, and statins (4, 13–15). Knock-out Abcc1−/− mouse studies have established that MRP1/Mrp1 can be an important determinant of drug disposition because of its presence in cells at the interface between many tissues and the systemic circulation (i.e. blood-organ barriers or pharmacological “sanctuary” sites). Thus, MRP1 has a chemoprotective role in multiple tissues (4, 16–18). Related to the protective properties of MRP1 are the increasing number of studies that report an association between the presence of certain ABCC1 single nucleotide polymorphisms and the occurrence of adverse drug reactions. An important example is the cardiotoxicity often experienced with anthracycline use in adult and pediatric cancer patients (4, 19–21).
In addition to contributing to drug sensitivity and resistance, MRP1 has been implicated in the etiology of a wide array of human pathologies. Thus, MRP1 may play a part in inflammatory and other immunological diseases, age-related macular degeneration, cardiovascular disease, and certain neurological disorders as well as tumor progression (4, 22–28). This potentially broader role of MRP1 in human health and disease has occurred in conjunction with a growing appreciation of its ability to mediate the efflux of bioactive OAs and regulating oxidative stress (25, 29–31). Thus, solutes effluxed by MRP1 also include hydrophilic conjugated xenobiotic and naturally occurring OA metabolites. Notable examples of the latter include the cysteinyl LTC4 and the conjugated estrogen, E217βG, as well as reduced and oxidized glutathione (GSH and GSSG) (4).
Distinguishing Structural Features of MRP1/ABCC1
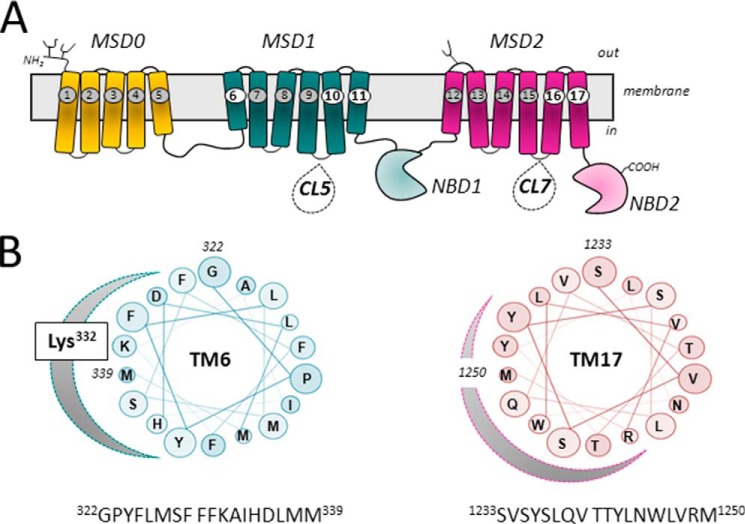
Typical mammalian ABC proteins comprise four domains, which are often encoded as a single polypeptide (e.g. P-glycoprotein) but are sometimes encoded as two polypeptides that form homodimers (e.g. ABCG2) or heterodimers (e.g. TAP1/TAP2). The 1531-amino acid MRP1 (and several ABCC homologs) has a five-domain structure with two NBDs and 17 TMs in three MSDs (MSD0, MSD1, and MSD2) (Fig. 1A). This structure differs from that of a typical ABC protein that has six TMs in each of two MSDs. The exact function of the NH2-terminal MSD0 in MRP1 remains uncertain and may well depend on the cell type (e.g. polarized epithelial cells versus tumor cells) in which it is expressed (32, 33). The “pore” in the membrane through which solutes (substrates) are effluxed is formed by the 12 TMs that comprise MSD1 and MSD2. The orientation of MSD0 toward the “core” MSD1/MSD2 structure of MRP1, as well as its influence on solute translocation through MSD1 and MSD2, is poorly understood (34).
FIGURE 1.
Topology of MRP1 and projections of amphipathic TM α-helices 6 and 17. A, shown is a schematic diagram of a predicted secondary structure of MRP1/ABCC1. The positions of TMs 6, 10, 11, 16, and 17, which have been identified as containing key determinants of MRP1 substrate specificity, are highlighted. Also indicated are CL5 and CL7, which contain amino acids involved in substrate specificity, proper folding, and plasma membrane trafficking, as well as the transport mechanism of MRP1. B, shown are helical wheel projections of 18 amino acids of the amphipathic TM6 (in MSD1) and TM17 (in MSD2) of MRP1. The clustering on one side of each TM helix of polar residues with side chains capable of H-bonding is indicated by the shaded curve. Highlighted on the projection of TM6 is Lys332, which is particularly critical for LTC4 and GSH transport (43, 51).
ATP-dependent Transport by MRP1
Transport by MRP1 (and other ABC transporters) is powered by the binding and hydrolysis of ATP, which facilitate the necessary protein conformation changes that enable solute translocation to occur. The NBDs each contain three key motifs: the Walker A and B motifs characteristic of P-loop ATPases and an “active transport” signature motif common to all ABC proteins (1, 35). The two NBDs form a “sandwich” dimer, and thus the two ATP-binding sites are effectively composed of the Walker motifs of one NBD and the active transport signature motif of the other (36). The ATP-binding sites of MRP1 are functionally nonequivalent with NBD1 having a higher affinity for ATP (but very low ATPase activity) (the so-called “degenerate” site), and NBD2 exhibiting a greater capacity for ATP hydrolysis (the so-called “consensus” site) (3, 10, 35, 37). This marked functional asymmetry seems to be a distinctive feature of ABCC proteins as well as several heterodimeric transporters. Such asymmetry is much less pronounced in non-ABCC full-length transporters such as P-glycoprotein and homodimeric transporters such as ABCG2. The asymmetry of the ABCC/MRP NBDs appears to be largely explainable by differences in the canonical sequences and spacing of the three key motifs (35).
Structural Determinants of Solute Transport by MRP1
The MSDs contain the greatest sequence divergence within all ABC transporters, and an abundance of evidence indicates that they largely (but not exclusively) dictate transporter substrate selectivity (38–40). In MRP1, the TMs of MSD1 and MSD2 contain a higher frequency of polar amino acids than do the analogous TMs of P-glycoprotein. In particular, the last two TMs of MSD1 and MSD2 of MRP1 (TMs 10 and 11 and TMs 16 and 17, respectively) are unusually amphipathic and contain a high proportion of amino acids with hydrogen-bonding side chains densely clustered on one side of the α-helix (Fig. 1B) (40–42). The MRP1 TMs also contain a relatively high number of ionizable amino acids, which is not expected because of the energetically unfavorable environment of the membrane bilayer for such residues (43). Multiple studies indicate that the hydrogen-bonding capacity (and hence ability to form intrahelical and interhelical ion pairs and hydrogen bonds) of the amino acids in these TMs underlies their importance in the substrate binding and transport properties of MRP1 (40, 42–45). The topologically comparable TMs of other OA-transporting ABCC proteins are similarly amphipathic, and many of the functionally important polar/ionizable amino acids of MRP1 are conserved (46–48). In contrast, the comparable TMs of P-glycoprotein (i.e. TMs 5, 6, 11, and 12), although containing key determinants of substrate specificity, are considerably less amphipathic (41, 49), consistent with its inability to transport hydrophilic OAs.
Amino acids in TMs other than TMs 10, 11, 16, and 17 have also been implicated in the transport properties of MRP1, but far less frequently (40). A notable example is Lys332 in the first TM of MSD1 (Fig. 1B) (43, 50). Mutation of TM6 Lys332 to an opposite, neutral, or even same-charge amino acid completely abrogates MRP1 binding of the high affinity physiological substrate LTC4, but has virtually no effect on the binding or transport of any OA substrate lacking a GSH moiety. Thus, it is thought that MRP1 Lys332 plays a direct role in the recognition of the γ-glutamate portion of GSH-containing substrates and modulators (51).
Although it has been widely accepted that the drug-transporting P-glycoprotein takes up its mostly hydrophobic substrates from the plasma membrane (so-called “vacuum cleaner” model) (52), it seems unlikely that this is the case for MRP1 because a substantial proportion of its substrates are relatively hydrophilic OAs that are formed inside the cell by conjugation reactions (see below). Therefore it is not surprising that various cytoplasmic amino acids have been identified to be directly involved in substrate binding and/or translocation by MRP1. One interesting example is Pro1150 located close to the beginning of CL7, which connects TM15 to TM16 (53). When Pro1150 is replaced by Ala (or Leu or Val), the mutant protein exhibits substantially increased levels of methotrexate and E217βG transport, due to a change in Kmapp (39). This increase is substrate-selective, however, because the transport of other OAs (e.g. LTC4) by the P1150A mutant is unchanged. Studies with 32P-labeled 8-azido-ATP also revealed changes in the nucleotide interactions of the mutant that were evident during E217βG (but not LTC4) transport (39). The functional importance of MRP1 Pro1150 is conserved in its homologs, MRP2/ABCC2 and MRP3/ABCC3 (54), but the mechanism by which this cytoplasmic mutation selectively “improves” the transport of a subset of OA substrates is not well understood. Structural studies may eventually be enlightening.
The CLs have roles in addition to their influence on substrate specificity. For example, Ala substitution of several charged amino acids in MSD1-CL5 and MSD2-CL7 causes misfolding of MRP1 resulting in reduced levels of the transporter at the plasma membrane (55–58). Although both CL5 and CL7 are important, MSD1-CL5 and its bonding interactions with NBD2 appear to play a more pivotal role in the proper folding and membrane trafficking and function of MRP1 than do the domain-domain interactions involving MSD2-CL7 (56).
Finally, homology models of mammalian ABC proteins based on structures of bacterial ABC transporters indicate that the coupling of substrate translocation through the MSDs to the ATPase (catalytic) activity of the NBDs is mediated by the CLs at the interfaces between the two domains (36). In MRP1, these are CL4 and CL5 in MSD1 and CL6 and CL7 in MSD2 (53). Particularly important are the short α-helical stretches of 16–18 residues within these CLs, now often referred to as the “coupling helices” (35, 36). Thus, although originally thought to serve simply as sequences that connect the TMs, it is now quite clear that the CLs of MRP1 are involved not only in determining its substrate specificity and its proper folding and stable expression at the plasma membrane but also in its transport mechanism.
Conjugated and Unconjugated Drug and OA Transport by MRP1/ABCC1
Although initial investigations of MRP1 mostly focused on its role in cancer multidrug resistance, the discovery that it could transport both conjugated and unconjugated OAs greatly broadened its pharmacological and physiological relevance (Fig. 2). The most studied OAs transported by MRP1 are those formed by conjugation with GSH, glucuronide, or sulfate. Conjugated xenobiotic OAs are typically products of Phase II drug metabolism, which are usually (but not always) nontoxic. Nevertheless, they require active transport (so-called Phase III) systems to be effluxed from the cell in which they are formed to enable their elimination from the body (59). Elimination of certain conjugated OAs, such as catechol metabolites, is particularly critical because they may retain the electrophilic or redox properties of their parent compounds, and thus their ability to exert tissue toxicity (30, 59, 60). For example, 5-(glutathion-S-yl)-N-methyl-α-methyldopamine and other GSH conjugates of the psychoactive chemical known as “ecstasy” have been identified as the causative agents in the selective neurotoxicity observed in abusers of this agent (60). These GSH conjugates are transported by MRP1 (and MRP2) in vitro (61), although the precise role of these transporters in the overall accumulation of these toxic metabolites in the intact brain is yet to be determined.
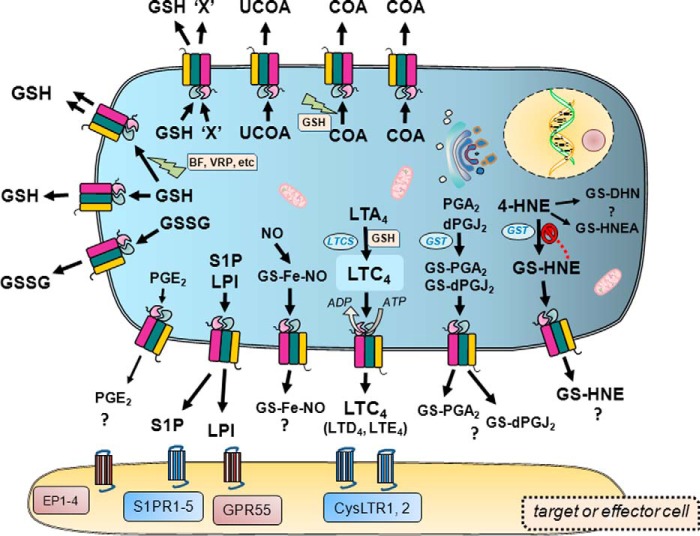
FIGURE 2.
ATP-dependent MRP1-mediated transport of endogenous and xenobiotic solutes. The diagram illustrates the diversity of molecules effluxed by MRP1 across the plasma membrane in an ATP-dependent fashion. Included are representative examples of the endogenous/physiological substrates, both proposed and demonstrated, effluxed by MRP1 such as lipid-derived effectors and mediators of cell signaling (and their receptors) implicated in the etiology and progression of multiple human diseases. The best characterized physiologic substrate of MRP1, viz. LTC4, is highlighted, and only in this case is the dependence of transport on ATP depicted. Also shown are the multiple and complex roles of GSH, both as a transported solute and as a stimulant of the transport of other solutes by MRP1. The ATP dependence of MRP1-mediated transport of these molecules is not depicted due to space constraints but is understood. EP1–4 refers to the four subtypes of PGE2 receptors. Abbreviations: GS-DHN, GS-1,4-dihydroxy-nonene; GS-HNEA, GS-4-hydroxy-2-nonenoic acid; BF, bioflavonoid; LTCS, LTC4 synthase; LTD4, leukotriene D4; LTE4, leukotriene E4; COA, conjugated organic anion; dPGJ2, 15-deoxy-Δ(12,14) PGJ2; S1PR1–5, S1P receptors 1–5; UCOA, unconjugated organic anion; VRP, verapamil; 'X', hydrophobic xenobiotic or drug.
Perhaps the best characterized endogenous conjugated OA substrates of MRP1 are the earlier mentioned GSH and glucuronide conjugates, LTC4 and E217βG, respectively (4, 17, 62, 63). In addition, the sulfated steroids estrone sulfate and dehydroepiandrostenedione sulfate are transported by MRP1. Transport of the latter two conjugates is distinguished by its dependence on GSH (64, 65). Several glucuronidated xenobiotics are also dependent on GSH for their transport, but none of the endogenous glucuronidated MRP1 substrates identified thus far require this tripeptide (4, 31, 66–68). The physicochemical properties that determine whether or not an OA will require GSH for its transport by MRP1 remain a mystery.
Lipid-derived Signaling Molecules as Substrates of MRP1
The CysLT LTC4 (and its extracellularly formed CysLT metabolites leukotriene D4 and leukotriene E4) are potent pro-inflammatory molecules derived from arachidonic acid through the 5-lipoxygenase/leukotriene C4 synthase pathway (69) (Figs. 2 and 3). CysLTs have roles in both innate and adaptive immune processes and exert their effects through interaction with the GPCRs, CysLTR1 and CysLTR2, which are found mostly in macrophages, mast cells, and the lung. In the airways, they induce smooth muscle contraction and increase vascular permeability and mucus secretion (70). The identification of CysLTs as in vitro substrates of MRP1 was first demonstrated using an inside-out membrane vesicle transport system in 1994 (62). The conjugated double bonds in its lipid “tail” endow LTC4 with photolability, and this, together with its high affinity for MRP1 (Kmapp 100 nm), allows direct and highly specific photolabeling of MRP1 that is readily detected by autoradiography. These properties of LTC4 have greatly facilitated the biochemical and pharmacological characterization of MRP1. Studies in Abcc1−/− mice confirmed that LTC4 was a physiologically relevant substrate as these animals exhibited decreased inflammatory responses consistent with impaired LTC4 export (17). It was suggested that MRP1 inhibitors might be developed that could be useful in the treatment of inflammatory disorders, including those involving the airways such as asthma and chronic obstructive pulmonary disease (17, 23). Unfortunately, this has not turned out to be the case. However, more recent genomic studies have indicated that certain ABCC1 polymorphisms may be associated with greater or lesser severity of chronic obstructive pulmonary disease, as well as possibly response of asthma patients to leukotriene modifiers (71, 72).
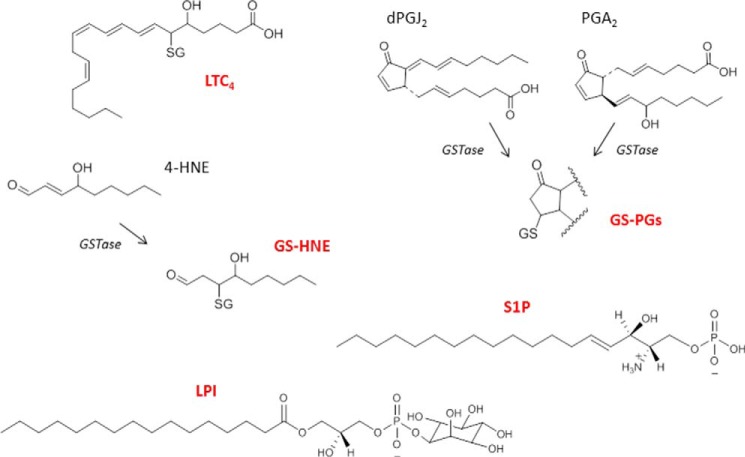
FIGURE 3.
Signaling molecules derived from endogenous lipids and transported by MRP1. Shown are the chemical structures of lipid-derived signaling molecules and their GSH conjugates that are reported to be transported by MRP1. Abbreviations: GS-HNE, GSH-conjugated 4-HNE; GS-PGs, GSH-conjugated prostaglandins; dPGJ2, 15-deoxy-Δ(12,14) prostaglandin J2; PGA2, prostaglandin A2.
Other signaling molecules derived from arachidonic acid and transported by MRP1, at least in vitro, include the GSH conjugates of the cyclopentenone PGs, prostaglandin A2 (PGA2), and 15-deoxy-Δ(12,14) PGJ2 (Figs. 2 and 3) (73, 74). The unconjugated forms of these electrophilic PGs have multiple cellular targets and exert both anti-proliferative and anti-inflammatory effects. Their α,β-unsaturated carbonyl groups enable them to covalently modify target proteins and react with GSH. Both GS-PG conjugates are transported by MRP1 in vitro with relatively high affinity (Kmapp ∼0.9 and 1.4 μm, respectively), and GSH conjugation and subsequent MRP1-mediated efflux of these PG conjugates protects cells from the cytotoxic effects of their parent PGs. Unlike LTC4, however, it has not yet been conclusively established that these GS-PGs are physiologically relevant MRP1 substrates. The unconjugated PGE2 has a saturated cyclopentanone structure (and thus is less reactive), and yet it is also transported by MRP1 (and MRP2) in vitro (75). Studies in knock-out mice, however, suggest that the homologous MRP4 (ABCC4) is more likely to be the physiological efflux transporter of this eicosanoid (76).
Like the class A and J PGs described above, 4-HNE is a relatively stable α,β-unsaturated electrophile produced by the peroxidation of polyunsaturated fatty acids in biological membranes in tissues under oxidative stress (Fig. 3). Because it can form adducts with DNA, proteins, and lipids, 4-HNE is both genotoxic and cytotoxic, and is causally involved in several human pathologies including cardiac and neurodegenerative diseases as well as cancer (77). Cellular 4-HNE levels are regulated through metabolism including its Phase II conjugation with GSH. GSH-conjugated 4-HNE is an inhibitor of GST, among other biological activities, and must therefore be exported from cells, a process likely mediated by MRP1 (78). In this way, MRP1, together with GSH, plays a protective role against oxidative stress. GSH-conjugated 4-HNE can be further metabolized to GS-1,4-dihydroxy-nonene and GS-4-hydroxy-2-nonenoic acid, but the biological activities of these GSH conjugates are not yet well characterized, nor has their cellular efflux by MRP1 been described (77, 79).
Two additional lipid-derived signaling molecules have been recently proposed as MRP1 substrates, S1P and LPI (Figs. 2 and 3). These lysophospholipid mediators participate in a myriad of cellular processes mostly through their actions on GPCRs, and disruptions in their signaling pathways have been implicated in an array of inflammatory, cardiovascular, neurological, and malignant diseases (80–82). However, how these signaling molecules are released from the cells where they are synthesized so that they can act on their target receptors is poorly understood, although it seems likely that multiple cell type-specific mechanisms are involved.
In the case of S1P, Mitra et al. (83) observed decreased export of this lipid mediator from mast cells after knocking down MRP1 with ABCC1 siRNA and after exposing the cells to the nonspecific MRP inhibitor MK-571. More recent studies indicate that MRP1 mediates S1P efflux from rat uterine leiomyoma cells and mouse adipocytes (84, 85), and a role for MRP1 in S1P-mediated signaling has also been suggested from studies using brain and spinal cord capillaries isolated from Abcc1−/− mice (86). Most of the activities of S1P are mediated through activation of the GPCRs S1PR1–5 (S1P receptors 1–5), which act in concert to regulate many key cellular processes in human health and disease as ably reviewed elsewhere (81, 82).
Pineiro et al. (87) also used siRNA to knock down ABCC1 mRNA and observed a 50% reduction in LPI export from PC-3 human prostate cancer cells that was associated with decreased proliferation. Consequently, they suggested that these cancer cells synthesize and export LPI (via MRP1), which then acts in an autocrine fashion to promote their own proliferation. LPI has an extensive range of both nonreceptor-mediated and receptor-mediated activities (80, 81). Most of its receptor-mediated effects occur via activation of the GPCR GPR55. GPR55 is also a metabolic regulator (88) and is proposed to have a signaling role in synaptic circuits in the brain (89). Hence MRP1-mediated LPI efflux has the potential to affect more cellular processes than just tumor cell proliferation and progression.
The ability of MRP1 to efflux the monophosphorylated lipids LPI and S1P is somewhat unexpected because phosphorylated OA transport is more typically associated with the four-domain MRP1 homologs MRP4 and MRP5 (90). However, MRP4 and MRP1 appear to share several OA substrates (e.g. PGE2, dehydroepiandrostenedione sulfate) (65, 75) despite their relatively distinct structures, and it will be of interest to determine whether S1P and LPI are also transported by MRP4. In this regard, pharmacological inhibitors that can distinguish between MRP1 and MRP4 may be useful (4, 91).
GSH and MRP1-mediated Transport
MRP1 was the first mammalian ABC transporter to be identified that requires GSH to efficiently transport some of its drug and conjugated OA substrates, and it remains unusual (but not unique) in this respect. Thus, unlike P-glycoprotein, MRP1-mediated efflux of natural product anticancer drugs (e.g. vincristine) is dependent on GSH, which is co-transported without the formation of conjugates (Fig. 2) (4, 63, 92–94). As mentioned earlier, transport of several conjugated OAs also requires GSH, but in these instances, GSH serves only a stimulatory role and GSH transport itself is not detected (4, 29, 95).
MRP1 can also mediate the ATP-dependent efflux of GSH even in the absence of drugs or other xenobiotics, but thus far, there is no evidence that under physiological conditions, another endogenous metabolite is co-effluxed with GSH. The affinity of MRP1 for GSH is low (Kmapp ∼10 mm) but nevertheless is biologically significant because elevated GSH levels were detected in organs of Abcc1−/− mice (96), and cells expressing elevated MRP1 often contain lower GSH levels (4, 29). GSH efflux by MRP1 can be stimulated in vitro not only by the hydrophobic drugs mentioned above but also by other xenobiotics and bioflavonoids (4, 97–99) (Fig. 2). In the case of apigenin, this stimulation is associated with a remarkable 10-fold decrease in the Kmapp of MRP1 for GSH, but the structural basis for this change remains unknown (98). Bioflavonoids are widely, if not always accurately (100), touted for their health benefits. It is not clear, however, whether and how normal cells (and tissues) could accrue a benefit from increased efflux of an antioxidant such as GSH, and the physiological relevance of bioflavonoid-stimulated MRP1-mediated GSH efflux is still uncertain. On the other hand, flavonoid-induced GSH efflux may be a way to sensitize cancer cells to antineoplastic agents (4, 99).
Despite being known for almost two decades, these multiple roles of GSH in the transport mechanism of MRP1 remain only partly understood (4, 29, 30). Unlike other GSH-binding proteins (e.g. GSTs), the sulfhydryl-reducing thiol moiety of GSH is not required for its interaction with MRP1 (94, 97). Thus, the central Cys residue of the GSH tripeptide can be replaced with a neutral amino acid (e.g. Leu) or modified by alkylation (e.g. S-methyl-GSH) and still retain its ability to stimulate drug and OA transport (97). There is some evidence that GSH (and S-methyl-GSH) cause a conformational change in MRP1 (specifically, its COOH terminus), but whether and how this change is related to its transport activity is still unclear (95). MRP1-mediated GSH efflux has been implicated as contributing to a variety of human pathologies including retinal pigment epithelial cell death associated with oxidative stress and age-related macular degeneration, and ischemic stroke (24, 26, 30).
MRP1 may also be involved in transporting biologically important GSH complexes, and in particular, those with NO, a short-lived chemically reactive signaling and effector molecule that exerts many of its varied physiological activities, including vasodilation, by interacting with iron-containing proteins. Given the short half-life of uncomplexed NO, and the need for it to be exported to carry out its extracellular functions, mechanisms for NO storage and transport are critical. Thus, Richardson and colleagues (101) have proposed that cells can form dinitrosyl-diglutathionyl-iron ((GS)2-Fe-(NO)2) complexes as long-lived NO intermediates and have provided genetic and pharmacological evidence that these complexes can be effluxed from cells by MRP1 (Fig. 2). Further studies that more precisely delineate the role of MRP1 in the actions of this important chemical messenger are eagerly anticipated.
In addition to GSH (and GSH complexes), MRP1 can transport the oxidized disulfide form of GSH, GSSG (Fig. 2) (102). GSSG is known to inhibit several enzymes (e.g. adenylate cyclase) while activating or stimulating others (e.g. aminolevulinate synthase), and thus it can play a regulatory role in cell metabolism. GSSG levels increase in cells under oxidative stress, and the efficient removal of this molecule with pro-oxidant activity is important for redox homeostasis (30). Unlike GSH, GSSG does not stimulate the transport of other MRP1 substrates but rather inhibits it (102). Low GSSG levels are normally maintained in the cell by GSH reductase, but GSSG export appears to be an alternative mechanism of removal. The affinity of MRP1 for GSSG is relatively high (Kmapp ∼70 μm) (102), and in vitro and genetic studies indicate that MRP1-mediated GSSG efflux is physiologically relevant, particularly in neural cells (103).
Concluding Remarks
We have come a long way since MRP1 was discovered more than 20 years ago during a search for causes of multidrug resistance in tumor cells not associated with overexpression of P-glycoprotein (3, 4). Much attention has remained focused on the drug-transporting activity of MRP1 because of the prospect of improving the responses of patients to antineoplastic agents through the co-administration of MRP1 inhibitors (4). Recent evidence that knowledge of ABCC1 polymorphisms may have some utility in predicting adverse drug reactions has enhanced interest in the role of MRP1 in therapeutic efficacy and toxicity (4). Within 5 years of its discovery, however, MRP1 was established as being more than just a drug transporter when both in vitro and knock-out mouse models revealed it to be the physiological efflux pump of the pro-inflammatory LTC4 as well as reduced and oxidized GSH. Interest in MRP1 as a mediator of the cellular efflux of these and other endogenous OAs has grown as other lipid-derived ligands such as PGs, PG conjugates, and lysophospholipids have been identified as MRP1 substrates. Whether these endogenous molecules exert their effects directly, or through GPCRs and associated signaling cascades, there is strong evidence that they are relevant and important in multiple cellular processes. Many actions of these MRP1 substrates are still far from fully understood, but their involvement in acute and chronic inflammation, cell metabolism, differentiation, proliferation, survival, and cell-cell communication, all processes that influence a host of human diseases including cancer, has been established. Thus, it seems clear that MRP1 (and its genetic variants) has emerged as a multitasking transporter that has critical roles in cell biology that are independent of its function as a drug efflux pump, and thus it has the potential to have a broader impact on human health and disease that still remains to be fully appreciated.
This work was supported by Grants MOP-10519 and MOP-133584 from the Canadian Institutes of Health Research.
- ABC
- ATP-binding cassette
- CysLT
- cysteinyl leukotriene
- CL
- cytoplasmic loop
- E217βG
- 17β-estradiol 17-(β-d-glucuronide)
- GPCR
- G-protein coupled receptor
- 4-HNE
- 4-hydroxy-nonenal
- LPI
- lysophosphatidylinositol
- LTC4
- leukotriene C4
- MRP
- multidrug resistance protein
- MSD
- membrane-spanning domain
- NBD
- nucleotide-binding domain
- OA
- organic anion
- PG
- prostaglandin
- PGE2
- prostaglandin E2
- S1P
- sphingosine-1-phosphate
- TM
- transmembrane α-helix
- CL
- cytoplasmic loop
- GS
- GSH-conjugated
- 8-azido-ATP
- 8-azidoadenosine-5′-triphosphate.
REFERENCES
- 1. Higgins C. F. (1992) ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 [DOI] [PubMed] [Google Scholar]
- 2. Slot A. J., Molinski S. V., Cole S. P. C. (2011) Mammalian multidrug resistance proteins (MRPs). Essays Biochem. 50, 179–207 [DOI] [PubMed] [Google Scholar]
- 3. Cole S. P. C., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M. V., Deeley R. G. (1992) Overexpression of a transporter gene in a multidrug resistant human lung cancer cell line. Science 258, 1650–1654 [DOI] [PubMed] [Google Scholar]
- 4. Cole S. P. C. (2014) Targeting the multidrug resistance protein (MRP1, ABCC1): past, present and future. Annu. Rev. Pharmacol. Toxicol. 54, 95–117 [DOI] [PubMed] [Google Scholar]
- 5. Szczypka M. S., Wemmie J. A., Moye-Rowley W. S., Thiele D. J. (1994) A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J. Biol. Chem. 269, 22853–22857 [PubMed] [Google Scholar]
- 6. Hamdoun A. M., Cherr G. N., Roepke T. A., Epel D. (2004) Activation of multidrug efflux transporter activity at fertilization in sea urchin embryos (Strongylocentrotus purpuratus). Dev. Biol. 276, 452–462 [DOI] [PubMed] [Google Scholar]
- 7. Klein M., Burla B., Martinoia E. (2006) The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett. 580, 1112–1122 [DOI] [PubMed] [Google Scholar]
- 8. Deeley R. G., Westlake C., Cole S. P. C. (2006) Transmembrane transport of endo- and xenobiotics by membrane ATP-binding cassette multidrug resistance proteins. Physiol. Rev. 86, 849–899 [DOI] [PubMed] [Google Scholar]
- 9. Li J., Li Z. N., Yu L. C., Bao Q. L., Wu J. R., Shi S. B., Li X. Q. (2010) Association of expression of MRP1, BCRP, LRP and ERCC1 with outcome of patients with locally advanced non-small cell lung cancer who received neoadjuvant chemotherapy. Lung Cancer 69, 116–122 [DOI] [PubMed] [Google Scholar]
- 10. Bagnoli M., Beretta G. L., Gatti L., Pilotti S., Alberti P., Tarantino E., Barbareschi M., Canevari S., Mezzanzanica D., Perego P. (2013) Clinicopathological impact of ABCC1/MRP1 and ABCC4/MRP4 in epithelial ovarian carcinoma. Biomed. Res. Int. 2013, 143202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hlaváč V., Brynychová V., Václavíková R., Ehrlichová M., Vrána D., Pecha V., Koževnikovová R., Trnková M., Gatěk J., Kopperová D., Gut I., Souček P. (2013) The expression profile of ATP-binding cassette transporter genes in breast carcinoma. Pharmacogenomics 14, 515–529 [DOI] [PubMed] [Google Scholar]
- 12. Haber M., Smith J., Bordow S. B., Flemming C., Cohn S. L., London W. B., Marshall G. M., Norris M. D. (2006) Association of high level MRP1 expression with poor clinical outcome in a large prospective study of primary neuroblastoma. J. Clin. Oncol. 24, 1546–1553 [DOI] [PubMed] [Google Scholar]
- 13. Lee S. H., Lee M.-S., Lee J. H., Kim S. W., Kang R.-H., Choi M.-J., Park S. J., Kim S. J., Lee J. M., Cole S. P. C., Lee M. G. (2010) MRP1 polymorphisms associated with citalopram response in patients with major depression. J. Clin. Psychopharmacol. 30, 116–125 [DOI] [PubMed] [Google Scholar]
- 14. Knauer M. J., Urquhart B. L., Meyer zu Schwabedissen H. E., Schwarz U. I., Lemke C. J., Leake B. F., Kim R. B., Tirona R. G. (2010) Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ. Res. 106, 297–306 [DOI] [PubMed] [Google Scholar]
- 15. Su W., Pasternak G. W. (2013) The role of multidrug resistance-associated protein in the blood-brain barrier and opioid analgesia. Synapse 67, 609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leslie E. M., Deeley R. G., Cole S. P. C. (2005) Multidrug resistance proteins in toxicology: role of P-glycoprotein, MRP1, MRP2 and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 204, 216–237 [DOI] [PubMed] [Google Scholar]
- 17. Wijnholds J., Evers R., van Leusden M. R., Mol C. A. A. M., Zaman G. J. R., Mayer U., Beijnen J. H., van der Valk M., Krimpenfort P., Borst P. (1997) Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat. Med. 3, 1275–1279 [DOI] [PubMed] [Google Scholar]
- 18. Wijnholds J., deLange E. C. M., Scheffer G. L., van den Berg D.-J., Mol C. A. A. M., van der Valk M., Schinkel A. H., Scheper R. J., Breimer D. D., Borst P. (2000) Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J. Clin. Invest. 105, 279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wojnowski L., Kulle B., Schirmer M., Schlüter G., Schmidt A., Rosenberger A., Vonhof S., Bickeböller H., Toliat M. R., Suk E. K., Tzvetkov M., Kruger A., Seifert S., Kloess M., Hahn H., Loeffler M., Nürnberg P., Pfreundschuh M., Trümper L., Brockmöller J., Hasenfuss G. (2005) NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation 112, 3754–3762 [DOI] [PubMed] [Google Scholar]
- 20. Semsei A. F., Erdelyi D. J., Ungvari I., Csagoly E., Hegyi M. Z., Kiszel P. S., Lautner-Csorba O., Szabolcs J., Masat P., Fekete G., Falus A., Szalai C., Kovacs G. T. (2012) ABCC1 polymorphisms in anthracycline-induced cardiotoxicity in childhood acute lymphoblastic leukaemia. Cell Biol. Int. 36, 79–86 [DOI] [PubMed] [Google Scholar]
- 21. Visscher H., Ross C. J., Rassekh S. R., Barhdadi A., Dubé M. P., Al-Saloos H., Sandor G. S., Caron H. N., van Dalen E. C., Kremer L. C., van der Pal H. J., Brown A. M., Rogers P. C., Phillips M. S., Rieder M. J., Carleton B. C., Hayden M. R., and Canadian Pharmacogenomics Network for Drug Safety Consortium (2012) Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J. Clin. Oncol. 30, 1422–1428 [DOI] [PubMed] [Google Scholar]
- 22. Blokzijl H., van Steenpaal A., Vander Borght S., Bok L. I. H., Libbrecht L., Tamminga M., Geuken M., Roskams T. A. D., Dijkstra G., Moshage H., Jansen P. L. M., Faber K. N. (2008) Up-regulation and cytoprotective role of epithelial multidrug resistance-associated protein 1 in inflammatory bowel disease. J. Biol. Chem. 283, 35630–35637 [DOI] [PubMed] [Google Scholar]
- 23. Yoshioka M., Sagara H., Takahashi F., Harada N., Nishio K., Mori A., Ushio H., Shimizu K., Okada T., Ota M., Ito Y. M., Nagashima O., Atsuta R., Suzuki T., Fukuda T., Fukuchi Y., Takahashi K. (2009) Role of multidrug resistance-associated protein 1 in the pathogenesis of allergic airway inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 296, L30–36 [DOI] [PubMed] [Google Scholar]
- 24. Park H. A., Kubicki N., Gnyawali S., Chan Y. C., Roy S., Khanna S., Sen C. K. (2011) Natural vitamin E α-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke 42, 2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Becher U. M., Ghanem A., Tiyerili V., Fürst D. O., Nickenig G., Mueller C. F. H. (2011) Inhibition of leukotriene C4 action reduces oxidative stress and apoptosis in cardiomyocytes and impedes remodeling after myocardial injury. J. Mol. Cell Cardiol. 50, 570–577 [DOI] [PubMed] [Google Scholar]
- 26. Sreekumar P. G., Spee C., Ryan S. J., Cole S. P. C., Kannan R., Hinton D. R. (2012) Mechanism of RPE cell death in α-crystallin deficient mice: a novel and critical role for MRP1-mediated GSH efflux. PLoS One 7, e33420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krohn M., Lange C., Hofrichter J., Scheffler K., Stenzel J., Steffen J., Schumacher T., Brüning T., Plath A. S., Alfen F., Schmidt A., Winter F., Rateitschak K., Wree A., Gsponer J., Walker L. C., Pahnke J. (2011) Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice. J. Clin. Invest. 121, 3924–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fletcher J. I., Haber M., Henderson M. J., Norris M. D. (2010) ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer 10, 147–156 [DOI] [PubMed] [Google Scholar]
- 29. Cole S. P. C., Deeley R. G. (2006) Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 27, 438–446 [DOI] [PubMed] [Google Scholar]
- 30. Ballatori N., Krance S. M., Marchan R., Hammond C. L. (2009) Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Aspects Med. 30, 13–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bellarosa C., Bortolussi G., Tiribelli C. (2009) The role of ABC transporters in protecting cells from bilirubin toxicity. Curr. Pharm. Des. 15, 2884–2892 [DOI] [PubMed] [Google Scholar]
- 32. Bakos E., Evers R., Calenda G., Tusnády G. E., Szakács G., Váradi A., Sarkadi B. (2000) Characterization of the amino-terminal regions in the human multidrug resistance protein (MRP1). J. Cell Sci. 113, 4451–4461 [DOI] [PubMed] [Google Scholar]
- 33. Westlake C. J., Cole S. P. C., Deeley R. G. (2005) Function of the NH2-terminal membrane spanning domain of multidrug resistance protein 1 in protein processing and plasma membrane targeting. Mol. Biol. Cell 16, 2483–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenberg M. F., Oleschuk C. J., Wu P., Mao Q., Deeley R. G., Cole S. P. C., Ford R. C. (2010) Structure of a human multidrug transporter in an inward-facing conformation. J. Struct. Biol. 170, 540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones P. M., George A. M. (2014) A reciprocating twin-channel model for ABC transporters. Q. Rev. Biophys. 47, 189–220 [DOI] [PubMed] [Google Scholar]
- 36. Hollenstein K., Dawson R. J., Locher K. P. (2007) Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 17, 412–418 [DOI] [PubMed] [Google Scholar]
- 37. Chang X. B. (2010) Molecular mechanisms of ATP-dependent solute transport by multidrug resistance-associated protein 1. Methods Mol. Biol. 596, 223–249 [DOI] [PubMed] [Google Scholar]
- 38. Ernst R., Kueppers P., Klein C. M., Schwarzmueller T., Kuchler K., Schmitt L. (2008) A mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter Pdr5. Proc. Natl. Acad. Sci. U.S.A. 105, 5069–5074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koike K., Conseil G., Leslie E. M., Deeley R. G., Cole S. P. C. (2004) Identification of proline residues in the core cytoplasmic and transmembrane regions of multidrug resistance protein 1 (MRP1/ABCC1) important for transport function, substrate specificity, and nucleotide interactions. J. Biol. Chem. 279, 12325–12336 [DOI] [PubMed] [Google Scholar]
- 40. Deeley R. G., Cole S. P. C. (2006) Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 580, 1103–1111 [DOI] [PubMed] [Google Scholar]
- 41. Seelig A., Blatter X. L., Wohnsland F. (2000) Substrate recognition by P-glycoprotein and the multidrug resistance-associated protein MRP1: a comparison. Int. J. Clin. Pharmacol. Ther. 38, 111–121 [DOI] [PubMed] [Google Scholar]
- 42. Ito K., Olsen S. L., Qiu W., Deeley R. G., Cole S. P. C. (2001) Mutation of a single conserved tryptophan in multidrug resistance protein 1 (MRP1/ABCC1) results in loss of drug resistance and selective loss of organic anion transport. J. Biol. Chem. 276, 15616–15624 [DOI] [PubMed] [Google Scholar]
- 43. Haimeur A., Deeley R. G., Cole S. P. C. (2002) Charged amino acids in the sixth transmembrane helix of multidrug resistance protein 1 (MRP1/ABCC1) are critical determinants of transport activity. J. Biol. Chem. 277, 41326–41333 [DOI] [PubMed] [Google Scholar]
- 44. Karwatsky J., Daoud R., Cai J., Gros P., Georges E. (2003) Binding of a photoaffinity analogue of glutathione to MRP1 (ABCC1) within two cytoplasmic regions (L0 and L1) as well as transmembrane domains 10–11 and 16–17. Biochemistry 42, 3286–3294 [DOI] [PubMed] [Google Scholar]
- 45. Zhang D. W., Nunoya K., Vasa M., Gu H. M., Cole S. P. C., Deeley R. G. (2006) Mutational analysis of polar amino acid residues within predicted transmembrane helices 10 and 16 of multidrug resistance protein 1 (ABCC1): effect on substrate specificity. Drug Metab. Dispos. 34, 539–546 [DOI] [PubMed] [Google Scholar]
- 46. Ito K., Oleschuk C. J., Westlake C., Vasa M. Z., Deeley R. G., Cole S. P. C. (2001) Mutation of Trp1254 in the multispecific organic anion transporter, multidrug resistance protein 2 (MRP2) (ABCC2), alters substrate specificity and results in loss of methotrexate transport activity. J. Biol. Chem. 276, 38108–38114 [DOI] [PubMed] [Google Scholar]
- 47. Moreau C., Gally F., Jacquet-Bouix H., Vivaudou M. (2005) The size of a single residue of the sulfonylurea receptor dictates the effectiveness of K ATP channel openers. Mol. Pharmacol. 67, 1026–1033 [DOI] [PubMed] [Google Scholar]
- 48. El-Sheikh A. A., van den Heuvel J. J., Krieger E., Russel F. G., Koenderink J. B. (2008) Functional role of arginine 375 in transmembrane helix 6 of multidrug resistance protein 4 (MRP4/ABCC4). Mol. Pharmacol. 74, 964–971 [DOI] [PubMed] [Google Scholar]
- 49. Crowley E., O'Mara M. L., Kerr I. D., Callaghan R. (2010) Transmembrane helix 12 plays a pivotal role in coupling energy provision and drug binding in ABCB1. FEBS J. 277, 3974–3985 [DOI] [PubMed] [Google Scholar]
- 50. Wu P., Oleschuk C. J., Mao Q., Keller B. O., Deeley R. G., Cole S. P. C. (2005) Analysis of human multidrug resistance protein 1 (ABCC1) by matrix-assisted laser desorption ionization/time of flight mass spectrometry: toward identification of leukotriene C4 binding sites. Mol. Pharmacol. 68, 1455–1465 [DOI] [PubMed] [Google Scholar]
- 51. Maeno K., Nakajima A., Conseil G., Rothnie A., Deeley R. G., Cole S. P. C. (2009) Molecular basis for reduced estrone sulfate transport and altered modulator sensitivity of TM6 and TM17 mutants of MRP1 (ABCC1). Drug Metab. Dispos. 37, 1411–1420 [DOI] [PubMed] [Google Scholar]
- 52. Gottesman M. M., Ling V. (2006) The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 580, 998–1009 [DOI] [PubMed] [Google Scholar]
- 53. DeGorter M. K., Conseil G., Deeley R. G., Campbell R. L., Cole S. P. C. (2008) Molecular modelling of the human multidrug resistance protein 1 (MRP1/ABCC1). Biochem. Biophys. Res. Commun. 365, 29–34 [DOI] [PubMed] [Google Scholar]
- 54. Létourneau I. J., Slot A. J., Deeley R. G., Cole S. P. C. (2007) Mutational analysis of a highly conserved proline residue in MRP1, MRP2 and MRP3 reveals a partially conserved function. Drug Metab. Dispos. 35, 1372–1379 [DOI] [PubMed] [Google Scholar]
- 55. Iram S. H., Cole S. P. C. (2011) Expression and function of human MRP1 (ABCC1) is dependent on amino acids in cytoplasmic loop 5 and its interface with nucleotide binding domain 2. J. Biol. Chem. 286, 7202–7213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Iram S. H., Cole S. P. C. (2012) Mutation of Glu521 or Glu535 in cytoplasmic loop 5 cause differential misfolding in multiple domains of the multidrug and organic anion transporter MRP1 (ABCC1). J. Biol. Chem. 287, 7543–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Conseil G., Rothnie A. J., Deeley R. G., Cole S. P. C. (2009) Multiple roles of charged amino acids in cytoplasmic loop 7 for expression and function of the multidrug and organic anion transporter MRP1 (ABCC1). Mol. Pharmacol. 75, 397–406 [DOI] [PubMed] [Google Scholar]
- 58. Conseil G., Deeley R. G., Cole S. P. C. (2006) Functional importance of three basic residues clustered at the cytosolic interface of transmembrane helix 15 in the multidrug and organic anion transporter MRP1 (ABCC1). J. Biol. Chem. 281, 43–50 [DOI] [PubMed] [Google Scholar]
- 59. Ishikawa T. (1992) The ATP-dependent glutathione S-conjugate export pump. Trends Biochem. Sci. 17, 463–468 [DOI] [PubMed] [Google Scholar]
- 60. Jones D. C., Duvauchelle C., Ikegami A., Olsen C. M., Lau S. S., de la Torre R., Monks T. J. (2005) Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J. Pharmacol. Exp. Ther. 313, 422–431 [DOI] [PubMed] [Google Scholar]
- 61. Slot A. J., Wise D. D., Deeley R. G., Monks T. J., Cole S. P. C. (2008) Modulation of human MRP1 (ABCC1) and MRP2 (ABCC2) transport by endogenous and exogenous glutathione-conjugated catechol metabolites. Drug Metab. Dispos. 36, 552–560 [DOI] [PubMed] [Google Scholar]
- 62. Leier I., Jedlitschky G., Buchholz U., Cole S. P. C., Deeley R. G., Keppler D. (1994) The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 269, 27807–27810 [PubMed] [Google Scholar]
- 63. Loe D. W., Almquist K. C., Deeley R. G., Cole S. P. C. (1996) Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles: demonstration of glutathione-dependent vincristine transport. J. Biol. Chem. 271, 9675–9682 [DOI] [PubMed] [Google Scholar]
- 64. Qian Y. M., Song W. C., Cui H., Cole S. P. C., Deeley R. G. (2001) Glutathione stimulates sulfated estrogen transport by multidrug resistance protein 1. J. Biol. Chem. 276, 6404–6411 [DOI] [PubMed] [Google Scholar]
- 65. Zelcer N., Reid G., Wielinga P., Kuil A., van der Heijden I., Schuetz J. D., Borst P. (2003) Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4). Biochem. J. 371, 361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sakamoto H., Hara H., Hirano K., Adachi T. (1999) Enhancement of glucuronosyl etoposide transport by glutathione in multidrug resistance-associated protein-overexpressing cells. Cancer Lett. 135, 113–119 [DOI] [PubMed] [Google Scholar]
- 67. Leslie E. M., Ito K., Upadhyaya P., Hecht S. S., Deeley R. G., Cole S. P. C. (2001) Transport of the β-O-glucuronide conjugate of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) by the multidrug resistance protein 1 (MRP1/ABCC1): requirement for glutathione or a non-sulfur-containing analog. J. Biol. Chem. 276, 27846–27854 [DOI] [PubMed] [Google Scholar]
- 68. Jedlitschky G., Leier I., Buchholz U., Barnouin K., Kurz G., Keppler D. (1996) Transport of glutathione, glucuronate, and sulphate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 56, 988–994 [PubMed] [Google Scholar]
- 69. Newcomer M. E., Gilbert N. C. (2010) Location, location, location: Compartmentalization of early events in leukotriene biosynthesis. J. Biol. Chem. 285, 25109–25114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Montuschi P., Peters-Golden M. L. (2010) Leukotriene modifiers for asthma treatment. Clin. Exp. Allergy 40, 1732–1741 [DOI] [PubMed] [Google Scholar]
- 71. Budulac S. E., Postma D. S., Hiemstra P. S., Kunz L. I., Siedlinski M., Smit H. A., Vonk J. M., Rutgers B., Timens W., Boezen H. M., and Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease (GLUCOLD) Study Group (2010) Multidrug resistance-associated protein-1 (MRP1) genetic variants, MRP1 protein levels and severity of COPD. Respir. Res. 11, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lima J. J., Blake K. V., Tantisira K. G., Weiss S. T. (2009) Pharmacogenetics of asthma. Curr. Opin. Pulm. Med. 15, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Evers R., Cnubben N. H., Wijnholds J., van Deemter L., van Bladeren P. J., Borst P. (1997) Transport of glutathione prostaglandin A conjugates by the multidrug resistance protein 1. FEBS Lett. 419, 112–116 [DOI] [PubMed] [Google Scholar]
- 74. Paumi C. M., Wright M., Townsend A. J., Morrow C. S. (2003) Multidrug resistance protein (MRP) 1 and MRP3 attentuate cytotoxic and transactivating effects of the cyclopentenone prostaglandin, 15-deoxy-Δ(12,14) prostaglandin J2 in MCF7 breast cancer cells. Biochemistry 42, 5429–5437 [DOI] [PubMed] [Google Scholar]
- 75. de Waart D. R., Paulusma C. C., Kunne C., Oude Elferink R. P. J. (2006) Multidrug resistance associated protein 2 mediates transport of prostaglandin E2. Liver Int. 26, 362–368 [DOI] [PubMed] [Google Scholar]
- 76. Lin Z. P., Zhu Y. L., Johnson D. R., Rice K. P., Nottoli T., Hains B. C., McGrath J., Waxman S. G., Sartorelli A. C. (2008) Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signalling and nociceptive response. Mol. Pharmacol. 73, 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dalleau S., Baradat M., Guéraud F., Huc L. (2013) Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 20, 1615–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Renes J., de Vries E. E., Hooiveld G. J., Krikken I., Jansen P. L., Müller M. (2000) Multidrug resistance protein MRP1 protects against the toxicity of the major lipid peroxidation product 4-hydroxynonenal. Biochem. J. 350, 555–561 [PMC free article] [PubMed] [Google Scholar]
- 79. Frohnert B. I., Bernlohr D. A. (2014) Glutathionylated products of lipid peroxidation: A novel mechanism of adipocyte to macrophage signaling. Adipocyte 3, 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yamashita A., Oka S., Tanikawa T., Hayashi Y., Nemoto-Sasaki Y., Sugiura T. (2013) The actions and metabolism of lysophophatidylinositol, an endogenous agonist for GPR55. Prostaglandins Other Lipid Mediat. 107, 103–116 [DOI] [PubMed] [Google Scholar]
- 81. Piñeiro R., Falasca M. (2012) Lysophosphatidylinositol signalling: new wine from an old bottle. Biochim. Biophys. Acta 1821, 694–705 [DOI] [PubMed] [Google Scholar]
- 82. Maceyka M., Harikumar K. B., Milstien S., Spiegel S. (2012) Sphingosine-1-phosphate signalling and its role in disease. Trends Cell Biol. 22, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mitra P., Oskeritzian C. A., Payne S. G., Beaven M. A., Milstien S., Spiegel S. (2006) Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. U.S.A. 103, 16394–16399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tanfin Z., Serrano-Sanchez M., Leiber D. (2011) ATP-binding cassette ABCC1 is involved in the release of sphingosine 1-phosphate from rat uterine leiomyoma ELT3 cells and late pregnant rat myometrium. Cell Signal. 23, 1997–2004 [DOI] [PubMed] [Google Scholar]
- 85. Ito S., Iwaki S., Koike K., Yuda Y., Nagasaki A., Ohkawa R., Yatomi Y., Furumoto T., Tsutsui H., Sobel B. E., Fujii S. (2013) Increased plasma sphingosine-1-phosphate in obese individuals and its capacity to increase the expression of plasminogen activator inhibitor-1 in adipocytes. Coron. Artery Dis. 24, 642–650 [DOI] [PubMed] [Google Scholar]
- 86. Cartwright T. A., Campos C. R., Cannon R. E., Miller D. S. (2013) Mrp1 is essential for sphingolipid signaling to P-glycoprotein in mouse blood-brain and blood-spinal cord barriers. J. Cereb. Blood Flow Metab. 33, 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Piñeiro R., Maffucci T., Falasca M. (2011) The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 30, 142–152 [DOI] [PubMed] [Google Scholar]
- 88. Liu B., Song S., Jones P. M., Persaud S. J. (2014) GPR55: From orphan to metabolic regulator? Pharmacol. Ther. 10.1016/j.pharmthera.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 89. Sylantyev S., Jensen T. P., Ross R. A., Rusakov D. A. (2013) Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc. Natl. Acad. Sci. U.S.A. 110, 5193–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Borst P., de Wolf C., van de Wetering K. (2007) Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 453, 661–673 [DOI] [PubMed] [Google Scholar]
- 91. Cheung L., Flemming C. L., Watt F., Masada N., Yu D. M. T., Huynh T., Conseil G., Tivnan A., Polinsky A., Gudkov A. V., Munoz M. A., Vishvanath A., Cooper D. M., Henderson M. J., Cole S. P. C., Fletcher J. I., Haber M., Norris M. D. (2014) High-throughput screening identifies Ceefourin 1 and Ceefourin 2 as highly selective inhibitors of multidrug resistance protein 4 (MRP4). Biochem. Pharmacol. 91, 97–108 [DOI] [PubMed] [Google Scholar]
- 92. Zaman G. J. R., Lankelma J., van Tellingen O., Beijnen J., Dekker H., Paulusma C., Oude Elferink R. P. J., Baas F., Borst P. (1995) Role of glutathione in the export of compounds from cells by the multidrug-resistance-associated protein. Proc. Natl. Acad. Sci. U.S.A. 92, 7690–7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rappa G., Lorico A., Flavell R. A., Sartorelli A. C. (1997) Evidence that the multidrug resistance protein (MRP) functions as a co-transporter of glutathione and natural product toxins. Cancer Res. 57, 5232–5237 [PubMed] [Google Scholar]
- 94. Loe D. W., Deeley R. G., Cole S. P. C. (1998) Characterization of vincristine transport by the 190 kDa multidrug resistance protein, MRP: evidence for co-transport with reduced glutathione. Cancer Res. 58, 5130–5136 [PubMed] [Google Scholar]
- 95. Rothnie A., Conseil G., Lau A. Y. T., Deeley R. G., Cole S. P. C. (2008) Mechanistic differences between GSH transport by MRP1 (ABCC1) and GSH modulation of MRP1-mediated transport. Mol. Pharmacol. 74, 1630–1640 [DOI] [PubMed] [Google Scholar]
- 96. Lorico A., Rappa G., Finch R. A., Yang D., Flavell R. A., Sartorelli A. C. (1997) Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 57, 5238–5242 [PubMed] [Google Scholar]
- 97. Leslie E. M., Bowers R. J., Deeley R. G., Cole S. P. C. (2003) Structural requirements for functional interaction of glutathione tripeptide analogs with the human multidrug resistance protein 1 (MRP1). J. Pharmacol. Exp. Ther. 304, 643–653 [DOI] [PubMed] [Google Scholar]
- 98. Leslie E. M., Deeley R. G., Cole S. P. C. (2003) Bioflavonoid stimulation of glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1). Drug Metab. Dispos. 31, 11–15 [DOI] [PubMed] [Google Scholar]
- 99. Brechbuhl H. M., Kachadourian R., Min E., Chan D., Day B. J. (2012) Chrysin enhances doxorubicin-induced cytotoxicity in human lung epithelial cancer cell lines: the role of glutathione. Toxicol. Appl. Pharmacol. 258, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bast A., Haenen G. R. (2013) Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 34, 430–436 [DOI] [PubMed] [Google Scholar]
- 101. Lok H. C., Suryo Rahmanto Y., Hawkins C. L., Kalinowski D. S., Morrow C. S., Townsend A. J., Ponka P., Richardson D. R. (2012) Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1–1 and multidrug resistance protein 1 via dinitrosyl iron complexes. J. Biol. Chem. 287, 607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Leier I., Jedlitschky G., Buchholz U., Center M., Cole S. P. C., Deeley R. G., Keppler D. (1996) ATP-dependent glutathione disulfide transport mediated by the MRP gene-encoded conjugate export pump. Biochem. J. 314, 433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Minich T., Riemer J., Schulz J. B., Wielinga P., Wijnholds J., Dringen R. (2006) The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J. Neurochem. 97, 373–384 [DOI] [PubMed] [Google Scholar]