Background: Little is known about the mechanism of action of MPA in the female genital tract.
Results: GR mediates MPA-induced up-regulation of IL-12 and down-regulation of IL-10 mRNA and protein levels.
Conclusion: MPA favors a pro-inflammatory milieu in ectocervical epithelial cells.
Significance: MPA used in hormonal therapy may modulate inflammation in the ectocervical environment via this genomic mechanism.
Keywords: Gene Regulation, Glucocorticoid, Glucocorticoid Receptor, Inflammation, Progesterone, Cervical Epithelium, Contraception, Cytokines, Medroxyprogesterone Acetate, Progestin
Abstract
Medroxyprogesterone acetate (MPA), designed to mimic the actions of the endogenous hormone progesterone (P4), is extensively used by women as a contraceptive and in hormone replacement therapy. However, little is known about the steroid receptor-mediated molecular mechanisms of action of MPA in the female genital tract. In this study, we investigated the regulation of the pro-inflammatory cytokine, interleukin (IL)-12, and the anti-inflammatory cytokine IL-10, by MPA versus P4, in an in vitro cell culture model of the female ectocervical environment. This study shows that P4 and MPA significantly increase the expression of the IL-12p40 and IL-12p35 genes, whereas IL-10 gene expression is suppressed in a dose-dependent manner. Moreover, these effects were abrogated when reducing the glucocorticoid receptor (GR) levels with siRNA. Using a combination of chromatin immunoprecipitation (ChIP), siRNA, and re-ChIP assays, we show that recruitment of the P4- and MPA-bound GR to the IL-12p40 promoter requires CCAAT enhancer-binding protein (C/EBP)-β and nuclear factor κB (NFκB), although recruitment to the IL-10 promoter requires signal transducer and activator of transcription (STAT)-3. These results suggest that both P4 and MPA may modulate inflammation in the ectocervix via this genomic mechanism.
Introduction
Progestogen is a term used to describe the endogenous hormone progesterone (P4)2 as well as synthetic progestins. The latter mimic the progestogenic activity of P4 and have been used in a number of therapeutic applications, such as contraception, hormone replacement therapy, and treatment of some gynecological disorders (1–3). Medroxyprogesterone acetate (MPA or Depo-Provera®) is an example of a synthetic progestin extensively used as a progestin-only injectable contraceptive in South Africa (4–7). At the molecular level, MPA elicits its biological effects by binding not only to the progesterone receptor (1, 8) but also to other members of the steroid receptor family such as the glucocorticoid receptor (GR), androgen receptor, and mineralocorticoid receptor (9–13). The subsequent off-target biological effects via these receptors may contribute to the undesirable side effects observed with its clinical use. For example, androgen receptor-mediated effects of MPA have been associated with an increased risk of breast cancer (14), although its activity via the GR has been linked to immunosuppression (1, 2, 8, 15) and apoptosis (16).
Clinical and epidemiological evidence suggests that the use of MPA as a contraceptive may increase the risk of acquiring genital tract infections such as herpes simplex virus type (HSV)-2 (17), Chlamydia (18), gonorrhea (19), and HIV-1 (6, 20–22). The lower female genital tract is the primary site of exposure to the majority of these sexually transmitted pathogens (23–26). Epithelial cells lining the female genital tract play a protective role when this site is exposed to pathogens (27–29). In addition to providing a physical barrier against sexually transmitted pathogens, these epithelial cells are also capable of producing a wide variety of cytokines and chemokines that regulate both innate and acquired local immune responses (27–31). This cytokine milieu in the female genital tract is a vital determinant of inflammation (30–32) and most likely susceptibility to infections (32–38).
MPA has previously been shown to regulate cytokine/chemokine gene expression in epithelial cell lines of the female genital tract in a ligand-, promoter-, and cell-specific manner (39). The possibility thus exists that MPA may disrupt normal immune responses in the female genital tract, thereby influencing inflammation at this site. This is consistent with some reports suggesting that hormonal contraception, such as MPA, is associated with an increase in inflammation at this site (18, 40, 41). Modulation of inflammation by MPA in the female genital tract is likely to affect susceptibility to sexually transmitted infections by altered recruitment of inflammatory cells (42).
In the light of the above, this study investigated the effects of MPA, relative to P4, on cytokine gene expression in a human ectocervical epithelial cell line. Specifically, we used gene-specific mRNA analysis, siRNA, and chromatin immunoprecipitation (ChIP) assays to explore the gene regulation of the pro-inflammatory cytokine, interleukin (IL)-12p40, and anti-inflammatory cytokine, IL-10, in response to P4 and MPA, and ELISA to determine IL-12 and IL-10 protein levels. IL-12, a 70-kDa heterodimeric protein composed of two disulfide-linked subunits, p40 and p35, is a key cytokine that promotes cellular immunity and the subsequent production of other pro-inflammatory cytokines (43, 44). However, IL-10 inhibits cellular immunity by suppressing the production of pro-inflammatory cytokines such as IL-12 and IL-8 (45, 46). Our results indicate that both P4 and MPA increase the expression of the IL-12p40 and IL-12p35 genes, although the IL-10 gene expression is decreased. A detailed investigation into the molecular mechanism, using a combination of chromatin immunoprecipitation (ChIP), siRNA, and re-ChIP assays, show that the GR is needed for the regulation of these cytokine genes and that recruitment of the P4- and MPA-bound GR to the IL-12p40 promoter requires CCAAT enhancer-binding protein (C/EBP)-β and nuclear factor κB (NFκB), whereas recruitment to the IL-10 promoter requires signal transducer and activator transcription (STAT)-3.
EXPERIMENTAL PROCEDURES
Cell Culture
The human Ect1/E6E7 ectocervical epithelial cell line was purchased from the ATCC and cultured and prepared as described previously (39, 47). The MDA-MB-231 human breast cancer cell line was a generous gift from Prof. Guy Haegemann (University of Gent, Belgium) and was cultured as described previously (48). Only mycoplasma-negative cells were used in experiments.
Materials
P4, MPA, cortisol, and tumor necrosis factor (TNF) were purchased from Sigma. [3H]Dexamethasone (specific activity of 82.8 Ci/mmol) was from AEC-Amersham Biosciences.
Immunoblotting
Ect1/E6E7 and MDA-MB-231 cells were seeded in 12-well plates at 1 × 105 cells per well. The cells were washed with ice-cold 1× PBS before lysis with sample buffer (100 mm Tris-HCl, pH 6.8, 20% glycerol, 5% SDS, 0.1% bromphenol blue, and 2% β-mercaptoethanol) (49). Protein samples were resolved by 10% SDS-PAGE, transferred to PVDF membranes (Millipore), and then blocked in 10% fat-free milk powder. The membranes were first probed with the primary antibodies, followed with HRP-conjugated secondary antibodies (goat anti-rabbit or anti-mouse) (Santa Cruz Biotechnology). Proteins were visualized using enhanced chemiluminescence (Pierce Thermo Scientific Inc.) and x-ray film (Africa X-Ray Industrial and Medical). The following primary antibodies all from Santa Cruz Biotechnology were used: anti-GR (H-300), anti-C/EBPβ (C-19), anti-NFκB p65 (C20), anti-STAT-3 (C-20), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (0411), and anti-heat shock protein (Hsp)-90α/β (H-114).
Whole Cell Binding Assay
Competitive whole cell binding assays were performed as described previously (11), with a few modifications. Briefly, Ect/E6E7 cells were seeded in 24-well plates at a density of 1 × 105 cells per well. After 48 h, the cells were washed three times with PBS and incubated for 6 h at 37 °C with 10 nm [3H]dexamethasone, in the absence (total binding) and presence of 1 μm unlabeled P4, MPA, or cortisol (nonspecific binding). Cells were washed three times with ice-cold PBS containing 0.2% bovine serum albumin (BSA), before lysis with reporter lysis buffer (Promega). Total binding was measured as counts/min (cpm), whereas the specific binding was determined by subtracting nonspecific binding from total binding. Specific binding was normalized to the protein concentration, determined using the Bradford protein assay method (50).
Quantitative Real Time PCR (qPCR)
Ect1/E6E7 and MDA-MB-231 cells were seeded in 12-well plates at a density of 1 × 105 cells per well and were incubated with test compounds for 6 h, before total RNA was isolated using Tri-Reagent (Sigma) according to the manufacturer's instructions. Duration of hormone treatment was chosen based on time course studies in Ect1/E6E7 cells showing maximum TNF-induced mRNA expression of these genes at this time (data not shown). Total RNA was reverse-transcribed using the Roche Applied Science transcriptor first strand cDNA synthesis kit. Real time qPCR was performed by using the LightCycler-FastStart DNA Masternon-plus SYBR Green I system (Roche Applied Science) according to the manufacturer's instructions. The mRNA expression of IL-12p40, IL-12p35, IL-10, and GAPDH (used as an internal standard) was measured using the primer sets as indicated in Table 1.
TABLE 1.
Primers used for real time qPCR

siRNA Transfections
Ect1/E6E7 and MDA-MB-231 cells were seeded in 12-well plates at a density of 1 × 105 cells per well. Cells were transfected with 10 nm siRNA using HiPerfect transfection reagent (Qiagen), according to the manufacturer's instructions, and incubated for 24 h (GR, C/EBPβ, and STAT-3) or 48 h (NFκB). Cells were subsequently treated for 6 h (qPCR) or 24 h (ELISA) with 0.02 μg/ml TNF in the absence and presence of 0.1% ethanol (control) or 1 μm test compound. For the quantification of mRNA expression by qPCR, RNA was harvested, and cDNA was synthesized. For the quantification of protein levels by ELISA, cell culture supernatants were collected and analyzed as described above. The following siRNAs were used: nonsilencing scrambled sequence control (NSC) or GR HS_NR3C1_6 or GR_HS_NR3C1_5 (all from Qiagen), or C/EBPβ, STAT-3, or NFκB p65 (all from Santa Cruz Biotechnology). Reduction in the protein levels was confirmed by Western blot analysis.
Chromatin Immunoprecipitation (ChIP) and Re-ChIP Assays
ChIP assays were performed as described earlier with minor modifications (51, 52). Briefly, Ect1/E6E7 cells were seeded in 14-cm2 dishes at a density of 1 × 107 cells. After 72 h, the supplemented KSFM was replaced with unsupplemented KSFM. Twenty four hours later, the cells were treated with 0.02 μg/ml TNF in the absence and presence of 0.1% ethanol (control) or 1 μm P4, MPA, or cortisol for 2 h, and then the proteins and the chromatin were cross-linked using 1% formaldehyde. Cells were washed twice with ice-cold PBS, harvested in PBS containing protease inhibitors (1× Complete Mini Protease Inhibitor Mixture tablet; Roche Applied Science), lysed, and sonicated. The sonicated chromatin was centrifuged at 15,000 × g for 10 min at 4 °C to pellet the cell debris. An aliquot of the lysate (30 μg) was removed and used as input, and 100 μg of the chromatin was immunoprecipitated with antibodies against GR (H-300) or anti-IgG (Santa Cruz Biotechnology). The immunoprecipitated chromatin was collected on protein A/G-agarose beads preblocked with salmon sperm DNA, extensively washed, and eluted with elution buffer (1% SDS and 100 mm NaHCO3). The cross-linking was reversed by adding NaCl (final concentration 300 nm) and incubating the samples overnight at 65 °C. Thereafter, the proteins were digested by treating the samples with proteinase K (Roche Applied Science, South Africa). Both immunoprecipitated and input DNA were purified using the NucleoSpin® Extract II kit (Thermo Scientific), and the purified immunoprecipitated DNA was quantified by qPCR, normalizing against input chromatin. Locations of primers used to amplify the DNA are shown in Figs. 4A and 5A, and the primer sequences are shown in Table 1.
FIGURE 4.
GR occupies the NFκB/C/EBPβ region of the IL-12p40 promoter in response to P4, MPA, and cortisol. Human Ect1/E6E7 cells were incubated with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH or 1 μm P4, MPA, or cortisol for 2 h, followed by the ChIP assay. A, schematic illustration of the IL-12p40 promoter, indicating all the cis-elements investigated in this study and position of the primers. B–D, cell lysates were subjected to immunoprecipitation with the GR-specific antibody or anti-IgG (negative control). The immunoprecipitated DNA fragments and input DNA were analyzed by real time qPCR. Data shown are normalized to input and expressed as the fold-response relative to EtOH (IgG control), which was set as 1. Results shown are the average (± S.E.) of at least four independent experiments. One-way ANOVA analysis of variance and Dunnett's (compares all columns versus control (IgG EtOH) column) post-tests were used for statistical analysis. ns, no statistical significance; *, **, and ***, p < 0.05, p < 0.01, or p < 0.001, respectively.
FIGURE 5.
GR occupies the Sp1/STAT-3 region of the IL-10 promoter in response to P4, MPA, and cortisol. Human Ect1/E6E7 cells were incubated with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH or 1 μm P4, MPA, or cortisol for 2 h, followed by the ChIP assay. A, schematic illustration of the IL-10 promoter, indicating all the cis-elements investigated in this study and position of the primers. B–D, cell lysates were subjected to immunoprecipitation with the GR-specific antibody or anti-IgG (negative control). The immunoprecipitated DNA fragments and input DNA were analyzed by real time qPCR. Data shown are normalized to input and expressed as the fold-response relative to EtOH (IgG control), which was set as 1. Results shown are the average (± S.E.) of at least four independent experiments. One-way ANOVA analysis of variance and Dunnett's test (compares all columns versus control (IgG EtOH) column) post-tests were performed as post-test. ns, no statistical significance; *, p < 0.05; **, p < 0.01.
For the chromatin reimmunoprecipitation (re-ChIP) assay, the immunoprecipitated DNA-protein complexes were eluted at 37 °C in elution buffer (1% SDS and 10 mm DTT). An aliquot of the supernatant was removed and used as a control for the first immunoprecipitation. The remaining sonicated chromatin was reimmunoprecipitated with antibodies specific for C/EBPβ, NFκB, or STAT-3 and analyzed as above.
ELISA
Ect1/E6E7 cells were seeded in 12-well plates at a density of 1 × 105 cells per well and incubated with test compounds for 24 h. Culture supernatants were collected and assayed for IL-12p70 and IL-10 by high sensitivity kits purchased from eBioscience according to the manufacturer's instructions. The optical density readings and standard concentrations were plotted, and the optical densities were converted to picograms/ml using linear regression analysis. The linearity range of the specific immunoassay kits used in this study were 0.16 to 10 pg/ml for IL-12p70 and 0.39 to 25 pg/ml for IL-10.
Data Manipulation and Statistical Analysis
GraphPad Prism® version 5 (GraphPad Software) was used for data manipulation, graphical presentations, and statistical analysis. One-way ANOVA, with Dunnett's (compares all columns versus control column) or Bonferroni's (compares all pairs of columns) post-tests, were used. Statistically significant differences are indicated by either *, **, and *** or #, ##, and ###, to indicate p < 0.05, p < 0.01, or p < 0.001, respectively, whereas p > 0.05 indicates no statistical significance (ns). The error bars represent the mean ± S.E. of at least three independent experiments.
RESULTS
Progestogen-activated GR Regulates mRNA Expression of IL-12p40, IL-12p35, and IL-10 Genes
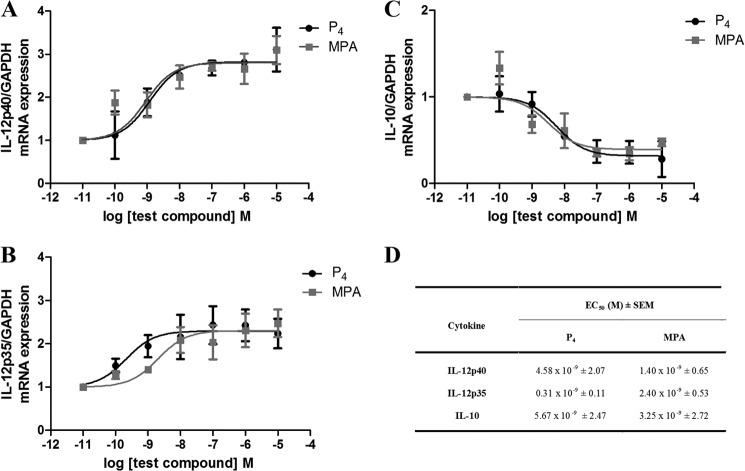
To assess the effects of P4 and MPA on inflammation in the female genital tract, the mRNA expression levels of endogenous IL-12p40, IL-12p35, and IL-10 were measured in the Ect1/E6E7 cells. These cells were used as an in vitro cell culture model for mucosal immunity in the female ectocervical environment, as they closely resemble the characteristics of their tissue of origin and primary cells (39, 47). An increase in pro-inflammatory cytokines such as IL-12 is critical for the progression of inflammation, although anti-inflammatory cytokines such as IL-10 control the course of the inflammatory process (45, 53–56). The cells were treated with increasing concentrations of P4 or MPA, and gene expression was measured using qPCR. P4 and MPA increased the gene expression of both IL-12p40 and IL-12p35 in a dose-dependent manner (Fig. 1, A and B), although these ligands dose-dependently decreased the expression of IL-10 (Fig. 1C). The potencies (EC50 values) of P4 and MPA were in the nanomolar range and are similar on all three genes (Fig. 1D).
FIGURE 1.
Effect of P4 and MPA on the TNF induced expression of IL-12p40, IL-12p35, and IL-10 in the human ectocervical cell line. The human Ect1/E6E7 cell line was incubated for 6 h with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH (vehicle control) or increasing concentrations of P4 or MPA. Total RNA was isolated and reverse-transcribed to cDNA. Real time qPCR was performed to determine the mRNA expression levels of IL-12p40 (A), IL-12p35 (B), and IL-10 (C), using GAPDH as the internal standard. Results shown are the average of at least four independent experiments (±S.E.). Relative IL-12p40, IL-12p35, and IL-10 mRNA expression of treated samples was calculated relative to the vehicle control (EtOH), which was set as 1. D, relative potency (EC50 ± S.E.) for each ligand for activation of the IL-12p40 and IL-12p35 genes and repression of the IL-10 gene was obtained from the data shown in A–C. Statistical analysis of the EC50 values for all genes indicated P4 versus MPA (p > 0.05).
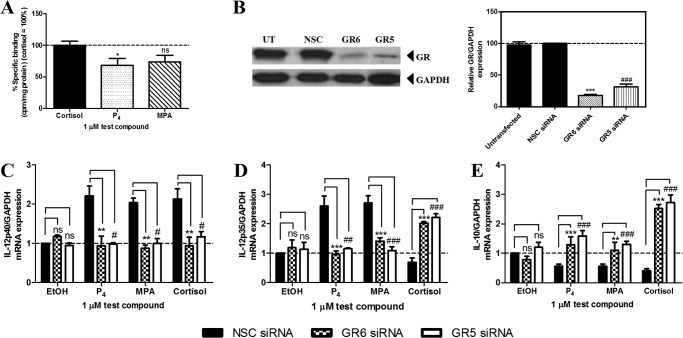
To gain insight into the mechanism whereby the progestogens regulate the mRNA expression of IL-12p40, IL-12p35, and IL-10, we investigated the involvement of the GR, because it is known that both P4 and MPA can bind to the GR (11). Competitive whole cell binding assays in this cell line confirmed that P4 and MPA bind to the native GR (Fig. 2A). To establish the involvement of the GR, cells were transfected with two different GR-specific siRNAs, or an NSC siRNA, prior to treatment with 0.02 μg/ml TNF in the absence and presence of 1 μm test compound for 24 h. Western blot analysis confirmed that both GR6 and GR5 siRNA reduced GR protein levels to a similar extent (p > 0.05) (Fig. 2B). Gene expression analysis by qPCR showed that the P4- and MPA-induced effects on IL-12p40, IL-12p35, and IL-10 were significantly reversed when the GR levels were decreased (Fig. 2, C–E). As expected, the effects of the natural glucocorticoid, cortisol, on IL-12p40, IL-12p35, and IL-10 gene expression were also significantly reduced by the decrease in GR protein levels (Fig. 2, C–E). Although similar GR-dependent effects were observed for P4 and MPA on IL-12p40 and IL-12p35 gene expression (Fig. 2, C and D), cortisol displayed differential effects on the expression of these genes, suggesting different mechanisms of regulation.
FIGURE 2.
Decreasing GR protein levels by siRNA indicates a role for the GR in mediating the effects of P4 and MPA on IL-12p40, IL-12p35, and IL-10 mRNA expression in the Ect1/E6E7 cell line. A, human Ect1/E6E7 cell line was incubated with 10 nm [3H]dexamethasone in the absence (total binding) and presence of 1 μm unlabeled (nonspecific binding) P4, MPA, or cortisol for 6 h. The percentage of specific binding (total binding minus nonspecific binding) is plotted. Binding of the test compounds to the GR is shown relative to binding of cortisol set as 100%. One-way ANOVA analysis of variance and Dunnett's test (compare all columns versus control (cortisol) column) were performed as post-test. B–E, untransfected human Ect1/E6E7 cells, as well as cells transfected with 10 nm NSC or two GR siRNA oligonucleotides, were either left untreated or treated with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH (control) or 1 μm P4, MPA, or cortisol for 6 h. B, for verification of GR knockdown, total protein from the untreated cells was harvested to perform Western blotting, using antibodies specific for the GR and GAPDH. The latter was used as a loading control. A representative blot is shown. GR expression levels relative to GAPDH were quantified using UN-SCAN-IT. Western blots of three independent experiments were quantified to determine the percentage GR protein knockdown. C–E, total RNA was isolated and reverse-transcribed to cDNA. Thereafter, real time qPCR was performed to determine the mRNA expression levels of IL-12p40 (C), IL-12p35 (D), and IL-10 (E), using GAPDH as the internal standard. Relative IL-12p40, IL-12p35, and IL-10 gene expression of treated samples was calculated relative to vehicle control (EtOH) of the NSC siRNA, which was set as 1. Statistically significant differences are indicated by *, **, or *** p < 0.05, p < 0.01, or p < 0.001, respectively, for GR6; #, p < 0.05; ##, p < 0.01; ###, p < 0.001, respectively, for GR5; ns, no statistical significance; UT, untransfected.
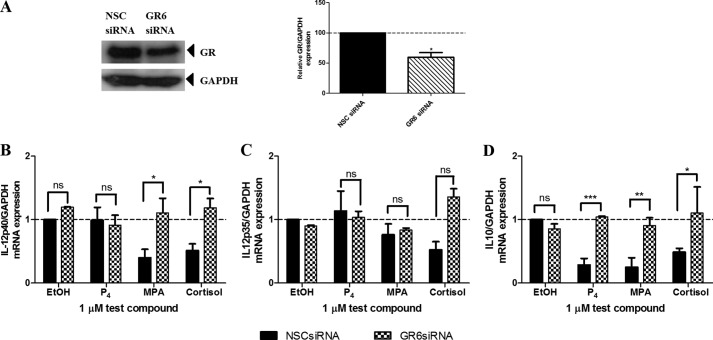
To determine whether the effects of the progestogens on IL-12 and IL-10 gene expression are specific to the Ect1/E6E7 cell line, the experiments were repeated in the MDA-MB-231 breast cancer cell line (Fig. 3). We used this cell line as it has previously been reported that the MDA-MB-231 cell line expresses IL-12p40, IL-12p35, and IL-10 mRNA (57). The results show that the effects of the progestogens on IL-12 gene expression are cell-specific in that P4 has no effect on IL-12p40 (Fig. 3B) and IL-12p35 (Fig. 3C) gene expression, although MPA decreases the mRNA expression of IL-12p40, but not IL-12p35, via a GR-mediated mechanism. A recent study by Hapgood and co-workers (58) observed similar GR-mediated anti-inflammatory effects of MPA in a human endocervical cell line. Interestingly, all the progestogens repressed IL-10 mRNA levels in both cell lines, in a GR-dependent manner (Figs. 2E and 3D), suggesting that the regulatory mechanisms for IL-10 are not cell-specific, unlike those for IL-12.
FIGURE 3.
MPA regulates IL-12p40 and IL-10, but not IL-12p35, mRNA levels in the MDA-MB-231 cell line in a GR-dependent manner. Human MDA-MB-231 cells transfected with 10 nm NSC or GR6 siRNA oligonucleotides were either left untreated or treated with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH (control) or 1 μm P4, MPA, or cortisol for 6 h. A, for verification of GR knockdown, total protein from the untreated cells was harvested to perform Western blotting, using antibodies specific for the GR and GAPDH. The latter was used as a loading control. A representative blot is shown. GR expression levels relative to GAPDH were quantified using UN-SCAN-IT. Western blots of at least two independent experiments were quantified to determine the percentage of GR protein knockdown. B–D, total RNA was isolated and reverse-transcribed to cDNA. Thereafter, real time qPCR was performed to determine the mRNA expression levels of IL-12p40 (B), IL-12p35 (C), and IL-10 (D) using GAPDH as the internal standard. Relative IL-12p40, IL-12p35, and IL-10 gene expression of treated samples was calculated relative to vehicle control (EtOH) of the NSC siRNA, which was set as 1. Results shown are the average (± S.E.) of at least two independent experiments. One-way ANOVA and Dunnett's (compares all pairs of columns versus control column) post-tests were used for statistical analysis. ns, no statistical significance; *, **, and ***, p < 0.05, p < 0.01, or p < 0.001, respectively.
P4 and MPA Promote the Recruitment of the GR to the Endogenous IL-12p40 and IL-10 Gene Promoters
Next, we wanted to elucidate the mechanism involved in GR-mediated regulation of IL-12 and IL-10 in response to P4 and MPA. Because IL12p35 is expressed in most cell types, unlike IL-12p40 (59, 60), we focused our attention on investigating promoter occupancy on IL-12p40, with a view to understanding the cell-specific mechanism of IL12 gene regulation. Thus, to investigate whether the GR is recruited to the endogenous IL-12p40 and IL-10 promoters, the Ect1/E6E7 cells were incubated with 0.02 μg/ml TNF in the absence or presence of 1 μm test compound for 2 h. The cell lysates were immunoprecipitated with a GR-specific antibody or anti-IgG (negative control), followed by qPCR analysis. Although it is generally accepted that the ligand-bound GR activates transcription of target genes by binding to glucocorticoid-response elements (GREs) (61) in the promoter region of these genes, no consensus GRE sequences are present within the proximal promoter region (−880 bp relative to the transcription start site) of the IL-12p40 gene (62, 63). Alternative cis-elements such as C/EBPβ and specific protein 1 (Sp1) were thus investigated, as previous studies have indicated that tethering of the GR to C/EBPβ or Sp1 transcription factors can activate transcription of genes containing C/EBPβ- (64, 65) or Sp1 (66, 67)-binding sites, respectively. For IL-10, we investigated the binding sites such as STAT-3 and activator protein (AP)-1, as tethering of the GR to STAT-3 and AP-1 has previously been associated with suppression of some genes (68, 69). A schematic diagram of all the cis-elements investigated in this study and the position of the primers are presented in Figs. 4A and 5A. Of note, some elements are located in close proximity to each other, and thus some primers span more than one cis-element.
Results showed that the GR occupies the NFκB/C/EBPβ region (Fig. 4B), but not the Sp1- or AP-1-binding sites (Fig. 4, C and D), of the IL-12p40 promoter when cells were treated with P4 and MPA. Interestingly, cortisol treatment resulted in GR recruitment to the NFκB/C/EBPβ- and Sp1-binding sites (Fig. 4, B and C). For IL-10, results show that the GR occupies the Sp1/STAT-3 region of the promoter (Fig. 5B) but not the AP-1- (Fig. 5C) or GRE/Sp1 (Fig. 5D)-binding sites. Collectively, the results suggest that in the Ect1/E6E7 cell line, P4, MPA, and cortisol-bound GR interact with the NFκB/C/EBPβ region of the IL-12p40 promoter to activate transcription of this gene, whereas recruitment of the P4, MPA, and cortisol-bound GR to the Sp1/STAT-3 region of the IL-10 promoter causes suppression of gene transcription.
GR Recruitment to the IL-12p40 Promoter in Response to P4 and MPA Is Dependent on Both the C/EBPβ and NFκB Transcription Factors
As we showed that the GR interacts with the NFκB/C/EBPβ region of the IL-12p40 promoter, we next performed re-ChIP assays to determine whether the GR forms a complex with the C/EBPβ and/or NFκB on the IL-12p40 promoter. Intact Ect1/E6E7 cells were treated with 0.02 μg/ml TNF in the absence or presence of 1 μm P4, MPA, or cortisol for 2 h. Cell lysates were subjected to immunoprecipitation with a GR-specific antibody and then with either the C/EBPβ- or NFκB-specific antibodies. Immunoprecipitation with anti-IgG served as negative control. Results in Fig. 6, A and B, suggest that in response to P4, MPA, and cortisol, the GR, C/EBPβ, and NFκB are co-localized on the endogenous IL-12p40 promoter.
FIGURE 6.
Recruitment of the progestogen-bound GR to the IL-12p40 promoter is dependent on both the transcription factors C/EBPβ and NFκB. A and B, human Ect1/E6E7 cells were incubated with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH or 1 μm P4, MPA, or cortisol for 2 h, followed by the re-ChIP assay. Cell lysates were subjected to immunoprecipitation with the GR-specific antibody and then with the C/EBPβ (A) or NFκB (B) antibody or anti-IgG (negative control). The immunoprecipitated DNA fragments and input DNA were analyzed by real time qPCR. Data shown are normalized to input and expressed as the fold-response relative to EtOH (IgG control), which was set as 1. Results shown are the average (± S.E.) of at least three independent experiments. One-way ANOVA analysis of variance and Dunnett's (compares all columns versus control (IgG EtOH) column) post-tests were used for statistical analysis. C and D, human Ect1/E6E7 cells, transfected with 10 nm NSC, C/EBPβ, or NFκB siRNA oligonucleotides, were treated for 6 h with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH (control) or 1 μm P4, MPA, or cortisol. For verification of C/EBPβ and NFκB knockdown, total protein from the untreated cells was harvested to perform Western blotting using antibodies specific for C/EBPβ and Hsp90 (C) and NFκB and GAPDH (D). Hsp90 and GAPDH were used as loading controls. A representative blot is shown for each knockdown. Total RNA was isolated and reverse-transcribed to cDNA. Thereafter, real time qPCR was performed to determine the mRNA expression levels of IL-12p40, using GAPDH as the internal standard. Relative IL-12p40 gene expression of treated samples was calculated relative to vehicle control (EtOH) of the NSC siRNA, which was set as 1. Results shown are the average (± S.E.) of at least three independent experiments. Two-way ANOVA analysis of variance and Bonferroni (compares all pairs of columns) post-tests were used for statistical analysis. ns, no statistical significance; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Both C/EBPβ and NFκB have previously been shown to be critical in the transcriptional activation of the IL-12p40 gene (70, 71). As the re-ChIP results indicate that the liganded GR interacts with both C/EBPβ and NFκB, we investigated whether both transcription factors are involved in the progestogen-induced up-regulation of IL-12p40 gene expression. The Ect1/E6E7 cell line was transfected with 10 nm NSC or validated C/EBPβ- or NFκB-specific siRNA oligonucleotides, followed by treatment with 0.02 μg/ml TNF in the absence or presence of 1 μm P4, MPA, or cortisol for 6 h. Western blot analysis showed ∼54 and ∼68% reduction in endogenous C/EBPβ (Fig. 6C) and NFκB (Fig. 6D) protein levels, respectively. Reducing C/EBPβ (Fig. 6C) and NFκB (Fig. 6D) protein levels significantly abolished the ligand-induced up-regulation of IL-12p40 gene expression, indicating that the progestogen-bound GR requires both transcription factors to activate transcription of the human IL-12p40 gene.
STAT-3 Is Required for GR-mediated Suppression of IL-10 Gene Expression in Response to P4 and MPA
The results indicating that the GR is recruited to the Sp1/STAT-3 region of the IL-10 promoter in response to P4 and MPA (Fig. 5B), but not the GRE/Sp1-binding sites (Fig. 5D), suggest that the STAT-3 and not the Sp1 element is important for the transcriptional suppression of IL-10 gene expression. Thus, re-ChIP assays were used to investigate whether the liganded GR forms a complex with STAT-3 on the IL-10 promoter. Intact Ect1/E6E7 cells were treated with 0.02 μg/ml TNF in the absence or presence of 1 μm P4, MPA, or cortisol for 2 h. Cell lysates were subjected to immunoprecipitation with a GR-specific antibody and subsequently a STAT-3-specific antibody. Immunoprecipitation with anti-IgG served as negative control. Results showed that in response to P4, MPA, and cortisol, the GR and STAT-3 were co-recruited to the endogenous IL-10 promoter (Fig. 7A).
FIGURE 7.

STAT-3 plays a role in the GR-mediated down-regulation of IL-10 gene expression in the Ect1/E6E7 cell line. A, human Ect1/E6E7 cells were incubated with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH or 1 μm P4, MPA, or cortisol for 2 h, followed by the re-ChIP assay. Cell lysates were subjected to immunoprecipitation with the GR-specific antibody and then with the STAT-3-specific antibody or anti-IgG (negative control). The immunoprecipitated DNA fragments and input DNA were analyzed by real time qPCR. Data shown are normalized to input and expressed as the fold-response relative to EtOH (IgG control), which was set as 1. Results shown are the average (± S.E.) of at least three independent experiments. One-way ANOVA and Dunnett's (compares all columns versus control (IgG EtOH) column) post-tests were used for statistical analysis. B, human Ect1/E6E7 cells, transfected with 10 nm NSC or STAT-3 siRNA oligonucleotides, respectively, were stimulated for 6 h with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH (control) or 1 μm P4, MPA, or cortisol. For verification of STAT-3 knockdown, total protein from the untreated cells was harvested to perform Western blotting using antibodies specific for STAT-3. GAPDH was used as a loading control. A representative blot is shown. Total RNA was isolated and reverse-transcribed to cDNA. Thereafter, real time qPCR was performed to determine the mRNA expression levels of IL-10, using GAPDH as the internal standard. Relative IL-10 gene expression of treated samples was calculated relative to vehicle control (EtOH) of the NSC siRNA, which was set as 1. Results shown are the average (± S.E.) of at least three independent experiments. Two-way ANOVA and Bonferroni (compares all pairs of columns) post-tests were used for statistical analysis. ns, no statistical significance; ***, p < 0.001.
To further confirm a role for STAT-3 in the progestogen-induced suppression of IL-10 gene transcription, the Ect1/E6E7 cell line was transfected with 10 nm NSC or validated STAT-3-specific siRNA oligonucleotides, followed by treatment with 0.02 μg/ml TNF in the absence or presence of 1 μm P4, MPA, or cortisol for 6 h. Western blot analysis showed 55% reduction in the endogenous STAT-3 protein levels (Fig. 7B). Reducing STAT-3 levels significantly attenuated the P4, MPA, and cortisol-induced suppression of IL-10 gene expression. Interestingly, STAT-3 knockdown appears to lift this suppression. In summary, these results suggest that the progestogen-bound GR and STAT-3 bind as a complex to the human IL-10 promoter, thereby suppressing transcription of the IL-10 gene.
Progestogen-activated GR Also Regulates IL-12 and IL-10 Secreted Protein Levels
Finally, we evaluated the effects of the progestogens on the secreted protein levels of these cytokines in the human ectocervical cell line. The Ect1/E6E7 cells were transfected with a control or GR-specific siRNA and treated with 0.02 μg/ml TNF in the absence and presence of 1 μm test compound for 24 h. Western blot analysis confirmed efficient reduction of GR protein levels (Fig. 8, A and B). IL-12, measured as the p70 heterodimer, and IL-10 protein levels secreted in the supernatants of the Ect1/E6E7 cells were quantified using commercially available ELISA kits. Consistent with the mRNA results, we show that P4 and MPA increases the protein levels of IL-12p70 (Fig. 8A), while decreasing IL-10 (Fig. 8B) protein levels. These responses were abrogated when the GR levels were reduced indicating that the GR dependence observed on the mRNA level is mimicked at the protein level.
FIGURE 8.
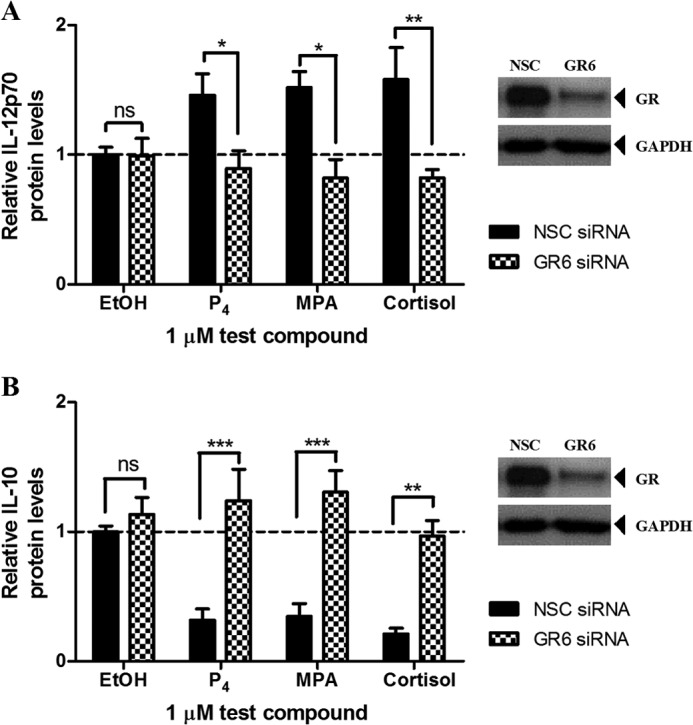
Human Ect1/E6E7 cells transfected with 10 nm NSC or GR6 siRNA oligonucleotides were either left untreated or treated with 0.02 μg/ml TNF in the absence or presence of 0.1% EtOH (control) or 1 μm P4, MPA, or cortisol for 24 h. For verification of GR knockdown, total protein from the untreated cells was harvested to perform Western blotting, using antibodies specific for the GR and GAPDH. The latter was used as a loading control, and a representative blot is shown. Cell culture supernatants were collected and the protein levels of IL-12p70 (A) and IL-10 (B) were measured using ELISA. The relative protein levels of the NSC siRNA vehicle control (EtOH) for IL-12p70 (∼1.5 pg/ml) and IL-10 (∼5 pg/ml) were set as 1, and the relative IL-12p70 and IL-10 protein levels of treated samples were calculated relative to this. Results shown are the average (± S.E.) of at least three independent experiments. Two-way ANOVA and Bonferroni (compares all pairs of columns) post-tests were used for statistical analysis. ns, no statistical significance; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
In this study, we investigated the effects of the progestin-only injectable contraceptive MPA relative to natural P4 on the transcriptional regulation of cytokine genes in a human ectocervical epithelial cell line treated with TNF to mimic infection. Our study is the first to show that P4 and MPA increase the mRNA and secreted protein levels of the pro-inflammatory cytokine IL-12, while decreasing the expression of the anti-inflammatory IL-10 gene, in the ectocervical cell line in a dose-dependent manner (Fig. 1). These pro-inflammatory effects are in line with our previous results in the ectocervical epithelial cell line, showing that P4 up-regulates the expression of the pro-inflammatory IL-6, IL-8, and RANTES (39) genes. However, in these cells MPA has either no effect (IL-6) or up-regulates (IL-8), or down-regulates (RANTES) gene expression. Furthermore, we show that the effects of the progestogens on IL-12 gene expression are cell type-specific, as similar effects were not observed in the MDA-MB-231 breast cancer cell line. However, the effects of the progestogens on IL-10 gene expression do not appear to be cell-specific, as similar GR-mediated effects were shown for the Ect1/E6E7 and MDA-MB-231 cell lines.
To delineate the molecular mechanism underlying the differential regulation of IL-12p40 and IL-10 by P4 and MPA, we investigated the role of the GR and its subsequent recruitment to these promoters. As the promoter of the IL-12p40 gene contains functional cis-acting sequences, such as response elements for NFκB, AP-1, Sp1, and C/EBP (62, 63, 70, 71), we used a combination of siRNA technology and ChIP assays to understand how P4 and MPA modulate IL-12p40 at the transcriptional level. Similar experiments were performed for IL-10 as its promoter also contains numerous cis-elements for AP-1, GRE, Sp1, and STAT-3 (72–77). Our results show that the GR is recruited to both the IL-12p40 (Fig. 4B) and IL-10 (Fig. 5B) promoters in response to P4 and MPA. Consistent with a role for the GR, we also demonstrate that the GR is recruited to these promoters in the presence of cortisol, the natural glucocorticoid (Figs. 4B and 5B), and that cortisol showed similar effects on the expression of these genes (Fig. 2, C and E). Moreover, we show for the first time that the liganded GR co-localizes with C/EBPβ and NFκB on the endogenous IL-12p40 promoter (Fig. 6). For IL-10, re-ChIP assays showed co-localization of the ligand-bound GR and STAT-3 on the endogenous IL-10 promoter (Fig. 7). Taken together, our investigations regarding the mechanism underlying the differential effects of P4 and MPA on IL-12p40 and IL-10 gene expression have revealed the role of different transcription factors, in particular the GR, NFκB, and C/EBPβ, in up-regulating IL-12p40 expression, although the GR and STAT-3 play a role in down-regulating IL-10 mRNA expression.
GR agonists such as cortisol and partial GR agonists such as P4 and MPA are usually reported to exert anti-inflammatory actions when acting via the GR by the classical mechanism of down-regulating pro-inflammatory genes and up-regulating anti-inflammatory genes (10–12, 69, 78). In agreement with this mechanism, some studies in human peripheral blood mononuclear cells show that glucocorticoids (62, 79, 80), P4 (81), and MPA (82) decrease IL-12p40 protein levels. However, evidence for the effects of these ligands on IL-10 gene expression is contradictory. Some studies show no effect (79, 82, 83), whereas others are in agreement with the classical mechanism showing an increase in IL-10 mRNA and protein expression (67, 79, 84–86), and some deviate from the classical mechanism by showing a decrease in IL-10 mRNA and protein levels (79, 87–90). Clearly, our results in the ectocervical epithelial cell line showing pro-inflammatory GR-mediated effects by P4, MPA, and cortisol via a unique tethering mechanism deviate from the classically accepted GR mechanism. Consistent with our results however, emerging evidence suggests that glucocorticoids can also elicit pro-inflammatory effects (91). Moreover, MPA has previously been shown to elicit pro-inflammatory effects in the cervix of mice by suppressing IL-10 mRNA and protein levels (90), although the mechanism and receptor mediating the response was not determined. Considering that P4 and MPA have previously been shown to have different binding affinities and transcriptional activities via the GR (11, 92), it was surprising that these progestogens displayed similar GR-mediated effects in the Ect1/E6E7 cell line. However, the relative affinities of P4 and MPA for the GR may be different in this cell line compared with other cell lines, as it has previously been shown that the concentration of GR determines the binding affinity of a ligand for the receptor (93). Moreover, as we have previously shown that P4 and MPA differentially regulate cytokine gene expression in a cell- and promoter-specific manner (39), discrepancies between the results from this study and others using synthetic GRE-containing promoters in COS-1 cells, for example (11), may be due to either cell- or promoter-specific effects.
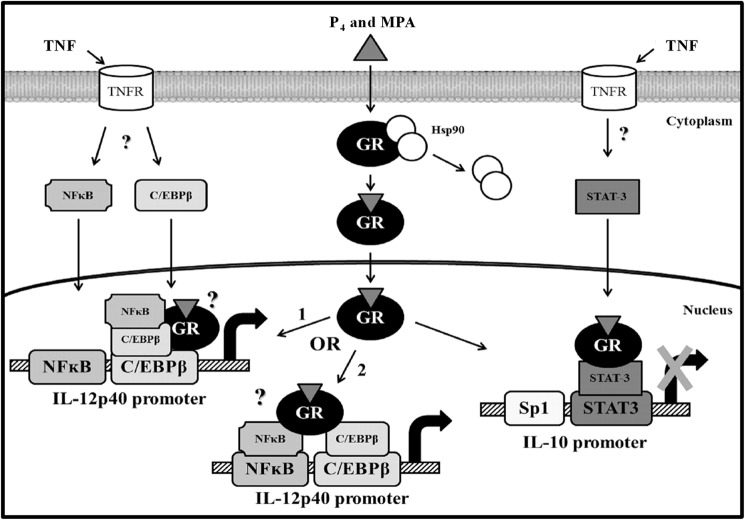
The precise signal transduction pathways leading to the activation of IL-12p40 gene transcription and the inhibition of IL-10 gene expression in the ectocervical cell line are not clear. However, some hypotheses can be formulated and are illustrated in Fig. 9. First, because TNF has previously been shown to activate and induce nuclear translocation of C/EBPβ and NFκB (94–96), as well as STAT-3 (97, 98), it is plausible that C/EBPβ and NFκB are recruited to the IL-12p40 promoter, and STAT-3 to the IL-10 promoter, upon TNF treatment. We further propose that the liganded GR interacts with the following: 1) NFκB, which tethers to C/EBPβ, the latter bound to its binding site in the IL-12p40 promoter, or 2) both NFκB and C/EBPβ, each bound to their respective binding sites. NFκB tethering to C/EBPβ bound to its binding site, and NFκB and C/EBPβ each bound to their respective sites are both mechanisms that have previously been proposed for the up-regulation of the pro-inflammatory IL-8 cytokine gene by TNF (99). Furthermore, at least two previous studies have shown that target gene expression is enhanced when glucocorticoid-bound GR tethers to DNA-bound C/EBPβ (64, 65). Further support of this mechanism is the fact that the IL-12p40 promoter does not appear to contain a functional GRE (62, 63) and that tethering of the GR to C/EBPβ bound to its binding site has previously been proposed as a mechanism for transactivation of glucocorticoid-responsive genes that lack functional GREs (100). In terms of IL-10 gene regulation, we propose that the liganded GR tethers to STAT-3 bound to its binding site within the IL-10 promoter. Tethering of the GR to DNA-bound STAT-3 has previously been implicated in transcriptional repression of genes, although direct binding of the GR to a STAT-3-binding site is associated with transcriptional activation of genes (68). Taken together, this biochemical study provides a novel mechanism whereby P4 and MPA are likely to modulate local immune function in the female genital tract.
FIGURE 9.
Schematic model for the progestogen-induced up-regulation of IL-12p40 and down-regulation of IL-10 gene expression via the GR in the ectocervical epithelial cell line. Upon P4 or MPA binding to the GR, the GR undergoes a conformational change and translocates to the nucleus where it occupies the IL-12p40 promoter to activate transcription of the IL-12p40 gene or occupies the IL-10 promoter to suppress IL-10 gene transcription. In response to P4 and MPA, the GR forms a complex with the transcription factors C/EBPβ and NFκB and occupies the NFκB/C/EBPβ region of the IL-12p40 promoter to activate transcription of this gene, although to decrease transcription of the IL-10 gene, the P4- and MPA-bound GR and the DNA-bound transcription factor STAT-3 bind as a complex to the IL-10 promoter. The questions marks indicate signaling pathways that are uncertain (see under “Discussion”). Hsp90, heat shock protein-90; STAT-3, signal transducer and activator of transcription-3.
Trying to understand the physiological implications of these results is not a simple task. Both IL-12 and IL-10 are key cytokines that play major roles in regulating inflammatory responses (54, 101–103). The IL-12 p40/p35 heterodimer is essential for the initiation of an effective immune response, although IL-10 protects the host from excessive inflammation (54, 101). Support for the critical role of these cytokines in regulating inflammatory responses is gained from studies showing that IL-10-deficient mice display dysregulated inflammatory responses and develop chronic inflammatory disorders, possibly due to their inability to counteract IL-12-driven inflammation (104, 105). Interestingly, enhanced production of IL-12p40 has been shown to prevent chronic enterocolitis in the intestinal epithelium of IL-10-deficient mice (56), due to the formation of IL-12p40 homodimers, suggesting that the IL-12 heterodimer is critical for chronic inflammatory responses. At first glance, it thus appears that our data showing increased levels of IL-12 and decreased levels of IL-10 by P4 and MPA suggest that these ligands would lead to increased inflammation in the ectocervical environment. However, it is important to remember that the defense function in the ectocervical environment is not only dependent on IL-12 and IL-10 but on a number of regulatory factors. Thus, the observed effects of P4 and MPA on IL-12 and IL-10 expression should be considered in the light of the fact that there is a constant release of various pro- and anti-inflammatory mediators in the cervical environment.
Another crucial point to ponder is whether the dosage of MPA used in hormonal therapy will exert similar effects on local immune function in the ectocervical environment in vivo. Serum concentrations of MPA range between 4.5 and 65 nm a few days after administration of the intramuscular injection, followed by a gradual decrease to ∼2.6 nm for about 3 months (15). As our dose response analysis shows that the potency (EC50 values) for MPA regulation of the IL-12p40, IL-12p35, and IL-10 genes in the ectocervical epithelial cells is in the nanomolar range (1.4, 7.47, and 3.25 nm, respectively), it is likely that the pro-inflammatory effects of MPA in these cells are relevant at serum doses of the injectable contraceptive Depo-Provera. Indeed, increased IL-12 protein levels have been reported in the vaginal lavage fluid of adolescent females using Depo-Provera as a contraceptive compared with non-users (106). In contrast to our in vitro data however, these authors reported increased levels of IL-10 for Depo-Provera users (106). The increased levels of both IL-12 and IL-10 may be due to increased concentrations of IL-12 stimulating the production of IL-10, a mechanism previously proposed for increasing levels of these cytokines in cervical specimens (107, 108). Serum concentrations of endogenous P4 have been reported to be low during the follicular phase (∼0.65 nm), increasing to ∼80 nm during the luteal phase, and ∼600 nm during pregnancy (1). As our dose response analysis shows that the potency for P4 regulation of IL-12p40, IL-12p35, and IL-10 is 4.58, 0.31, and 5.67 nm, respectively, it is probable that P4, like MPA, would modulate immune responses in the ectocervical environment in vivo. Evidence in the literature suggests that high P4 concentrations, such as those in the luteal phase and during pregnancy, are associated with increased HIV-1 shedding in cervical secretions (21, 109, 110) and increased susceptibility to HIV-1 infections (21, 111–113). Similarly, both human and animal studies suggest a link between high P4 levels and increased risk of sexually transmitted diseases like HSV-2, Chlamydia, and Candidiasis (24, 114). Considering that the concentrations of P4 fluctuate due to reproductive processes (27, 114, 115), whereas the serum levels of MPA used as contraceptive peak after injection (4.5–65 nm) but then stay constant (∼ 2.6 nm) for approximately 3 months, P4 may induce transient inflammation of the ectocervical environment during times of high P4 concentrations, although MPA may cause a more sustained inflammation. Our results are consistent with a model whereby pregnant and pre-menopausal nonpregnant women in the luteal phase could be vulnerable to sexually transmitted diseases due to pro-inflammatory actions of P4 via the GR, although women on depo-medroxyprogesterone acetate could be vulnerable at all times, but especially after injection, via the same mechanism.
Taken together, our study shows a GR-dependent mechanism for the differential regulation of IL-12 and IL-10 by both P4 and MPA in the ectocervical epithelial cell line and reveals the role of different transcription factors, including NFκB and C/EBPβ to enhance transcription of the IL-12p40 gene and STAT-3 to suppress IL-10 gene transcription. Furthermore, we suggest that both P4 and physiological doses of Depo-Provera may disrupt normal immune function in the ectocervix via this mechanism. Although we have not investigated how these progestogen-induced effects would impact on HIV infectivity, our results, taken together with evidence in the literature indicating that modulation of mucosal immunity in the female genital tract may increase susceptibility to HIV-1 (35, 38, 42), suggest that P4 and MPA could increase susceptibility to genital tract infections. The clinical implications of these results may be significant and warrant further investigation.
Acknowledgment
We thank Carmen Langeveldt for maintaining the Ect1/E6E7, COS-1, and MDA-MB-231 cell lines.
This work was supported by grants from the Medical Research Council, the National Research Foundation in South Africa, and Stellenbosch University (to D. A.).
- P4
- progesterone
- MPA
- medroxyprogesterone acetate
- GR
- glucocorticoid receptor
- GRE
- glucocorticoid response element
- C/EBP-β
- CCAAT enhancer-binding protein β
- STAT-3
- signal transducer and activator of transcription
- NFκB
- nuclear factor κB
- ANOVA
- analysis of variance
- NSC
- nonsilencing scrambled sequence control
- qPCR
- quantitative PCR
- re-CHIP
- chromatin reimmunoprecipitation
- RANTES
- regulated on activation normal T cell expressed and secreted.
REFERENCES
- 1. Africander D., Verhoog N., Hapgood J. P. (2011) Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 76, 636–652 [DOI] [PubMed] [Google Scholar]
- 2. Hapgood J. P., Koubovec D., Louw A., Africander D. (2004) Not all progestins are the same: implications for usage. Trends Pharmacol. Sci. 25, 554–557 [DOI] [PubMed] [Google Scholar]
- 3. Speroff L., Darney P. D. (2010) in A Clinical Guide for Contraception (Seigafuse S., ed) 5th Ed., pp. 217–238, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 4. Bailie R., Katzenellenbogen J., Hoffman M., Schierhout G., Truter H., Dent D., Gudgeon A., van Zyl J., Rosenberg L., Shapiro S. (1997) A case control study of breast cancer risk and exposure to injectable progestogen contraceptives. Methods and patterns of use among controls. S. Afr. Med. J. 87, 302–304 [PubMed] [Google Scholar]
- 5. Smit J., Gray A., McFadyen L., Zuma K. (2001) Counting the costs: comparing depot medroxyprogesterone acetate and norethisterone oenanthate utilisation patterns in South Africa. BMC Health Serv. Res. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrison C. S., Skoler-Karpoff S., Kwok C., Chen P. L., van de Wijgert J., Gehret-Plagianos M., Patel S., Ahmed K., Ramjee G., Friedland B., Lahteenmaki P. (2012) Hormonal contraception and the risk of HIV acquisition among women in South Africa. AIDS 26, 497–504 [DOI] [PubMed] [Google Scholar]
- 7. Kleinschmidt I., Rees H., Delany S., Smith D., Dinat N., Nkala B., McIntyre J. A. (2007) Injectable progestin contraceptive use and risk of HIV infection in a South African family planning cohort. Contraception 75, 461–467 [DOI] [PubMed] [Google Scholar]
- 8. Stanczyk F. Z., Hapgood J. P., Winer S., Mishell D. R., Jr. (2013) Progestogens used in postmenopausal hormone therapy: Differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr. Rev. 34, 171–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Africander D. J., Storbeck K. H., Hapgood J. P. (2014) A comparative study of the androgenic properties of progesterone and the progestins, medroxyprogesterone acetate (MPA), and norethisterone acetate (NET-A). J. Steroid Biochem. Mol. Biol. 143, 404–415 [DOI] [PubMed] [Google Scholar]
- 10. Koubovec D., Vanden Berghe W., Vermeulen L., Haegeman G., Hapgood J. P. (2004) Medroxyprogesterone acetate downregulates cytokine gene expression in mouse fibroblast cells. Mol. Cell. Endocrinol. 221, 75–85 [DOI] [PubMed] [Google Scholar]
- 11. Koubovec D., Ronacher K., Stubsrud E., Louw A., Hapgood J. P. (2005) Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol. Cell. Endocrinol. 242, 23–32 [DOI] [PubMed] [Google Scholar]
- 12. Bamberger C. M., Else T., Bamberger A. M., Beil F. U., Schulte H. M. (1999) Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J. Clin. Endocrinol. Metab. 84, 4055–4061 [DOI] [PubMed] [Google Scholar]
- 13. Africander D., Louw R., Hapgood J. P. (2013) Investigating the anti-mineralocorticoid properties of synthetic progestins used in hormone therapy. Biochem. Biophys. Res. Commun. 433, 305–310 [DOI] [PubMed] [Google Scholar]
- 14. Birrell S. N., Butler L. M., Harris J. M., Buchanan G., Tilley W. D. (2007) Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J. 21, 2285–2293 [DOI] [PubMed] [Google Scholar]
- 15. Hapgood J. P. (2013) Immunosuppressive biological mechanisms support reassessment of usage of the injectable contraceptive medroxyprogesterone acetate. Endocrinology 154, 985–988 [DOI] [PubMed] [Google Scholar]
- 16. Tomasicchio M., Avenant C., Du Toit A., Ray R. M., Hapgood J. P. (2013) The progestin-only contraceptive medroxyprogesterone acetate, but not norethisterone acetate, enhances HIV-1 Vpr-mediated apoptosis in human CD4+ T cells through the glucocorticoid receptor. Plos ONE 8, e62895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mostad S. B., Kreiss J. K., Ryncarz A. J., Mandaliya K., Chohan B., Ndinya-Achola J., Bwayo J. J., Corey L. (2000) Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J. Infect. Dis. 181, 58–63 [DOI] [PubMed] [Google Scholar]
- 18. Baeten J. M., Nyange P. M., Richardson B. A., Lavreys L., Chohan B., Martin H. L., Jr., Mandaliya K., Ndinya-Achola J. O., Bwayo J. J., Kreiss J. K. (2001) Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am. J. Obstet. Gynecol. 185, 380–385 [DOI] [PubMed] [Google Scholar]
- 19. Morrison C. S., Bright P., Wong E. L., Kwok C., Yacobson I., Gaydos C. A., Tucker H. T., Blumenthal P. D. (2004) Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm. Dis. 31, 561–567 [DOI] [PubMed] [Google Scholar]
- 20. Morrison C. S., Chen P. L., Kwok C., Richardson B. A., Chipato T., Mugerwa R., Byamugisha J., Padian N., Celentano D. D., Salata R. (2010) Hormonal contraception and HIV acquisition: reanalysis using margincal structural modeling. AIDS 24, 1778–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hel Z., Stringer E., Mestecky J. (2010) Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr. Rev. 31, 79–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heffron R., Donnell D., Rees H., Celum C., Mugo N., Were E., de Bruyn G., Nakku-Joloba E., Ngure K., Kiarie J., Coombs R. W., Baeten J. M. (2012) Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect. Dis. 12, 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacDonald E. M., Savoy A., Gillgrass A., Fernandez S., Smieja M., Rosenthal K. L., Ashkar A. A., Kaushic C. (2007) Susceptibility of human female primary genital epithelial cells to herpes simplex virus, type-2 and the effect of TLR3 ligand and sex hormones on infection. Biol. Reprod. 77, 1049–1059 [DOI] [PubMed] [Google Scholar]
- 24. Brabin L. (2002) Interactions of the female hormonal environment, susceptibility to viral infections, and disease progression. AIDS Patient Care STDS 16, 211–221 [DOI] [PubMed] [Google Scholar]
- 25. Cummins J. E., Dezzutti C. S. (2000) Sexual HIV-1 transmission and mucosal defense mechanisms. AIDS Rev. 2, 144–154 [Google Scholar]
- 26. Quayle A. J. (2002) The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J. Reprod. Immunol. 57, 61–79 [DOI] [PubMed] [Google Scholar]
- 27. Wira C. R., Fahey J. V., Sentman C. L., Pioli P. A., Shen L. (2005) Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol. Rev. 206, 306–335 [DOI] [PubMed] [Google Scholar]
- 28. Wira C. R., Grant-Tschudy K. S., Crane-Godreau M. A. (2005) Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am. J. Reprod. Immunol. 53, 65–76 [DOI] [PubMed] [Google Scholar]
- 29. Wira C. R., Fahey J. V., Ghosh M., Patel M. V., Hickey D. K., Ochiel D. O. (2010) Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. 63, 544–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fichorova R. N., Anderson D. J. (1999) Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60, 508–514 [DOI] [PubMed] [Google Scholar]
- 31. Ochiel D. O., Fahey J. V., Ghosh M., Haddad S. N., Wira C. R. (2008) Innate immunity in the female reproductive tract: role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr. Womens Health Rev. 4, 102–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fichorova R. N. (2004) Guiding the vaginal microbicide trials with biomarkers of inflammation. J. Acquir. Immune Defic. Syndr. 37, S184–S193 [PMC free article] [PubMed] [Google Scholar]
- 33. Kreiss J., Willerford D. M., Hensel M., Emonyi W., Plummer F., Ndinya-Achola J., Roberts P. L., Hoskyn J., Hillier S., Kiviat N. (1994) Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J. Infect. Dis. 170, 1597–1601 [DOI] [PubMed] [Google Scholar]
- 34. Cummins J. E., Christensen L., Lennox J. L., Bush T. J., Wu Z., Malamud D., Evans-Strickfaden T., Siddig A., Caliendo A. M., Hart C. E., Dezzutti C. S. (2006) Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res. Hum. Retroviruses 22, 788–795 [DOI] [PubMed] [Google Scholar]
- 35. Gumbi P. P., Nkwanyana N. N., Bere A., Burgers W. A., Gray C. M., Williamson A. L., Hoffman M., Coetzee D., Denny L., Passmore J. A. (2008) Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T-cell responses in the female genital tract during chronic HIV infection. J. Virol. 82, 8529–8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blish C. A., Baeten J. M. (2011) Hormonal contraception and HIV-1 transmission. Am. J. Reprod. Immunol. 65, 302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roberts L., Liebenberg L., Barnabas S., Passmore J. A. (2012) Vaginal microbicides to prevent human immunodeficiency virus infection in women: perspectives on the female genital tract, sexual maturity and mucosal inflammation. Best Pract. Res. Clin. Obstet. Gynaecol. 26, 441–449 [DOI] [PubMed] [Google Scholar]
- 38. Hickey D. K., Patel M. V., Fahey J. V., Wira C. R. (2011) Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J. Reprod. Immunol. 88, 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Africander D., Louw R., Verhoog N., Noeth D., Hapgood J. P. (2011) Differential regulation of endogenous pro-inflammatory cytokine genes by medroxyprogesterone acetate and norethisterone acetate in cell lines of the female genital tract. Contraception 84, 423–435 [DOI] [PubMed] [Google Scholar]
- 40. Ghanem K. G., Shah N., Klein R. S., Mayer K. H., Sobel J. D., Warren D. L., Jamieson D. J., Duerr A. C., Rompalo A. M. (2005) Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J. Infect. Dis. 191, 358–366 [DOI] [PubMed] [Google Scholar]
- 41. Morrison C., Fichorova R. N., Mauck C., Chen P. L., Kwok C., Chipato T., Salata R., Doncel G. F. (2014) Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J. Acquir. Immune Defic. Syndr. 66, 109–117 [DOI] [PubMed] [Google Scholar]
- 42. Ildgruben A. K., Sjöberg I. M., Hammarström M. L. (2003) Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet. Gynecol. 102, 571–582 [DOI] [PubMed] [Google Scholar]
- 43. Trinchieri G., Scott P. (1995) Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res. Immunol. 146, 423–431 [DOI] [PubMed] [Google Scholar]
- 44. Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. (1989) Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170, 827–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asadullah K., Sterry W., Volk H. D. (2003) Interleukin-10 therapy–review of a new approach. Pharmacol. Rev. 55, 241–269 [DOI] [PubMed] [Google Scholar]
- 46. Kelly R. W., Carr G. G., Critchley H. O. (1997) A cytokine switch induced by human seminal plasma: an immune modulation with implications for sexually transmitted disease. Hum. Reprod. 12, 677–681 [DOI] [PubMed] [Google Scholar]
- 47. Fichorova R. N., Rheinwald J. G., Anderson D. J. (1997) Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57, 847–855 [DOI] [PubMed] [Google Scholar]
- 48. Ludwig K., Parsons S. J. (2011) The tumor suppressor, p190RhoGAP, differentially initiates apoptosis and confers docetaxel sensitivity to breast cancer cells. Genes Cancer 2, 20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sambrook J., Fritsch E. F., Maniatus T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., pp. 18–60-18.75, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 50. Bradford M. M. (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 254, 248–254 [DOI] [PubMed] [Google Scholar]
- 51. Verhoog N. J., Du Toit A., Avenant C., Hapgood J. P. (2011) Glucocorticoid-independent repression of tumor necrosis factor (TNF) α-stimulated interleukin (IL)-6 expression by the glucocorticoid receptor: a potential mechanism for protection against an excessive inflammatory response. J. Biol. Chem. 286, 19297–19310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Avenant C., Kotitschke A., Hapgood J. P. (2010) Glucocorticoid receptor phosphorylation modulates transcription efficacy through GRIP-1 recruitment. Biochemistry 49, 972–985 [DOI] [PubMed] [Google Scholar]
- 53. Chang H. D., Radbruch A. (2007) The pro- and anti-inflammatory potential of interleukin-12. Ann. N.Y. Acad. Sci. 1109, 40–46 [DOI] [PubMed] [Google Scholar]
- 54. Cao S., Liu J., Chesi M., Bergsagel P. L., Ho I. C., Donnelly R. P., Ma X. (2002) Differential regulation of IL-12 and IL-10 gene expression in macrophages by the basic leucine zipper transcription factor c-Maf fibrosarcoma. J. Immunol. 169, 5715–5725 [DOI] [PubMed] [Google Scholar]
- 55. Yilma A. N., Singh S. R., Fairley S. J., Taha M. A., Dennis V. A. (2012) The anti-inflammatory cytokine, interleukin-10, inhibits inflammatory mediators in human epithelial cells and mouse macrophages exposed to live and UV-inactivated Chlamydia trachomatis. Mediators Inflamm. 2012, 520174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shiraki M., Aihara H., Kinouchi Y., Takahashi S., Oki M., Noguchi M., Takahashi K., Miyazaki J., Shimosegawa T. (2004) IL-12 p40 prevents the development of chronic enterocolitis in IL-10-deficient mice. Lab. Invest. 84, 1491–1500 [DOI] [PubMed] [Google Scholar]
- 57. Heckel M. C., Wolfson A., Slachta C. A., Schwarting R., Salgame P., Katsetos C. D., Platsoucas C. D. (2011) Human breast tumor cells express IL-10 and IL-12p40 transcripts and proteins, but do not produce IL-12p70. Cell. Immunol. 266, 143–153 [DOI] [PubMed] [Google Scholar]
- 58. Govender Y., Avenant C., Verhoog N. J., Ray R. M., Grantham N. J., Africander D., Hapgood J. P. (2014) The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. Plos ONE 9, e96497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Watford W. T., Moriguchi M., Morinobu A., O'Shea J. J. (2003) The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 14, 361–368 [DOI] [PubMed] [Google Scholar]
- 60. Gubler U., Chua A. O., Schoenhaut D. S., Dwyer C. M., McComas W., Motyka R., Nabavi N., Wolitzky A. G., Quinn P. M., Familletti P. C. (1991) Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc. Natl. Acad. Sci. U.S.A. 88, 4143–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Webster J. C., Cidlowski J. A. (1999) Mechanisms of glucocorticoid-receptor-mediated repression of gene expression. Trends Endocrinol. Metab. 10, 396–402 [DOI] [PubMed] [Google Scholar]
- 62. Ma W., Gee K., Lim W., Chambers K., Angel J. B., Kozlowski M., Kumar A. (2004) Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-κB transcription factors. J. Immunol. 172, 318–330 [DOI] [PubMed] [Google Scholar]
- 63. Ma X., Chow J. M., Gri G., Carra G., Gerosa F., Wolf S. F., Dzialo R., Trinchieri G. (1996) The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J. Exp. Med. 183, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johansson-Haque K., Palanichamy E., Okret S. (2008) Stimulation of MAPK-phosphatase 1 gene expression by glucocorticoids occurs through a tethering mechanism involving C/EBP. J. Mol. Endocrinol. 41, 239–249 [DOI] [PubMed] [Google Scholar]
- 65. Arambasić J., Poznanović G., Ivanović-Matić S., Bogojević D., Mihailović M., Uskoković A., Grigorov I. (2010) Association of the glucocorticoid receptor with STAT3, C/EBPβ, and the hormone-responsive element within the rat haptoglobin gene promoter during the acute phase response. IUBMB Life 62, 227–236 [DOI] [PubMed] [Google Scholar]
- 66. Ou X. M., Chen K., Shih J. C. (2006) Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J. Biol. Chem. 281, 21512–21525 [DOI] [PubMed] [Google Scholar]
- 67. Mozo L., Suárez A., Gutiérrez C. (2004) Glucocorticoids up-regulate constitutive interleukin-10 production by human monocytes. Clin. Exp. Allergy 34, 406–412 [DOI] [PubMed] [Google Scholar]
- 68. Langlais D., Couture C., Balsalobre A., Drouin J. (2012) The Stat3/GR interaction code: predictive value of direct/indirect DNA recruitment for transcription outcome. Mol. Cell 47, 38–49 [DOI] [PubMed] [Google Scholar]
- 69. De Bosscher K., Haegeman G. (2009) Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol. Endocrinol. 23, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Becker C., Wirtz S., Ma X., Blessing M., Galle P. R., Neurath M. F. (2001) Regulation of IL-12 p40 promoter activity in primary human monocytes: roles of NF-κB, CCAAT/enhancer-binding protein β, and PU. 1 and identification of a novel repressor element (GA-12) that responds to IL-4 and prostaglandin E(2). J. Immunol. 167, 2608–2618 [DOI] [PubMed] [Google Scholar]
- 71. Plevy S. E., Gemberling J. H., Hsu S., Dorner A. J., Smale S. T. (1997) Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol. 17, 4572–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mosser D. M., Zhang X. (2008) Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 226, 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Benkhart E. M., Siedlar M., Wedel A., Werner T., Ziegler-Heitbrock H. W. (2000) Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J. Immunol. 165, 1612–1617 [DOI] [PubMed] [Google Scholar]
- 74. Kube D., Platzer C., von Knethen A., Straub H., Bohlen H., Hafner M., Tesch H. (1995) Isolation of the human interleukin 10 promoter. Characterization of the promoter activity in Burkitt's lymphoma cell lines. Cytokine 7, 1–7 [DOI] [PubMed] [Google Scholar]
- 75. Ma W., Lim W., Gee K., Aucoin S., Nandan D., Kozlowski M., Diaz-Mitoma F., Kumar A. (2001) The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 276, 13664–13674 [DOI] [PubMed] [Google Scholar]
- 76. Unterberger C., Staples K. J., Smallie T., Williams L., Foxwell B., Schaefer A., Kempkes B., Hofer T. P., Koeppel M., Lohrum M., Stunnenberg H., Frankenberger M., Ziegler-Heitbrock L. (2008) Role of STAT3 in glucocorticoid-induced expression of the human IL-10 gene. Mol. Immunol. 45, 3230–3237 [DOI] [PubMed] [Google Scholar]
- 77. Kremer K. N., Kumar A., Hedin K. E. (2007) Haplotype-independent costimulation of IL-10 secretion by SDF-1/CXCL12 proceeds via AP-1 binding to the human IL-10 promoter. J. Immunol. 178, 1581–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kurebayashi J., Otsuki T., Tanaka K., Yamamoto Y., Moriya T., Sonoo H. (2003) Medroxyprogesterone acetate decreases secretion of interleukin-6 and parathyroid hormone-related protein in a new anaplastic thyroid cancer cell line, KTC-2. Thyroid 13, 249–258 [DOI] [PubMed] [Google Scholar]
- 79. Visser J., van Boxel-Dezaire A., Methorst D., Brunt T., de Kloet E. R., Nagelkerken L. (1998) Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood 91, 4255–4264 [PubMed] [Google Scholar]
- 80. Mirani M., Elenkov I., Volpi S., Hiroi N., Chrousos G. P., Kino T. (2002) HIV-1 protein Vpr suppresses IL-12 production from human monocytes by enhancing glucocorticoid action: potential implications of Vpr coactivator activity for the innate and cellular immunity deficits observed in HIV-1 infection. J. Immunol. 169, 6361–6368 [DOI] [PubMed] [Google Scholar]
- 81. Kleynhans L., Du Plessis N., Black G. F., Loxton A. G., Kidd M., van Helden P. D., Walzl G., Ronacher K. (2011) Medroxyprogesterone acetate alters Mycobacterium bovis BCG-induced cytokine production in peripheral blood mononuclear cells of contraceptive users. PLoS ONE 6, e24639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huijbregts R. P., Helton E. S., Michel K. G., Sabbaj S., Richter H. E., Goepfert P. A., Hel Z. (2013) Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology 154, 1282–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shrier L. A., Bowman F. P., Lin M., Crowley-Nowick P. A. (2003) Mucosal immunity of the adolescent female genital tract. J. Adolesc. Health 32, 183–186 [DOI] [PubMed] [Google Scholar]
- 84. Gayo A., Mozo L., Suárez A., Tuñon A., Lahoz C., Gutiérrez C. (1998) Glucocorticoids increase IL-10 expression in multiple sclerosis patients with acute relapse. J. Neuroimmunol. 85, 122–130 [DOI] [PubMed] [Google Scholar]
- 85. John M., Lim S., Seybold J., Jose P., Robichaud A., O'Connor B., Barnes P. J., Chung K. F. (1998) Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1α, granulocyte-macrophage colony-stimulating factor, and interferon-γ release from alveolar macrophages in asthma. Am. J. Respir. Crit. Care Med. 157, 256–262 [DOI] [PubMed] [Google Scholar]
- 86. Yates M. A., Li Y., Chlebeck P., Proctor T., Vandenbark A. A., Offner H. (2010) Progesterone treatment reduces disease severity and increases IL-10 in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 220, 136–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang C., Nixon M., Kenyon C. J., Livingstone D. E., Duffin R., Rossi A. G., Walker B. R., Andrew R. (2011) 5α-Reduced glucocorticoids exhibit dissociated anti-inflammatory and metabolic effects. Br. J. Pharmacol. 164, 1661–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rowland T. L., McHugh S. M., Deighton J., Dearman R. J., Ewan P. W., Kimber I. (1998) Differential regulation by thalidomide and dexamethasone of cytokine expression in human peripheral blood mononuclear cells. Immunopharmacology 40, 11–20 [DOI] [PubMed] [Google Scholar]
- 89. Skjolaas K. A., Grieger D. M., Hill C. M., Minton J. E. (2002) Glucocorticoid regulation of type 1 and type 2 cytokines in cultured porcine splenocytes. Vet. Immunol. Immunopathol. 87, 79–87 [DOI] [PubMed] [Google Scholar]
- 90. Elovitz M. A., Gonzalez J. (2008) Medroxyprogesterone acetate modulates the immune response in the uterus, cervix and placenta in a mouse model of preterm birth. J. Matern. Fetal Neonatal Med. 21, 223–230 [DOI] [PubMed] [Google Scholar]
- 91. Lannan E. A., Galliher-Beckley A. J., Scoltock A. B., Cidlowski J. A. (2012) Proinflammatory actions of glucocorticoids: glucocorticoids and TNFα coregulate gene expression in vitro and in vivo. Endocrinology 153, 3701–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ronacher K., Hadley K., Avenant C., Stubsrud E., Simons S. S., Jr., Louw A., Hapgood J. P. (2009) Ligand-selective transactivation and transrepression via the glucocorticoid receptor: role of cofactor interaction. Mol. Cell. Endocrinol. 299, 219–231 [DOI] [PubMed] [Google Scholar]
- 93. Robertson S., Rohwer J. M., Hapgood J. P., Louw A. (2013) Impact of glucocorticoid receptor density on ligand-independent dimerization, cooperative ligand-binding and basal priming of transactivation: a cell culture model. Plos ONE 8, e64831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Adcock I. M., Caramori G. (2001) Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 79, 376–384 [DOI] [PubMed] [Google Scholar]
- 95. Cardinaux J. R., Allaman I., Magistretti P. J. (2000) Pro-inflammatory cytokines induce the transcription factors C/EBPβ and C/EBPδ in astrocytes. Glia 29, 91–97 [PubMed] [Google Scholar]
- 96. Kim M. H., Minton A. Z., Agrawal V. (2009) C/EBPβ regulates metastatic gene expression and confers TNF-α resistance to prostate cancer cells. Prostate 69, 1435–1447 [DOI] [PubMed] [Google Scholar]
- 97. Miscia S., Marchisio M., Grilli A., Di Valerio V., Centurione L., Sabatino G., Garaci F., Zauli G., Bonvini E., Di Baldassarre A. (2002) Tumor necrosis factor α (TNF-α) activates Jak1/Stat3-Stat5B signaling through TNFR-1 in human B cells. Cell Growth Differ. 13, 13–18 [PubMed] [Google Scholar]
- 98. Robinson K., Vona-Davis L., Riggs D., Jackson B., McFadden D. (2006) Peptide YY attenuates STAT1 and STAT3 activation induced by TNF-α in acinar cell line AR42J. J. Am. Coll. Surg. 202, 788–796 [DOI] [PubMed] [Google Scholar]
- 99. Stein B., Baldwin A. S., Jr. (1993) Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-κB. Mol. Cell. Biol. 13, 7191–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Roos A. B., Nord M. (2012) The emerging role of C/EBPs in glucocorticoid signaling: lessons from the lung. J. Endocrinol. 212, 291–305 [DOI] [PubMed] [Google Scholar]
- 101. Zhou L., Nazarian A. A., Smale S. T. (2004) Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol. Cell. Biol. 24, 2385–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lin W. W., Karin M. (2007) A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 117, 1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Watson D. C., Sargianou M., Panos G. (2012) Interleukin-12 (IL-12)/IL-10 ratio as a marker of disease severity in Crimean-Congo hemorrhagic fever. Clin. Vaccine Immunol. 19, 823–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 [DOI] [PubMed] [Google Scholar]
- 105. Davidson N. J., Hudak S. A., Lesley R. E., Menon S., Leach M. W., Rennick D. M. (1998) IL-12, but not IFN-γ, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J. Immunol. 161, 3143–3149 [PubMed] [Google Scholar]
- 106. Barousse M. M., Theall K. P., Van Der Pol B., Fortenberry J. D., Orr D. P., Fidel P. L., Jr. (2007) Susceptibility of middle adolescent females to sexually transmitted infections: impact of hormone contraception and sexual behaviors on vaginal immunity. Am. J. Reprod. Immunol. 58, 159–168 [DOI] [PubMed] [Google Scholar]
- 107. Crowley-Nowick P. A., Ellenberg J. H., Vermund S. H., Douglas S. D., Holland C. A., Moscicki A. B. (2000) Cytokine profile in genital tract secretions from female adolescents: impact of human immunodeficiency virus, human papillomavirus, and other sexually transmitted pathogens. J. Infect. Dis. 181, 939–945 [DOI] [PubMed] [Google Scholar]
- 108. Jeannin P., Delneste Y., Seveso M., Life P., Bonnefoy J. Y. (1996) IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J. Immunol. 156, 3159–3165 [PubMed] [Google Scholar]
- 109. Benki S., Mostad S. B., Richardson B. A., Mandaliya K., Kreiss J. K., Overbaugh J. (2004) Cyclic shedding of HIV-1 RNA in cervical secretions during the menstrual cycle. J. Infect. Dis. 189, 2192–2201 [DOI] [PubMed] [Google Scholar]
- 110. Reichelderfer P. S., Coombs R. W., Wright D. J., Cohn J., Burns D. N., Cu-Uvin S., Baron P. A., Coheng M. H., Landay A. L., Beckner S. K., Lewis S. R., Kovacs A. A. (2000) Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. Aids 14, 2101–2107 [DOI] [PubMed] [Google Scholar]
- 111. Quinn T. C., Overbaugh J. (2005) HIV/AIDS in women: an expanding epidemic. Science 308, 1582–1583 [DOI] [PubMed] [Google Scholar]
- 112. Gray R. H., Li X., Kigozi G., Serwadda D., Brahmbhatt H., Wabwire-Mangen F., Nalugoda F., Kiddugavu M., Sewankambo N., Quinn T. C., Reynolds S. J., Wawer M. J. (2005) Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 366, 1182–1188 [DOI] [PubMed] [Google Scholar]
- 113. Saba E., Origoni M., Taccagni G., Ferrari D., Doglioni C., Nava A., Lisco A., Grivel J. C., Margolis L., Poli G. (2013) Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol. 6, 1081–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gravitt P., Ghanem K. (2010) in Sex Hormones and Immunity to Infection (Klein S. L., Roberts C., eds) pp. 257–280, Springer Publication Inc., New York [Google Scholar]
- 115. Bouman A., Heineman M. J., Faas M. M. (2005) Sex hormones and the immune response in humans. Hum. Reprod. Update 11, 411–423 [DOI] [PubMed] [Google Scholar]