Background: miR-141, miR-146b-5p, and the RNA-binding protein AUF1 are post-transcriptional regulators that play important roles in carcinogenesis.
Results: miR-141 and miR-146b-5p repress AUF1, an inducer of mesenchymal features, through stabilizing the transcription factor ZEB1 and activating the serine-threonine kinase AKT.
Conclusion: AUF1 is a prometastatic gene regulated by miR-141 and miR-146b-5p.
Significance: miR-141, miR-146b-5p, and AUF1 could be of great cancer prognostic/therapeutic values.
Keywords: Cell Invasion, Cell Migration, Epithelial-Mesenchymal Transition (EMT), Post-transcriptional Regulation, RNA-binding Protein, AKT, AUF1, ZEB1, miR-141, miR-146b-5p
Abstract
miR-141 and miR-146b-5p are two important tumor suppressor microRNAs, which control several cancer-related genes and processes. In the present report, we have shown that these microRNAs bind specific sites at the 3′-untranslated region (UTR) of the mRNA-binding protein AUF1, leading to its down-regulation. This inverse correlation between the levels of these microRNAs and AUF1 has been identified in various osteosarcoma cell lines. Additionally, we present clear evidence that AUF1 promotes mesenchymal features in osteosarcoma cells and that miR-141 and miR-146b-5p suppress this prometastatic process through AUF1 repression. Indeed, both microRNAs suppressed the invasion/migration and proliferation abilities of osteosarcoma cells through inhibiting the AKT protein kinase in an AUF1-dependent manner. We have also shown that AUF1 binds to and stabilizes the mRNA of the AKT activator phosphoinositide-dependent kinase-1 (PDK1). Furthermore, miR-141 and miR-146b-5p positively regulate the epithelial markers (E-cadherin and Epcam) and repress the mesenchymal markers (N-cadherin, Vimentin, Twist2, and ZEB1). These effects were mediated via the repression of the epithelial-to-mesenchymal inducer ZEB1 through targeting AUF1, which binds the 3′-UTR of the ZEB1 mRNA and reduces its turnover. These results indicate that at least some tumor suppressor functions of miR-141 and miR-146b-5p are mediated through the repression of the oncogenic potentials of AUF1. Therefore, these 3′-UTR-directed post-transcriptional gene expression regulators constitute promising new targets for diagnostic and/or therapeutic interventions.
Introduction
The mRNA 3′-UTR plays an important role in the post-transcriptional regulation of gene expression, which is controlled by two main types of mRNA-interacting factors: RNA-binding proteins and microRNAs (miRNAs)2 (1). Recent studies have uncovered reciprocal regulations at various levels between these two factors (1–3). One of the best characterized RNA-binding proteins is AUF1 (AU-binding factor 1), also known as heterogenous nuclear ribonucleoprotein D (hnRNPD). AUF1 is a family of four isoforms (37, 40, 42, and 45 kDa), which result from alternative splicing of a single pre-mRNA (4). Although all of these isoforms contain two RNA recognition motifs, they each exhibit different affinity for target transcripts, with the p37 isoform having the strongest affinity (4, 5). AUF1 forms direct complexes with a variety of AU-rich conserved elements in the 3′-UTR of many transcripts (6). Although AUF1 is predominantly associated with mRNA-destabilizing activity (7–11), various studies have shown that AUF1 can also promote the stability and translation of some target transcripts (12–14). Interestingly, AUF1 target genes are implicated in several processes, such as cell proliferation, stress responses, apoptosis, and transcription (6, 15, 16). Furthermore, several lines of evidence support a role of AUF1 in the initiation and/or progression of cancer (15). Indeed, high AUF1 levels were detected in numerous malignancies, including cancers of the breast, skin, thyroid, and liver (15, 17). However, many potential roles of deregulated AUF1 expression or activity in tumor initiation and/or progression remain unresolved. In addition, little is known about the mechanisms that control AUF1 expression in many cellular contexts, including those that enhance AUF1 levels during carcinogenesis.
The other important 3′-UTR-related post-transcriptional regulators are miRNAs, which belong to a large family of non-coding RNAs. Mature miRNAs are 21–23-bp-long molecules, which regulate the expression of a plethora of genes involved in various biochemical pathways. Therefore, alteration of their expression is related to various diseases, including cancer (18). Indeed, miRNAs act as tumor suppressors or oncogenes and are involved in various steps of the carcinogenesis process, including metastasis. Recent data showed their implication in the epithelial-to-mesenchymal transition process (19). During EMT, cells lose their epithelial markers, such as E-cadherin and Epcam, whereas the mesenchymal markers, such as vimentin and N-cadherin, are up-regulated, resulting in loss of cell-cell contact and increase in the migration/invasion abilities. The EMT program is regulated by several transcription factors, including SNAIL, ZEB1, and TWIST (20).
We have recently shown that the tumor suppressor protein p16INK4a, hereafter referred to as p16, negatively regulates the expression of AUF1 through activating the turnover of its mRNA (10) and also positively regulates the expression of miR-141 and miR-146b-5p, two important tumor suppressor miRNAs (21). Interestingly, the two miRNAs have been computationally predicted to have binding sites in the 3′-UTR of the AUF1 transcript (22) but have not been experimentally validated. Therefore, we sought to explore here the possible implication of miR-141 and miR-146b-5p in the post-transcriptional regulation of AUF1.
We have shown here that miR-141 and miR-146b-5p repress mesenchymal features through targeting AUF1-ZEB1 in osteosarcoma cells.
EXPERIMENTAL PROCEDURES
Cell Lines, Cell Culture, and Chemicals
The p16-defective osteosarcoma U2OS cell line and its isogenic EH1, which expresses CDKN2A under the control of an isopropyl 1-thio-β-d-galactopyranoside-inducible promoter, are a generous gift from Dr. G. Peters (23), and HFSN1 (primary normal human skin fibroblast) cells were routinely cultured in DMEM/F-12 medium supplemented with 10% FCS. Osteosarcoma cell lines (HOS, MG63, 143B, and SaOS2) were obtained from ATCC (Manassas, VA) and were cultured following the instructions of the company. All supplements were purchased from Invitrogen. Cells were maintained at 5% CO2 in a 37 °C humidified incubator. Actinomycin D was purchased from Sigma.
miRNA Target Prediction
miRNA targets were predicted using algorithms, including miRanda Human miRNA targets, miRDB, RNA22, and miROrg. To identify the genes commonly predicted by these different algorithms, the results of predicted targets were intersected using miRWalk.
RNA Purification and Quantitative RT-PCR
Total RNA, containing miRNA, was purified using the miRNeasy minikit (Qiagen) according to the manufacturer's instructions and was treated with RNase-free DNase before cDNA synthesis using either the Advantage RT-PCR kit (Clontech) or miScript II RT kit (Qiagen) for mature miRNAs. Quantitative RT-PCR was performed using RT2 Real-TimeTM SYBR Green qPCR Mastermix (Qiagen), and the amplifications were performed utilizing the Bio-Rad iQ5 multicolor real-time PCR detection system. The melting curve data were collected to check PCR specificity, the amount of PCR products was measured by threshold cycle (Ct) values, and the relative ratio of specific genes to GAPDH or U6 for each sample was then calculated. The respective primers were as follows: AUF1, 5′-GATCAAGGGGTTTTGGCTTT-3′ (forward) and 5′-GTTGTCCATGGGGACCTCTA-3′ (reverse); CDKN1A, 5′-CAGAGGAGGCGCCAAGACAG-3′ (forward) and 5′-CCTGACGGCGGAAAACGC-3′ (reverse); ZEB1, 5′-GGCAGAGAATGAGGGAGAAG-3′ (forward) and 5′-CTTCAGACACTTGCTCACTACTC-3′ (reverse); PDK1, 5′-CATGTCACGCTGGGTAATGAGG-3′ (forward) and 5′-CTCAACACGAGGTCTTGGTGCA-3′ (reverse); GAPDH, 5′-GAGTCCACTGGCGTCTTC-3′ (forward) and 5′-GGGGTGCTAAGCAGTTGGT-3′ (reverse); mature miR-141, UAACACUGUCUGGUAAAGAUGG; and mature miR-146b-5p, UGAGAACUGAAUUCCAUAGGCU.
Immunoprecipitation and RT-PCR
Cell lysates were prepared from confluent cells, and 3 mg were incubated in the lysis buffer (50 mm Tris (pH 8), 100 mm NaCl, 10% glycerol, protease inhibitors, 5 mm DTT, and 2 units/ml RNasin), and 5 μg of AUF1 mouse monoclonal antibody (mouse IgG1 was used as control) were added and mixed at 4 °C for 4 h. An equal volume of protein A-agarose was added per immunoprecipitation and mixed overnight at 4 °C. After centrifugation, the pellet was resuspended in 1 ml of TRI reagent used for RNA extraction. RT-PCRs were performed as described above.
Transfection and Viral Infection
pSILENCER-AUF1 siRNA, which targets all AUF1 isoforms (24), was used at 0.5 μg/ml for transfection utilizing Lipofectamine 2000 following the protocol recommended by the manufacturer (Invitrogen). pLKO.1-miRZip146b-5p (inhibitor of miR-146b-5p), pLKO.1-miRZip141 (inhibitor of miR-141), pCDH-miR-141 (expressing pre-miR-141), pCDH-miR-146b-5p (expressing pre-miR-146b-5p) (System Biosciences), pLenti-GIII-CMV-hHNRNPD-GFP-2A-Puro (expressing the p37AUF1 isoform) (Applied Biological Materials Inc.), pGFP-C-shLenti-ZEB1-shRNA (specific down-regulation of ZEB1) (Origene), and their control plasmids were used at 1 μg/ml each for transfection of 293FT cells. Lentiviral supernatants were collected 48 h post-transfection. Culture media were removed from the target cells and replaced with the lentiviral supernatant and incubated for 24 h in the presence of 1 μg/ml Polybrene (Sigma-Aldrich). Transduced cells were selected after 48 h with puromycin or G418. AKT siRNA (specific down-regulation of AKT) (Qiagen) was used at 20 nm, and transfection was performed using RNAiFect, following the protocol recommended by the manufacturer (Qiagen).
Dual-Luciferase Reporter Assay
U2OS cells were plated at 1 × 105 cells/well on 6-well plates and transfected with 3 μg of the luciferase/Renilla reporter vector containing either human AUF1 3′-UTR (871 bp), mutated sequence of the miR-141 or miR-146b-5p seed sequence, human ZEB1 3′-UTR (75 bp), mutated sequence of the AUF1 binding site in the corresponding sequence, human PDK1 3′-UTR, or the mutated sequence of the AUF1 binding site as well as a control sequence with no AU-rich conserved elements (GeneCopoeia). Transfection was carried out using Lipofectamine 2000, as recommended by the manufacturer (Invitrogen). At 24 h post-transfection, cells were seeded in a 96-well plate, and firefly and Renilla luciferase activities were consecutively measured using the Dual-Luciferase assay as recommended by the manufacturer (GeneCopoeia). The firefly luciferase signal was normalized to the Renilla luciferase signal for each individual analysis. The mean and S.E. were calculated from three wells for each 3′-UTR activity and presented as -fold change over the non-stimulated control.
Northern Blot
Northern blot analysis was performed using the High Sensitive miRNA Northern blot assay kit as recommended by the manufacturer (Signosis, Inc.). In brief, total RNA was prepared using miRNeasy (Qiagen), and 5 μg was separated using precast 15% TBE-urea gel (Signosis, Inc.). After transfer, nylon membranes (Signosis, Inc.) were UV-cross-linked and hybridized with DNA oligonucleotides complementary to miRNA or U6 that had been end-labeled with Biotin (Signosis, Inc.). Images were acquired with a CCD camera (LAS 4000, GE Healthcare).
Biotin Pull-down Analysis
The probes used to prepare biotinylated transcripts spanning the ZEB1 3′-UTR are CAAGGCUCUAACCCGCCUUCAUCCAAUGUGUGGCCUACAAUAACUAGCAUUUGUUGAUUUGUCUCUUGUAUCAAA (wild type) and CAAGGCUCUAACCCGCCUUCAUCCAAUGUGUGGCCUACAAUAACUAGCAUUUGGGUCGCCGUCUCUUGUAUCAAA (mutated). The probes used to prepare biotinylated transcripts spanning the PDK1 3′-UTR are UCUUACCUCUGAGGUUAAUUUACCAUUUUUAAA (wild type) and UCUUAGCUCUGAGGGGCCGCAACCAUUUUUAAA (mutated). Biotinylation was performed using the RNA 3′-end biotinylation kit as instructed by the manufacturer (Thermo Scientific). Cytoplasmic lysates (200 μg/sample) were incubated with 3 μg of purified biotinylated transcripts for 30 min at room temperature, and then the complexes were precipitated with streptavidin-coupled Dynabeads (Invitrogen) as described previously (3). Proteins present in the pull-down material were analyzed by immunoblotting.
Cellular Lysate Preparation and Immunoblotting
This has been performed as described previously (5). Antibodies directed against AUF1, E-cadherin (HECD-1), N-cadherin, Twist2, and vimentin (RV202) were purchased from Abcam; ZEB1 (4C4) antibody was from Abnova; p21 (F-5) and GAPDH (FL-335) antibodies were purchased from Santa Cruz Biotechnology, Inc.; p16 antibody was purchased from BD Biosciences; and AKT (C73H10), phospho-AKT (Thr-308), PDK1, phospho-PDK1 (Ser-241), and Epcam (VU1D9) antibodies were purchased from Cell Signaling Technology.
Quantification of Protein Expression Level
The expression levels of the immunoblotted proteins were measured using the densitometer (Bio-Rad GS-800 calibrated densitometer) as described previously (5).
Analysis of mRNA Stability
Cells were challenged with actinomycin D (5 μg/ml) for various periods of time (0–6 h), and then total RNA was purified and assessed using qRT-PCR. One-phase exponential decay curve analysis (GraphPad Prism) (GraphPad software 5.03, Inc.) was used to assess mRNA decay kinetics (25).
Cell Migration, Invasion, and Proliferation
These assays were performed in label-free real-time settings using the xCELLigence RTCA technology (Roche Applied Science), which measures impedance changes in a meshwork of interdigitated gold microelectrodes located at the well bottom (E-plate) or at the bottom side of a microporous membrane (CIM plate 16) (26, 27). Cell migration and invasion were assessed as per the manufacturer's instructions. In brief, 2 × 104 cells in serum-free medium were added to the upper wells of the CIM plate coated with a thin layer of Matrigel (BD Biosciences) basement membrane matrix diluted 1:20 in serum-free medium (for invasion) or non-coated (for migration). A complete medium was used as a chemoattractant in the lower chamber. Subsequently, the plates were incubated in the RTCA for 24 h, and the impedance value of each well was automatically monitored by the xCELLigence system and expressed as a cell index value, which represents cell status based on the measured electrical impedance change divided by a background value. Each assay was biologically performed in triplicate.
For the proliferation assay, exponentially growing cells (2 × 104) were seeded in an E-plate with complete medium as per the manufacturer's instructions. Cell proliferation was assessed for 48 h. All data were recorded and analyzed by the RTCA software. Cell index was used to measure the change in the electrical impedance divided by background value, which represents cell status. Each assay was biologically performed in triplicate (28).
Statistical Analysis
Statistical analysis was performed using Student's t test, and p values of 0.05 and less were considered as statistically significant.
RESULTS
miR-141 and miR-146b-5p Negatively Regulate the Expression of AUF1
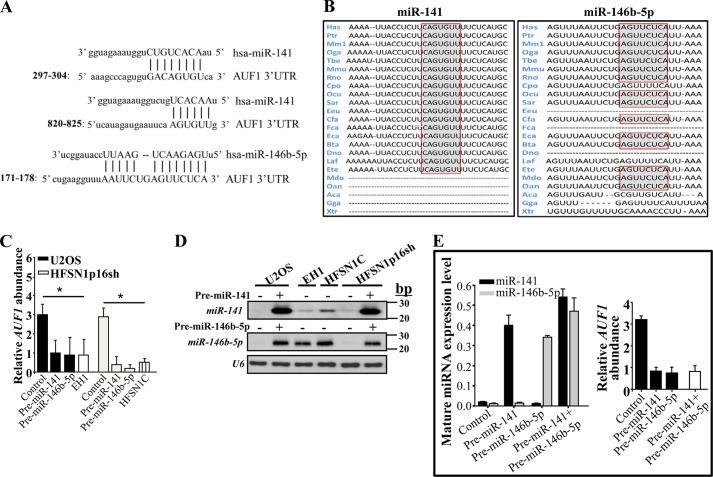
In order to investigate the possible implication of miR-141 and miR-146b-5p in p16-dependent regulation of AUF1, we first made use of miRNA databases to analyze the 3′-UTR of the AUF1 transcript. The AUF1 3′-UTR contains two potential binding sites for miR-141 located at bases 297–304 (mirSVR score = −0.2859) and 820–825 (mirSVR score = −0.0174) and one potential binding site for miR-146b-5p with high complementarity located at bases 171–178 (mirSVR score = −1.3007) (Fig. 1A). These regions are highly conserved among different species (Fig. 1B).
FIGURE 1.
miR-141 and miR-146b-5p repress the expression of AUF1. A, sequence alignment of human miR-141 and miR-146b-5p binding sites in the AUF1 3′-UTR. B, the binding sites of miR-141 and miR-146b-5p in the AUF1 3′-UTR in different species. C and E, total RNA was purified from the indicated cells harboring the indicated constructs, and the indicated RNAs were amplified by qRT-PCR using specific primers. Error bars, S.E. values of three different experiments; *, p ≤ 0.001. D, total RNA was prepared from the indicated cells and was used for Northern blot analysis.
To study the effect of miR-141 and miR-146b-5p on AUF1 expression, the respective precursors were ectopically expressed in the p16-defective U2OS cells, and the skin fibroblast HFSN1 cells expressing CDKN2A shRNA (HFSN1p16sh). Both types of cells express low levels of these miRNAs (21) but high levels of AUF1 (10). EH1 (U2OS isogenic cells, which express low levels of p16) and HFSN1 cells expressing a scrambled shRNA sequence (HFSN1C) were used as respective controls. Next, total RNA was prepared from these cells, and the level of the AUF1 mRNA was assessed by quantitative RT-PCR (qRT-PCR). Fig. 1C shows that the increase in the levels of pre-miR-141 and pre-miR-146b-5p reduced the level of the AUF1 mRNA 4- and 4.8-fold in U2OS and HFSN1p16sh cells, respectively. This level is similar to the AUF1 level observed in the EH1 and HFSN1C cells, which express normal levels of both miRNAs. These data indicate that miR-141 and miR-146b-5p are potential negative regulators of AUF1, and their expression mirrors the level of p16.
To confirm the increase in the level of the mature forms of these miRNAs following the ectopic expression of their precursors, we made use of the RNA prepared above to assess the levels of miR-141 and miR-146b-5p using Northern blotting. Fig. 1D shows that ectopic expression of pre-miR-141 and pre-miR-146b-5p in U2OS and HFSN1p16sh cells increased the expression of the mature forms of these miRNAs.
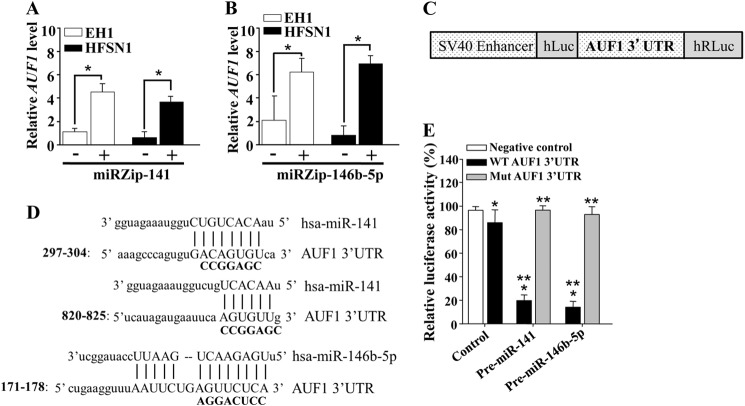
To further evaluate the contribution of miR-141 and miR-146b-5p in the negative regulation of the AUF1 expression, miR-141 and miR-146b-5p were inhibited by specific anti-miRNAs (miRZips) in p16-proficient HFSN1 and EH1 cells, which express both miRNAs. A nonspecific sequence was used as control. Fig. 2A shows that the inhibition of miR-141 increased the expression level of the AUF1 mRNA in EH1 and HFSN1 cells. Similar results were obtained upon the inhibition of miR-146b-5p (Fig. 2B). These results indicate that, like p16, miR-141 and miR-146b-5p negatively regulate the AUF1 expression.
FIGURE 2.
miR-141 and miR-146b-5p negatively regulate the expression of the AUF1 mRNA via its 3′-UTR. Cells were transfected with miRZip-141 (A) and miRZip-146b-5p (B), and then total RNA was purified from the indicated cells and amplified by qRT-PCR. Error bars, S.E. values of three different experiments. *, p < 0.0002. C, schematic representation of the luciferase reporter vector bearing the AUF1 3′-UTR. D, sequence alignment of human miR-141 and miR-146b-5p binding sites in the AUF1 3′-UTR showing the mutated sequences (boldface type). E, U2OS cells expressing pre-miR-141, pre-miR-146b-5p, or a control plasmid were stably transfected with the luciferase reporter vector bearing either the wild type AUF1 3′-UTR or a mutated sequence for one of the binding sites of miR-141 (residues 297–304) or miR-146b-5p. The reporter activity was assessed at 48 h post-transfection. Data (mean ± S.E., n = 4) are presented as percentage change in reporter activity as compared with the negative control cells (*) or to the wild type 3′-UTR (**). * and **, p < 0.0004.
Next, we sought to evaluate the combined effect of both miR-141 and miR-146b-5p on the expression of AUF1. To this end, U2OS cells were sequentially co-transfected with pre-miR-141 and pre-miR-146b-5p, and total RNA was prepared from these cells and their controls. Subsequently, the level of mature miRNAs was assessed by qRT-PCR. Fig. 1E (left) shows clear up-regulation of both miRNAs in cells co-transfected with their precursors. Fig. 1E (right) shows that the level of the AUF1 mRNA did not further decrease in U2OS cells co-expressing both pre-miR-141 and pre-miR-146b-5p as compared with the AUF1 level in cells expressing only one of these miRNAs.
miR-141 and miR-146b-5p Control the AUF1 mRNA Expression via Its 3′-UTR
Next, we sought to evaluate the potential contribution of the miR-141 and miR-146b-5p binding sites in the AUF1 mRNA 3′-UTR to the regulation of AUF1 expression. To this end, intact AUF1 3′-UTR or mutated sequence for these binding sites was inserted into a luciferase/Renilla reporter vector (Fig. 2C) and introduced into U2OS cells stably expressing pre-miR-141, pre-miR-146b-5p, or empty vector (control). The reporter activity fused to the intact sequence of the AUF1 3′-UTR was significantly reduced in U2OS cells expressing pre-miR-141 or pre-miR-146b-5p as compared with the control cells (Fig. 2E). Interestingly, this effect was abolished by mutating the putative miR-141 or miR-146b-5p binding sites within the 3′-UTR of the AUF1 mRNA (Fig. 2, D and E). This indicates that the effect of miR-141 and miR-146b-5p on AUF1 is mediated through interaction with their seeding sequence in the AUF1 3′-UTR.
The AUF1 Protein Level Is Modulated in a miR-141/miR-146b-5p-dependent Manner
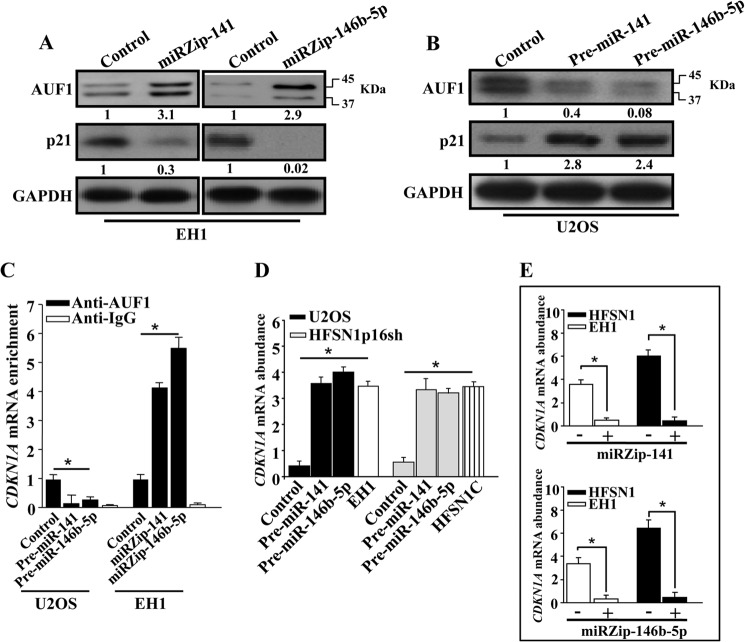
To investigate the effect of miR-141 and miR-146b-5p on the expression of the AUF1 protein, whole cell extracts were first prepared from EH1 cells expressing either miRZip-141, miRZip-146b-5p, or their control plasmid. Subsequently, the level of the AUF1 protein was assessed by immunoblotting. Fig. 3A shows that the inhibition of miR-141 increased 3-fold the level of the AUF1 protein. Similar results were obtained with the inhibition of miR-146b-5p (Fig. 3A). However, the expression of pre-miR-141 or pre-miR-146b-5p in U2OS cells decreased the level of the AUF1 protein 2.5- and 12.5-fold, respectively (Fig. 3B). To further show this, we investigated the effect of these miRs on p21, one of the major AUF1 targets (8). Fig. 3A shows that the increase in the level of AUF1 with inhibition of miR-141 and miR-146b-5p led to a strong decrease in the expression of the p21 protein. However, the level of p21 was up-regulated in response to the expression of pre-miR-141 and pre-miR-146b-5p in U2OS cells, as compared with its level in the control cells (Fig. 3B). These results confirm the role of miR-141 and miR-146b-5p as negative regulators of AUF1.
FIGURE 3.
miR-141 and miR-146b-5p repress the expression of the AUF1 protein. A and B, whole protein extracts were prepared and used for immunoblotting analysis utilizing antibodies against the indicated proteins. The numbers below the bands indicate the corresponding expression levels after loading correction against GAPDH. C, whole cell lysates were prepared from U2OS and EH1 expressing the indicated constructs, and 400 μg of proteins were used for immunoprecipitation with anti-AUF1 antibody or anti-IgG (Control). The level of the CDKN1A mRNA was assessed by qRT-PCR of the corresponding immunoprecipitation material using specific primers. Error bars, S.E. values of three different experiments. D and E, total RNA was purified from the indicated cells, and the CDKN1A mRNA was amplified by qRT-PCR using specific primers. Error bars, S.E. values of three different experiments; *, p ≤ 0.001.
Because AUF1 binds the CDKN1A mRNA (8), we studied the effect of miR-141 and miR-146b-5p on this binding. Therefore, AUF1/mRNA ribonucleoprotein complexes were obtained by immunoprecipitation using anti-AUF1 antibody from EH1 cells expressing miRZip-141 or miRZip-146b-5p and from U2OS cells expressing either pre-miR-141 or pre-miR-146b-5p and their control counterparts. Subsequently, the CDKN1A mRNA was amplified by qRT-PCR using specific primers. Fig. 3C shows amplification of the CDKN1A mRNA, indicating the binding of the AUF1 protein to this molecule. Importantly, the level of the CDKN1A mRNA that was bound to AUF1 was higher in miRZip-141- and miRZip-146b-5p-expressing cells than in the control cells (Fig. 3C). However, the level of the CDKN1A mRNA decreased upon expression of pre-miR-141 or pre-miR-146b-5p in U2OS cells (Fig. 3C). Immunoprecipitation using IgG as a negative control showed undetectable signals. These data demonstrate that miR-141 and miR-146b-5p negatively control the expression of AUF1.
Interestingly, miR-141- and miR-146b-5p-dependent inhibition of AUF1 expression increased the level of the CDKN1A transcript in both U2OS and HFSN1p16sh to a level similar to that observed in EH1 and HFSN1C (Fig. 3D). On the other hand, the inhibition of miR-141 and miR-146b-5p, which increased the level of the AUF1 protein, decreased the level of the CDKN1A mRNA (Fig. 3E). This confirms that miR-141 and miR-146b-5p control the expression of AUF1.
Inverse Correlation between the Levels of miR-141/miR-146b-5p and AUF1 in Osteosarcoma Cell Lines
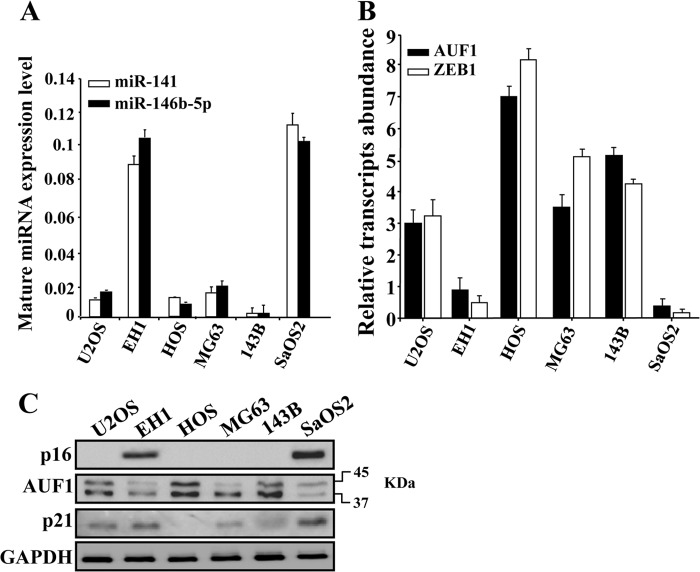
Next, we sought to assess the levels of p16, miR-141, miR-146b-5p, AUF1, and its target p21 in a panel of osteosarcoma cell lines. To this end, total RNA and proteins were extracted from these cells, and the levels of mature miR-141 and miR-146b-5p as well as the level of the AUF1 mRNA were assessed by qRT-PCR, whereas the levels of p16, AUF1, and p21 proteins were assessed by immunoblotting. Fig. 4A shows that, among these various cell lines, only those expressing p16 (EH1 and SaOS2) (Fig. 4C) expressed high levels of mature miR-141 and miR-146b-5p. Interestingly, these two cell lines expressed low levels of both the AUF1 mRNA and protein as compared with the other cell lines (U2OS, HOS, and 143B), which express low levels of miR-141 and miR-146b-5p (Fig. 4, A–C). However, MG63 cells, wherein the expression level of miR-141 and miR-146b-5p were low, expressed a moderate level of AUF1 (Fig. 4, B and C). These results show the presence of an inverse correlation between the levels of miR-141/miR-146b-5p and the level of AUF1 in osteosarcoma cells. As expected, Fig. 4C shows also an inverse correlation between the level of AUF1 and that of p21.
FIGURE 4.
miR-141 and miR-146b-5p levels are negatively correlated with the AUF1 level in osteosarcoma cells. A and B, total RNA was prepared from the indicated cells and utilized to amplify the indicated transcripts by qRT-PCR using specific primers. C, whole cell lysates were prepared from the indicated cells and used for immunoblotting analysis utilizing antibodies against the indicated proteins.
miR-141 and miR-146b-5p Suppress the Proliferation and Migration/Invasion Abilities of U2OS Cells in an AUF1/AKT-dependent Manner
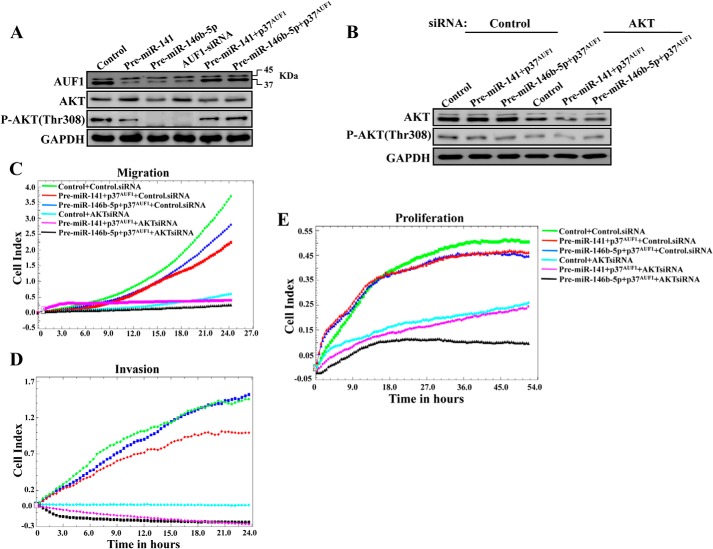
Because miR-141 and miR-146b-5p are implicated in various cancer-related processes (29, 30), we sought to investigate the role of AUF1 in these processes and test whether AUF1 is an effector of these miRNAs. Therefore, U2OS cells were transfected with plasmids bearing pre-miR-141, pre-miR-146b-5p, or an empty vector used as control. After 72 h of transfection, cell migration/invasion were assessed. Cells were seeded in the upper wells of the CIM plate in the presence of Matrigel basement membrane matrix (invasion) or without (migration), and the invasion/migration were assessed for 24 h. Fig. 5, A and B, shows that the expression of pre-miR-141 or pre-miR-146b-5p, which reduced the level of the AUF1 protein similarly to the level observed upon down-regulation of AUF1 using specific siRNA (Fig. 6A), strongly reduced both the migration and the invasion of U2OS cells, with a higher effect of miR-146b-5p. A similar result was obtained when AUF1 was down-regulated using specific siRNA (Fig. 5, A and B). Interestingly, when the p37AUF1 isoform, which has the strongest affinity for the target transcript among the other isoforms (4, 5), was ectopically expressed in cells expressing pre-miR-141 or pre-miR-146b-5p, which increased the level of the AUF1 protein similar to that observed in the control cells (Fig. 6A), the migration and invasion capabilities of U2OS cells were restored (Fig. 5, A and B). This indicates that AUF1 enhances the migration/invasion abilities of U2OS cells and that miR-141 and miR-146b-5p inhibit these abilities through the repression of AUF1.
FIGURE 5.
miR-141 and miR-146b-5p repress the invasion/migration potential of U2OS cells through inhibition of AUF1. A and B, U2OS cells were transfected with the indicated constructs, and cells (2 × 104) were added in serum-free medium to the upper wells of the CIM plates (Roche Applied Science) separated by an 8-μm pore size PET membrane with a thin layer of Matrigel basement membrane matrix (Invasion) or without (Migration), and the migration/invasion were assessed for 24 h using the RTCA instrument. Each assay was performed in triplicate. The results were expressed as mean cells/well. C, cells were transfected with the indicated constructs, and the proliferation rate was measured several passages post-transfection using the RTCA instrument and E-plates.
FIGURE 6.
AUF1 activates the migration/invasion abilities of U2OS through targeting AKT. A, whole cell lysates were prepared from the indicated cells and were used for immunoblotting using antibodies against the indicated proteins. B, cells were treated with either control or AKT siRNA, and whole cell lysates were prepared and used for immunoblotting analysis using antibodies against the indicated proteins. C–E, as described in the legend to Fig. 5, A–C, respectively.
We next investigated the effect on cell proliferation after serial passaging of cells post-transfection. Fig. 5C shows that the expression of pre-miR-141 or pre-miR-146b-5p strongly reduced the proliferation rate of U2OS cells, with a higher effect of miR-146b-5p. A similar result was obtained when AUF1 was down-regulated using specific siRNA (Fig. 5C). Interestingly, when the p37AUF1 isoform was ectopically expressed in cells expressing pre-miR-141 or pre-miR-146b-5p, the proliferation rate was restored (Fig. 5C). This indicates that AUF1 enhances the proliferation of U2OS cells and that miR-141 and miR-146b-5p inhibit the proliferation of these cells through the repression of AUF1.
To explore the molecular mechanisms that underlay the modulation of the proliferation and the migratory/invasiveness capacities of U2OS by AUF1, miR-141, and miR-146b-5p, we investigated the possible activation of the pro-invasive/migratory and proliferative protein kinase AKT (31, 32). Fig. 6A shows that whereas the expression of pre-miR-141 and pre-miR-146b-5p as well as AUF1 down-regulation had only a slight effect on the expression level of total AKT, the level of the active/phosphorylated form of this kinase AKT (Thr-308) was strongly reduced. As for the migration/invasion, ectopic expression of AUF1 in pre-miR-141- or pre-miR-146b-5p-expressing cells suppressed the effect of these miRNAs and restored the normal level of phospho-AKT (Thr-308) (Fig. 6A). This implies that the miR-141- and miR-146b-5p-dependent repression of the invasiveness and migratory abilities of U2OS cells is mediated through the activation of AKT in an AUF1-dependent manner.
To confirm the role of AKT in this process, AKT was down-regulated using specific siRNA in the U2OS cells expressing either pre-miR-141 or pre-miR-146b-5p along with the p37AUF1 isoform. A scrambled sequence was used as control. Whole cell extracts were prepared from these cells, and the levels of the AKT and the phospho-AKT (Thr-308) proteins were assessed by immunoblotting. Fig. 6B shows that AKT siRNA decreased the levels of both total and phospho-AKT (Thr-308) proteins. Subsequently, the invasion/migration as well as proliferation of these cells were assessed for 24 h as described above. Fig. 6, C–E, shows that down-regulation of AKT strongly repressed the migration/invasion and proliferation abilities of cells expressing p37AUF1 in the presence of miR-141 or miR-146b-5p as compared with control cells. This indicates that the AUF1-dependent activation of the invasion/migration and proliferation abilities of U2OS cells is mediated through the activation of AKT.
AUF1 Binds and Stabilizes the PDK1 mRNA
To explore the molecular mechanism underlying AUF1-dependent positive regulation of phospho-AKT (Thr-308), we studied the effect of AUF1 on the expression of PDK1, which phosphorylates AKT on Thr-308 (33). To this end, whole cell extracts were prepared from U2OS cells expressing either AUF1 siRNA or a control plasmid, and the levels of the PDK1, phospho-PDK1 (Ser-241), and phospho-AKT (Thr-308) proteins were assessed by immunoblotting. Fig. 7A shows that AUF1 siRNA decreased the levels of both total and phospho-PDK1 (Ser-241) as well as phospho-AKT (Thr-308) proteins. Subsequently, the level of the PDK1 mRNA was assessed in these cells by qRT-PCR. Fig. 7B shows a clear decrease in the level of the PDK1 mRNA in AUF1 siRNA-expressing cells as compared with their control counterparts. In addition, the level of the PDK1 mRNA was assessed in EH1 cells expressing either the p37AUF1 isoform or a control plasmid. Fig. 7B shows that the expression of the p37AUF1 isoform in EH1 cells increases the PDK1 mRNA to a level higher than that in U2OS cells, indicating that AUF1 is a positive regulator of PDK1.
FIGURE 7.
AUF1 binds and stabilizes the PDK1 mRNA. A, whole cell lysates were prepared from the indicated cells and used for immunoblotting analysis utilizing antibodies against the indicated proteins. B, total RNA was prepared from the indicated cells and utilized to amplify the indicated transcripts by qRT-PCR using specific primers. C, U2OS and EH1 cells expressing the indicated constructs were treated with actinomycin D and then reincubated for the indicated periods of time. Total RNA was extracted, and the remaining amount of the PDK1 mRNA was assessed using qRT-PCR. The dashed lines indicate the PDK1 mRNA half-life. Error bars, S.E. values of three different experiments. D, biotinylated PDK1 3′-UTR bearing either wild type or mutated sequence of the AUF1 binding site was incubated with cytoplasmic cellular lysate from the indicated cells, and the association of AUF1 with these RNAs was detected by immunoblotting using anti-AUF1 antibody. E, U2OS cells expressing AUF1 siRNA or a control plasmid were stably transfected with the luciferase reporter vector bearing either the wild-type PDK1 3′-UTR or a mutated sequence for the binding site of AUF1 (residues 556–562). The reporter activity was assessed at 48 h post-transfection. Data (mean ± S.E., n = 4) were presented as a percentage change in reporter activity as compared with the negative control cells (*) or with the wild-type 3′-UTR (**). * and **, p < 0.00003.
Because AUF1 is an RNA-binding protein, we sought to investigate whether AUF1 has any role in the stability of the PDK1 mRNA. Therefore, U2OS cells expressing AUF1 siRNA or a control plasmid as well as EH1 cells expressing either the p37AUF1 isoform or a control plasmid were treated with the transcription inhibitor actinomycin D and then reincubated for different periods of time (0–6 h). Total RNA was purified, and the mRNA level of PDK1 was assessed by qRT-PCR. Fig. 7C shows that the down-regulation of AUF1 in U2OS cells led to a clear decrease in the PDK1 mRNA half-life as compared with control cells. However, the ectopic expression of the p37AUF1 isoform in EH1 cells increased the PDK1 mRNA half-life as compared with the corresponding control cells (Fig. 7C). This shows that AUF1 stabilizes the PDK1 mRNA. Next, we searched for an AUF1 binding site(s) on the 3′-UTR of the PDK1 mRNA, and we found a single AUF1 binding site (Fig. 7D, top). Therefore, we studied the binding of AUF1 to the PDK1 mRNA. To this end, biotinylated PDK1 3′-UTR bearing either the wild type or the mutated AUF1 binding site was synthesized and incubated with cytoplasmic cellular lysates prepared from U2OS expressing either AUF1 siRNA or the control plasmid. The 3′-UTR/AUF1 ribonucleoprotein complexes were precipitated, and the level of the AUF1 protein was assessed by immunoblotting. Fig. 7D (bottom) shows that AUF1 was associated with the PDK1 3′-UTR in U2OS cells, and this association was potently reduced when AUF1 was down-regulated in AUF1-deficient U2OS cells. Interestingly, the presence of a mutated AUF1 binding site abolished the binding of AUF1 to the PDK1 3′-UTR (Fig. 7D). This shows that AUF1 binds to the PDK1 3′-UTR in vitro.
To further show this, we investigated the potential contribution of the AUF1 binding sites in the PDK1 mRNA 3′-UTR to the regulation of PDK1 expression. To this end, wild-type PDK1 3′-UTR or the mutated sequence at the AUF1 binding site was inserted into a luciferase/Renilla reporter vector and introduced into U2OS cells stably expressing AUF1 siRNA or empty vector (control). The reporter activity fused to the intact sequence of the PDK1 3′-UTR was significantly reduced in U2OS cells expressing AUF1 siRNA as compared with the control cells (Fig. 7E). Interestingly, the activity was abolished by mutating the putative AUF1 binding site within the 3′-UTR of the PDK1 mRNA (Fig. 7E). This further indicates that the effect of AUF1 is mediated through interaction with its seeding sequence in the PDK1 3′-UTR. Together, these data indicate that AUF1 regulates the phosphorylation of AKT via positive regulation of the PDK1 mRNA.
miR-141 and miR-146b-5p Suppress the Mesenchymal Features of Osteosarcoma Cells in an AUF1-dependent Manner
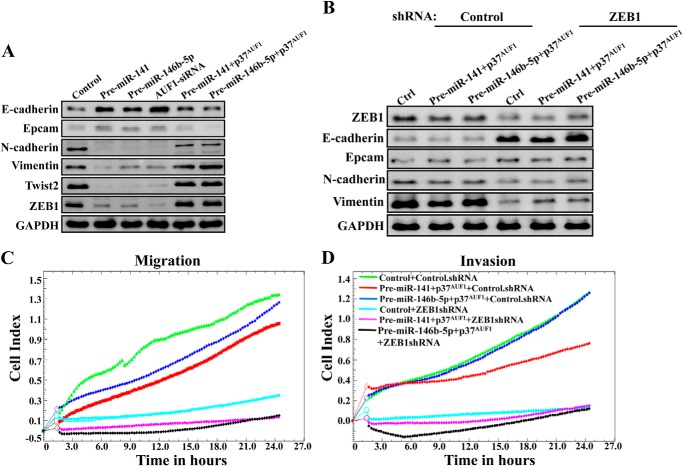
The loss of epithelial markers and the up-regulation of mesenchymal genes is related to an increase in the invasion/migration as well as the proliferation capacities of cells. Therefore, we sought to confirm the role of miR-141, miR-146b-5p, and their downstream effector AUF1 on the expression level of the main EMT-related proteins by immunoblotting. Fig. 8A shows a clear increase in the level of the epithelial markers E-cadherin and Epcam in pre-miR-141, pre-miR-146b-5p, and AUF1 siRNA-expressing U2OS cells as compared with control cells. However, the levels of the mesenchymal N-cadherin, vimentin, Twist2, and ZEB1 proteins were reduced in these construct-expressing U2OS cells as compared with control cells (Fig. 8A). This indicates that whereas miR-141 and miR-146b-5p activate the epithelial features, AUF1 is an activator of the mesenchymal characteristics. Interestingly, when AUF1 was expressed in cells expressing pre-miR-141 or pre-miR-146b-5p, the level of the epithelial E-cadherin and Epcam proteins decreased, whereas the levels of the mesenchymal N-cadherin, vimentin, Twist2, and ZEB1 proteins increased (Fig. 8A). These results suggest that miR-141 and miR-146b-5p repress the transition from epithelial to mesenchymal features through down-regulation of AUF1, which is an activator of this transition.
FIGURE 8.
miR-141 and miR-146b-5p promote epithelial features in U2OS cells through inhibition of AUF1 targeting ZEB1. A and B, whole cell lysates were prepared from the indicated cells and were used for immunoblotting analysis using antibodies against the indicated proteins. C and D, as described in the legend to Fig. 5, A and B, respectively.
To explore the molecular mechanism that underlies AUF1-dependent activation of the mesenchymal features, we studied the effect of ZEB1 down-regulation on the expression of the EMT markers. Therefore, ZEB1 was knocked down using specific shRNA in the U2OS cells expressing either pre-miR-141 or pre-miR-146b-5p along with the p37AUF1 isoform. A scrambled sequence was used as control. Whole cell extracts were prepared from these cells, and the levels of the ZEB1 and the other EMT markers were assessed by immunoblotting. Fig. 8B shows that ZEB1-shRNA decreased the level of ZEB1 as well as the levels of the mesenchymal N-cadherin and vimentin proteins, whereas the levels of E-cadherin and Epcam increased. Subsequently, the migration/invasion of these cells were assessed for 24 h. Fig. 8, C and D, shows that down-regulation of ZEB1 strongly repressed the migration/invasion abilities of these cells, as compared with control cells. This indicates that the AUF1-dependent activation of the mesenchymal markers in U2OS cells is mediated through ZEB1 up-regulation.
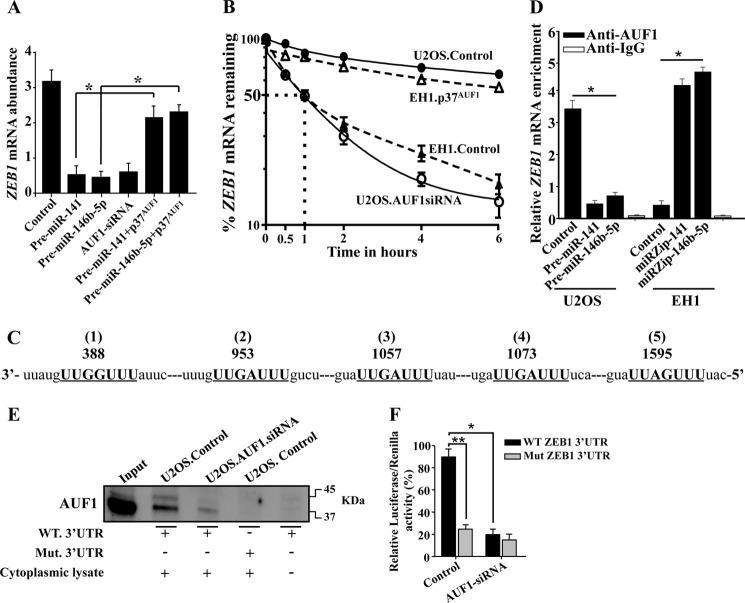
AUF1 Binds and Stabilizes the ZEB1 mRNA
To explore the molecular mechanism underlying AUF1-dependent-positive regulation of ZEB1, we studied the effect of miR-141, miR-146b-5p, and AUF1 on the expression of the ZEB1 mRNA. Therefore, the level of the ZEB1 mRNA was assessed in U2OS cells expressing pre-miR-141, pre-miR-146b-5p, or AUF1 siRNA by qRT-PCR. Fig. 9A shows a clear decrease in the level of the ZEB1 mRNA in these cells as compared with control cells. This indicates that whereas miR-141 and miR-146b-5p are repressors of the ZEB1 expression, AUF1 is a positive regulator of this EMT transcription factor. Interestingly, the expression of AUF1 in U2OS cells expressing pre-miR-141 or pre-miR-146b-5p restored the expression of the ZEB1 mRNA to a level similar to that observed in control cells (Fig. 9A). Furthermore, we have shown a strong correlation between the expression levels of AUF1 and ZEB1 in various osteosarcoma cell lines (Fig. 4B). These results indicate that miR-141 and miR-146b-5p repress the expression of ZEB1 in an AUF1-dependent manner.
FIGURE 9.
AUF1 binds and stabilizes the ZEB1 mRNA. A, total RNA was prepared from U2OS cells expressing the indicated constructs and the level of the ZEB1 mRNA was assessed by qRT-PCR. Error bars, means ± S.D. *, p < 0.001. B, U2OS and EH1 cells expressing the indicated constructs were treated with actinomycin D and then reincubated for the indicated periods of time. Total RNA was extracted, and the remaining amount of the ZEB1 mRNA was assessed using qRT-PCR. The dotted lines indicate the ZEB1 mRNA half-life. Error bars, S.E. values of three different experiments. C, sequence alignment of human ZEB1 3′-UTR showing the AUF1 binding sites. D, RNAs bound to the AUF1 protein were isolated by immunoprecipitation from U2OS and EH1 cells expressing the indicated constructs using anti-AUF1 antibody or anti-IgG (control), and then the ZEB1 mRNA was amplified by qRT-PCR. Error bars, S.E. values of three different experiments; *, p ≤ 0.001. E, biotinylated ZEB1 3′-UTR bearing either wild type or mutated sequence of the second AUF1 binding site was incubated with cytoplasmic cellular lysate from the indicated cells, and the association of AUF1 with these RNAs was detected by immunoblotting using anti-AUF1 antibody. F, U2OS cells expressing AUF1 siRNA or a control plasmid were stably transfected with the luciferase reporter vector bearing either wild-type ZEB1 3′-UTR or a mutated sequence for one of the binding sites of AUF1 (residues 953–959). The reporter activity was assessed at 48 h post-transfection. Data (mean ± S.E., n = 4) are presented as a percentage change in reporter activity as compared with the negative control cells (*) or with the wild-type 3′-UTR (**). * and **, p < 0.00003.
Because AUF1 is an RNA-binding protein, we sought to investigate whether AUF1 has any role in the stability of the ZEB1 mRNA. Therefore, U2OS cells expressing AUF1 siRNA or a control plasmid as well as EH1 cells expressing either the p37AUF1 isoform or a control plasmid were treated with the transcription inhibitor actinomycin D and then reincubated for different periods of time (0–6 h). Total RNA was purified, and the mRNA level of ZEB1 was assessed by qRT-PCR. Fig. 9B shows that the down-regulation of AUF1 in U2OS cells led to a significant decrease in the ZEB1 mRNA half-life similar to that observed in the EH1 cells. However, the expression of the p37AUF1 isoform in EH1 cells stabilizes the ZEB1 mRNA to a level similar to that in U2OS cells (Fig. 9B). This shows that AUF1 reduced the turnover of the ZEB1 mRNA. Next, we searched for AUF1 binding site(s) on the 3′-UTR of the ZEB1 mRNA, and we found five different AUF1 binding sites (Fig. 9C). Therefore, we studied the binding of AUF1 to the ZEB1 mRNA and the effect of miR-141 and miR-146b-5p on the amount of the AUF1-ZEB1 mRNA ribonucleoprotein complex using U2OS cells expressing either pre-miR-141, pre-miR-146b-5p, or the control plasmid as well as EH1 cells expressing either miRZip-141, miRZip-146b-5p, or the corresponding control plasmid. AUF1 mRNA ribonucleoprotein complexes were obtained by immunoprecipitation using anti-AUF1 antibody and were used for qRT-PCR amplification using specific primers. Fig. 9D shows amplification of the ZEB1 mRNA, indicating the binding of the AUF1 protein to this transcript. Importantly, the level of the ZEB1 mRNA that was bound to AUF1 decreased in U2OS cells expressing pre-miR-141 or pre-miR-146b-5p, as compared with the control cells (Fig. 9D). In contrast, the level of the ZEB1 mRNA that was bound to AUF1 increased in EH1 cells expressing miRZip-141 or miRZip-146b-5p, as compared with the control cells (Fig. 9D). These results show that the amount of the AUF1-ZEB1 mRNA ribonucleoprotein complex is modulated in a miR-141- and miR-146b-5p-dependent manner and that these two miRNAs repress ZEB1 via down-regulation of its stabilizer, AUF1.
To further show the binding of AUF1 to the ZEB1 3′-UTR, biotinylated ZEB1 3′-UTR spanning either the wild type or the mutated AUF1 binding site were synthesized and incubated with cytoplasmic cellular lysates prepared from U2OS expressing either AUF1 siRNA or the control plasmid. The 3′-UTR-AUF1 ribonucleoprotein complexes were precipitated, and the level of AUF1 was assessed by immunoblotting. Fig. 9E shows that AUF1 was associated with the ZEB1 3′-UTR in U2OS cells, and this association was potently reduced when AUF1 was down-regulated in p16-deficient U2OS cells. Interestingly, mutation of its binding site abolished AUF1 binding to the ZEB1 3′-UTR (Fig. 9E). This result shows the binding of AUF1 to the ZEB1 3′-UTR in vitro.
To further confirm this, we investigated the potential contribution of the AUF1 binding sites in the ZEB1 mRNA 3′-UTR to the regulation of the ZEB1 expression. To this end, wild-type ZEB1 3′-UTR or the mutated sequence for the second AUF1 binding site was inserted into a luciferase/Renilla reporter vector and introduced into U2OS cells stably expressing AUF1 siRNA or empty vector (control). The reporter activity fused to the intact sequence of the ZEB1 3′-UTR was significantly reduced in U2OS cells expressing AUF1 siRNA as compared with the control cells (Fig. 9F). Interestingly, the activity was abolished by mutating the putative AUF1 binding site within the 3′-UTR of the ZEB1 mRNA (Fig. 9F). This further indicates that the effect of AUF1 is mediated through interaction with its seeding sequence in the ZEB1 3′-UTR.
DISCUSSION
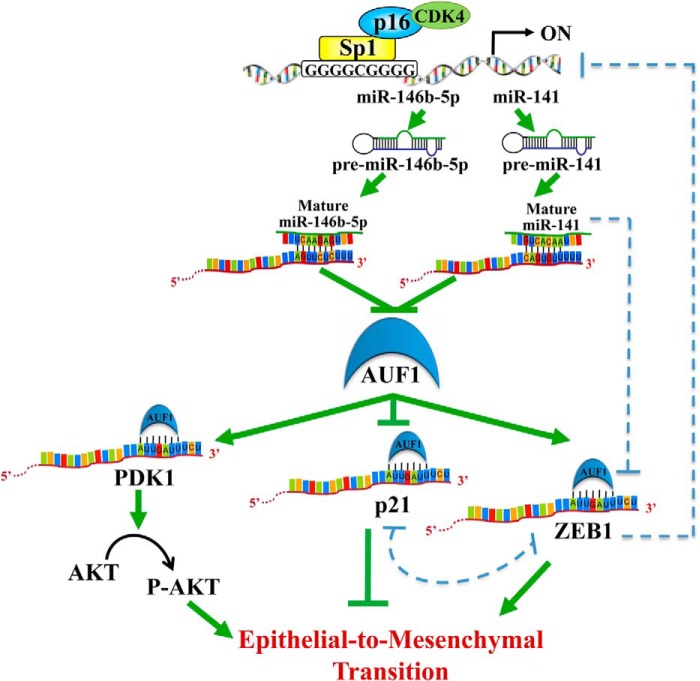
After showing that p16 post-transcriptionally regulates the expression of AUF1 and is also a positive regulator of miR-141 and miR-146b-5p (10, 21), we present here several lines of evidence indicating that the RNA-binding protein AUF1 is a target of miR-141 and miR-146b-5p, two essential tumor suppressor miRNAs. First, there is high complementarity between mature miR-141 and miR-146b-5p and the 3′-UTR of the AUF1 mRNA. Second, the AUF1 3′-UTR was responsive to both miR-141 and miR-146b-5p. Third, mutated miR-141 and miR-146b-5p binding sites in the AUF1 3′-UTR abolished their regulatory effects on AUF1. Fourth, the inhibition of miR-141 or miR-146b-5p with specific anti-miR inhibitors increased the expression of AUF1. Finally, ectopic expression of pre-miR-141 or pre-miR-146b-5p decreased the AUF1 level, which affects the expression of its target CDKN2A. These results provide the first indication that miR-141 and miR-146b-5p negatively regulate the expression of AUF1 and show that p16-dependent regulation of AUF1 is miR-141/miR146b-5p-related (summarized in Fig. 10). In line with this finding, we have shown the presence of an inverse correlation between the expression of p16/miR-141/miR-146b-5p and the level of their target AUF1 in various osteosarcoma cell lines. Interestingly, osteosarcoma cells that express low levels of miR-141 and miR-146b-5p (U2OS, HOS, MG63, and 143B) are highly aggressive and prometastatic as compared with the SaOS2 cell line (34), which express high levels of these miRNAs and a reduced amount of AUF1. Similarly, these miRNAs are almost undetectable in the highly invasive and p16-defective breast cancer MDA-MB-231 cells, whereas AUF1 is highly expressed (15). This indicates that this inverse relationship between miR-141/miR-146b-5p and AUF1 exists in different cancer cells and that the increase in the AUF1 level is related to more aggressive and prometastatic features.
FIGURE 10.
Schematic representation of the effect of the p16-Sp1-CDK4 complex on miR-141 and miR-146b-5p and their downstream targets AUF1, p21, ZEB1, and AKT and the role of this pathway in modulating the EMT process.
In fact, several lines of evidence indicate that AUF1 has various procarcinogenic functions. Indeed, many AUF1-targeted transcripts encode products that control pro- and antioncogenic processes, and AUF1 levels are enhanced in numerous cancers. Furthermore, overexpression of AUF1 enhanced tumorigenesis in murine models (15), and mice engineered to overexpress the p37 isoform of AUF1 developed undifferentiated sarcomas with high vascularization and cellularity (36). Moreover, cytoplasmic expression of AUF1 was higher in malignant thyroid tissues as compared with benign tissues, and total or exon-selective knockdown of AUF1 led to growth inhibition accompanied by the induction of cell cycle inhibitors (37). Additionally, in breast carcinoma cells, AUF1 showed increased binding to a number of mRNAs linked to the transformation of epithelial cells (38). Finally, signaling cascades that modulate the AUF1 function are deregulated in several cancerous tissues (15). Importantly, the present findings added further weight to the oncogenic significance of AUF1 by presenting the first indication that this RNA-binding protein promotes the prometastatic mesenchymal features in osteosarcoma cells, whereas miR-141/miR-146b-5p-dependent suppression of AUF1 represses these features. To reach this conclusion, we have first shown that AUF1 enhances the migration/invasion and proliferation capabilities of U2OS cells. Indeed, similar to the ectopic expression of miR-141 or miR-146b-5p, down-regulation of AUF1 repressed the migration/invasion abilities as well as the proliferation rate of U2OS cells in an AKT-dependent manner. This indicates that AUF1 promotes the activation of this oncoprotein, which belongs to the important PI3K/AKT/mTOR oncogenic pathway, one of the most frequently deregulated pathways in cancer (39). The activation of AKT induces EMT in various cancer types (40–42). In squamous cell carcinoma lines, AKT induced EMT through transcriptionally down-regulating E-cadherin by inducing Snail (41). To address the molecular mechanism underlying AUF1-dependent activation of the AKT protein kinase, we have shown that AUF1 binds to and stabilizes the mRNA of PDK1, which activates/phosphorylates AKT on Thr-308 (33) (Fig. 10). It is also possible that AUF1 negatively targets the tumor suppressor PTEN phosphatase or activates the protein kinase PI3K, which are two major regulators of the AKT activity (43).
Second, miR-141 and miR-146b-5p induce epithelial features through repression of AUF1, which favors the mesenchymal ones. Indeed, whereas ectopic expression of miR-141 and miR-146b-5p up-regulated the epithelial markers E-cadherin and Epcam in osteosarcoma cells, the introduction of p37AUF1 suppressed these genes and induced the mesenchymal markers N-cadherin, vimentin, Twist2, and ZEB1. By contrast, AUF1 knockdown also restored the epithelial features of osteosarcoma cells. In addition, we have shown that AUF1 positively regulates the expression of the E-cadherin repressor ZEB1. Further analysis revealed five putative AUF1-binding AU-rich conserved elements in the ZEB1 3′-UTR region (Fig. 9C). Therefore, we have shown that AUF1 directly binds to the ZEB1 mRNA and reduces its turnover. Furthermore, the analysis of AUF1 and ZEB1 expression by qRT-PCR showed that their expression levels are remarkably correlative in the various osteosarcoma cell lines that have been studied (Fig. 4), which further confirms the AUF1-dependent stabilization of ZEB1. These data suggest that ZEB1 contributes to the AUF1-related down-regulation of E-cadherin and the consequent induction of EMT in osteosarcoma cells. In addition to ZEB1, miR-141 and miR-146b-5p as well as their target AUF1 modulate the expression of other EMT-related markers, which could be mediated through direct interaction or indirectly via an EMT regulator.
It has been shown previously that miR-141 suppresses the EMT process through direct inhibition of ZEB1 (44–46). Therefore, miR-141 can represses ZEB1 either directly or indirectly through down-regulation of its stabilizer AUF1. Furthermore, ZEB1 negatively controls miR-141, indicating the presence of a reciprocal negative feedback loop between ZEB1 and miR-141 (45) (Fig. 10). The fact that miR-141 and AUF1 bind the ZEB1 3′-UTR suggests the presence of a third level of ZEB1 post-transcriptional regulation through possible competitive or cooperative interaction between this miRNA and AUF1.
The other effect of miR-141/miR-146b-5p/AUF1 on epithelial/mesenchymal characteristics could be mediated through the tumor suppressor p21 protein (Fig. 10). Indeed, AUF1 negatively regulates p21 (8), which is an inhibitor of EMT in breast and colorectal cancer cells (47, 48) through formation of a complex with ZEB1 (49). This is consistent with the present findings, because U2OS cells that express a high level of AUF1 and low level of p21 exhibit high migration/invasion capabilities and more mesenchymal features. On the other hand, ectopic expression of pre-miR-141 or pre-miR-146b-5p decreased the AUF1 level and up-regulated p21 (Fig. 3), which inhibited the mesenchymal markers and favored epithelial features (Fig. 8). Furthermore, a direct effect of miR-146b-5p on p21 is also possible, as suggested by the miRTarBase prediction algorithm. However, using luciferase reporter assays, Borgdorff et al. (35) have reported a lack of direct interaction between miR-146b-5p and the CDKN1A mRNA.
In summary, we have shown here that miR-141 and miR-146b-5p inhibit the prometastatic mesenchymal features through repression of the RNA-binding protein AUF1. The AUF1-dependent promotion of these features in osteosarcoma cells is mediated through stabilization/accumulation of ZEB1 and the activation of AKT via PDK1 up-regulation. These results shed more light on the capital role of the 3′-UTR-related posttranscriptional gene expression regulation in carcinogenesis, and suggest that miR-141, miR-146b-5p, and AUF1 may be of potential diagnostic and/or therapeutic value for treatment of osteosarcomas and possibly other types of cancer.
Acknowledgments
We are grateful to Dr. Myriam Gorospe for kindly providing the pSILENCER-AUF1 siRNA plasmid. We also thank the Research Centre administration for continuous help and support.
This work was performed under Research Advisory Council (RAC) Proposal 2090027.
- miRNA
- microRNA
- hnRNPD
- heterogenous nuclear ribonucleoprotein D
- EMT
- epithelial-mesenchymal transition
- CIM
- cell invasion and migration
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Ciafrè S. A., Galardi S. (2013) microRNAs and RNA-binding proteins: a complex network of interactions and reciprocal regulations in cancer. RNA Biol. 10, 935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim H. H., Kuwano Y., Srikantan S., Lee E. K., Martindale J. L., Gorospe M. (2009) HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 23, 1743–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tominaga K., Srikantan S., Lee E. K., Subaran S. S., Martindale J. L., Abdelmohsen K., Gorospe M. (2011) Competitive regulation of nucleolin expression by HuR and miR-494. Mol. Cell Biol. 31, 4219–4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagner B. J., DeMaria C. T., Sun Y., Wilson G. M., Brewer G. (1998) Structure and genomic organization of the human AUF1 gene: alternative pre-mRNA splicing generates four protein isoforms. Genomics 48, 195–202 [DOI] [PubMed] [Google Scholar]
- 5. Kajita Y., Nakayama J., Aizawa M., Ishikawa F. (1995) The UUAG-specific RNA binding protein, heterogeneous nuclear ribonucleoprotein D0: common modular structure and binding properties of the 2xRBD-Gly family. J. Biol. Chem. 270, 22167–22175 [DOI] [PubMed] [Google Scholar]
- 6. White E. J., Brewer G., Wilson G. M. (2013) Post-transcriptional control of gene expression by AUF1: mechanisms, physiological targets, and regulation. Biochim. Biophys. Acta 1829, 680–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gratacós F. M., Brewer G. (2010) The role of AUF1 in regulated mRNA decay. Wiley Interdiscip. Rev. RNA 1, 457–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lal A., Mazan-Mamczarz K., Kawai T., Yang X., Martindale J. L., Gorospe M. (2004) Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 23, 3092–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al-Khalaf H. H., Aboussekhra A. (2013) p16INK4A positively regulates p21WAF1 expression by suppressing AUF1-dependent mRNA decay. PLoS One 8, e70133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al-Khalaf H. H., Colak D., Al-Saif M., Al-Bakheet A., Hendrayani S. F., Al-Yousef N., Kaya N., Khabar K. S., Aboussekhra A. (2011) p16INK4a positively regulates cyclin D1 and E2F1 through negative control of AUF1. PLoS One 6, e21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brewer G., Saccani S., Sarkar S., Lewis A., Pestka S. (2003) Increased interleukin-10 mRNA stability in melanoma cells is associated with decreased levels of A + U-rich element binding factor AUF1. J. Interferon Cytokine Res. 23, 553–564 [DOI] [PubMed] [Google Scholar]
- 12. Liao B., Hu Y., Brewer G. (2007) Competitive binding of AUF1 and TIAR to MYC mRNA controls its translation. Nat. Struct. Mol. Biol. 14, 511–518 [DOI] [PubMed] [Google Scholar]
- 13. Sarkar S., Sinsimer K. S., Foster R. L., Brewer G., Pestka S. (2008) AUF1 isoform-specific regulation of anti-inflammatory IL10 expression in monocytes. J. Interferon Cytokine Res. 28, 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palanisamy V., Park N. J., Wang J., Wong D. T. (2008) AUF1 and HuR proteins stabilize interleukin-8 mRNA in human saliva. J. Dent. Res. 87, 772–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zucconi B. E., Wilson G. M. (2011) Modulation of neoplastic gene regulatory pathways by the RNA-binding factor AUF1. Front. Biosci. 16, 2307–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arcondéguy T., Lacazette E., Millevoi S., Prats H., Touriol C. (2013) VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res. 41, 7997–8010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdelmohsen K., Tominaga-Yamanaka K., Srikantan S., Yoon J. H., Kang M. J., Gorospe M. (2012) RNA-binding protein AUF1 represses Dicer expression. Nucleic Acids Res. 40, 11531–11544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iorio M. V., Croce C. M. (2012) MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 4, 143–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hao J., Zhang Y., Deng M., Ye R., Zhao S., Wang Y., Li J., Zhao Z. (2014) MicroRNA control of epithelial-mesenchymal transition in cancer stem cells. Int. J. Cancer 135, 1019–1027 [DOI] [PubMed] [Google Scholar]
- 20. Lamouille S., Xu J., Derynck R. (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Khalaf H. H., Mohideen P., Nallar S. C., Kalvakolanu D. V., Aboussekhra A. (2013) The cyclin-dependent kinase inhibitor p16INK4a physically interacts with transcription factor Sp1 and cyclin-dependent kinase 4 to transactivate microRNA-141 and microRNA-146b-5p spontaneously and in response to ultraviolet light-induced DNA damage. J. Biol. Chem. 288, 35511–35525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asirvatham A. J., Gregorie C. J., Hu Z., Magner W. J., Tomasi T. B. (2008) MicroRNA targets in immune genes and the Dicer/Argonaute and ARE machinery components. Mol. Immunol. 45, 1995–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McConnell B. B., Gregory F. J., Stott F. J., Hara E., Peters G. (1999) Induced expression of p16INK4a inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol. Cell Biol. 19, 1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang W., Martindale J. L., Yang X., Chrest F. J., Gorospe M. (2005) Increased stability of the p16 mRNA with replicative senescence. EMBO Rep. 6, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al-Haj L., Blackshear P. J., Khabar K. S. (2012) Regulation of p21/CIP1/WAF-1 mediated cell-cycle arrest by RNase L and tristetraprolin, and involvement of AU-rich elements. Nucleic Acids Res. 40, 7739–7752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knopfová L., Beneš P., Pekarčíková L., Hermanová M., Masařík M., Pernicová Z., Souček K., Smarda J. (2012) c-Myb regulates matrix metalloproteinases 1/9, and cathepsin D: implications for matrix-dependent breast cancer cell invasion and metastasis. Mol. Cancer 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jurmeister S., Baumann M., Balwierz A., Keklikoglou I., Ward A., Uhlmann S., Zhang J. D., Wiemann S., Sahin Ö. (2012) MicroRNA-200c represses migration and invasion of breast cancer cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Mol. Cell Biol. 32, 633–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams C. J., Pike A. C., Maniam S., Sharpe T. D., Coutts A. S., Knapp S., La Thangue N. B., Bullock A. N. (2012) The p53 cofactor Strap exhibits an unexpected TPR motif and oligonucleotide-binding (OB)-fold structure. Proc. Natl. Acad. Sci. U.S.A. 109, 3778–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao G., Wang B., Liu Y., Zhang J. G., Deng S. C., Qin Q., Tian K., Li X., Zhu S., Niu Y., Gong Q., Wang C. Y. (2013) miRNA-141, downregulated in pancreatic cancer, inhibits cell proliferation and invasion by directly targeting MAP4K4. Mol. Cancer Ther. 12, 2569–2580 [DOI] [PubMed] [Google Scholar]
- 30. Geraldo M. V., Yamashita A. S., Kimura E. T. (2012) MicroRNA miR-146b-5p regulates signal transduction of TGF-β by repressing SMAD4 in thyroid cancer. Oncogene 31, 1910–1922 [DOI] [PubMed] [Google Scholar]
- 31. Klemke R. L., Cai S., Giannini A. L., Gallagher P. J., de Lanerolle P., Cheresh D. A. (1997) Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoeli-Lerner M., Yiu G. K., Rabinovitz I., Erhardt P., Jauliac S., Toker A. (2005) Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell 20, 539–550 [DOI] [PubMed] [Google Scholar]
- 33. Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 34. Lauvrak S. U., Munthe E., Kresse S. H., Stratford E. W., Namløs H. M., Meza-Zepeda L. A., Myklebost O. (2013) Functional characterisation of osteosarcoma cell lines and identification of mRNAs and miRNAs associated with aggressive cancer phenotypes. Br. J. Cancer 109, 2228–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borgdorff V., Lleonart M. E., Bishop C. L., Fessart D., Bergin A. H., Overhoff M. G., Beach D. H. (2010) Multiple microRNAs rescue from Ras-induced senescence by inhibiting p21Waf1/Cip1. Oncogene 29, 2262–2271 [DOI] [PubMed] [Google Scholar]
- 36. Gouble A., Grazide S., Meggetto F., Mercier P., Delsol G., Morello D. (2002) A new player in oncogenesis: AUF1/hnRNPD overexpression leads to tumorigenesis in transgenic mice. Cancer Res. 62, 1489–1495 [PubMed] [Google Scholar]
- 37. Trojanowicz B., Brodauf L., Sekulla C., Lorenz K., Finke R., Dralle H., Hoang-Vu C. (2009) The role of AUF1 in thyroid carcinoma progression. Endocr. Relat. Cancer 16, 857–871 [DOI] [PubMed] [Google Scholar]
- 38. Mazan-Mamczarz K., Hagner P. R., Dai B., Wood W. H., Zhang Y., Becker K. G., Liu Z., Gartenhaus R. B. (2008) Identification of transformation-related pathways in a breast epithelial cell model using a ribonomics approach. Cancer Res. 68, 7730–7735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chappell W. H., Steelman L. S., Long J. M., Kempf R. C., Abrams S. L., Franklin R. A., Bäsecke J., Stivala F., Donia M., Fagone P., Malaponte G., Mazzarino M. C., Nicoletti F., Libra M., Maksimovic-Ivanic D., Mijatovic S., Montalto G., Cervello M., Laidler P., Milella M., Tafuri A., Bonati A., Evangelisti C., Cocco L., Martelli A. M., McCubrey J. A. (2011) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2, 135–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suman S., Kurisetty V., Das T. P., Vadodkar A., Ramos G., Lakshmanaswamy R., Damodaran C. (2014) Activation of AKT signaling promotes epithelial-mesenchymal transition and tumor growth in colorectal cancer cells. Mol. Carcinog. 53, E151–E160 [DOI] [PubMed] [Google Scholar]
- 41. Grille S. J., Bellacosa A., Upson J., Klein-Szanto A. J., van Roy F., Lee-Kwon W., Donowitz M., Tsichlis P. N., Larue L. (2003) The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63, 2172–2178 [PubMed] [Google Scholar]
- 42. Almhanna K., Strosberg J., Malafa M. (2011) Targeting AKT protein kinase in gastric cancer. Anticancer Res. 31, 4387–4392 [PubMed] [Google Scholar]
- 43. Liao Y., Hung M. C. (2010) Physiological regulation of Akt activity and stability. Am. J. Transl. Res. 2, 19–42 [PMC free article] [PubMed] [Google Scholar]
- 44. Korpal M., Lee E. S., Hu G., Kang Y. (2008) The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 283, 14910–14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burk U., Schubert J., Wellner U., Schmalhofer O., Vincan E., Spaderna S., Brabletz T. (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 9, 582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gregory P. A., Bert A. G., Paterson E. L., Barry S. C., Tsykin A., Farshid G., Vadas M. A., Khew-Goodall Y., Goodall G. J. (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 10, 593–601 [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y., Yan W., Jung Y. S., Chen X. (2013) PUMA cooperates with p21 to regulate mammary epithelial morphogenesis and epithelial-to-Mesenchymal transition. PLoS One 8, e66464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu M., Casimiro M. C., Wang C., Shirley L. A., Jiao X., Katiyar S., Ju X., Li Z., Yu Z., Zhou J., Johnson M., Fortina P., Hyslop T., Windle J. J., Pestell R. G. (2009) p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 19035–19039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li X. L., Hara T., Choi Y., Subramanian M., Francis P., Bilke S., Walker R. L., Pineda M., Zhu Y., Yang Y., Luo J., Wakefield L. M., Brabletz T., Park B. H., Sharma S., Chowdhury D., Meltzer P. S., Lal A. (2014) A p21-ZEB1 complex inhibits epithelial-mesenchymal transition through the microRNA 183-96-182 cluster. Mol. Cell Biol. 34, 533–550 [DOI] [PMC free article] [PubMed] [Google Scholar]