Background: Secreted Frizzled-related protein 1 is a secreted Wnt antagonist.
Results: The kidneys from Sfrp1 knock-out mice showed significant increase in the renal fibrosis after unilateral ureteral obstruction.
Conclusion: Deletion of Sfrp1 makes mice more susceptible to renal damage through non-canonical Wnt/PCP pathway.
Significance: The relationship between kidney damage and Wnt/non-canonical pathway definitely opens a new field to study mechanisms of renal diseases.
Keywords: Beta-catenin (B-catenin), Cell Signaling, Gene Knockout, Kidney, Pathology, Wnt Signaling
Abstract
Renal fibrosis is responsible for progressive renal diseases that cause chronic renal failure. Sfrp1 (secreted Frizzled-related protein 1) is highly expressed in kidney, although little is known about connection between the protein and renal diseases. Here, we focused on Sfrp1 to investigate its roles in renal fibrosis using a mouse model of unilateral ureteral obstruction (UUO). In wild-type mice, the expression of Sfrp1 protein was markedly increased after UUO. The kidneys from Sfrp1 knock-out mice showed significant increase in expression of myofibrobast markers, α-smooth muscle actin (αSMA). Sfrp1 deficiency also increased protein levels of the fibroblast genes, vimentin, and decreased those of the epithelial genes, E-cadherin, indicated that enhanced epithelial-to-mesenchymal transition. There was no difference in the levels of canonical Wnt signaling; rather, the levels of phosphorylated c-Jun and JNK were more increased in the Sfrp1−/− obstructed kidney. Moreover, the apoptotic cell population was significantly elevated in the obstructed kidneys from Sfrp1−/− mice following UUO but was slightly increased in those from wild-type mice. These results indicate that Sfrp1 is required for inhibition of renal damage through the non-canonical Wnt/PCP pathway.
Introduction
Renal tubulointerstitial fibrosis is regarded as a final common pathway of progressive chronic kidney diseases (1–4). Fibrotic damage is characterized by the increase of interstitial fibroblasts and myofibroblasts (5, 6). In a recent study, epithelial-to-mesenchymal transition is essential for the development of renal fibrosis, in which tubular epithelial cells are transformed into interstitial fibroblasts and myofibroblasts by activating signaling pathways, including the Wnt and TGF-β pathway (7–11).
Wnt family members secrete glycoproteins that play crucial roles in various cellular functions (12, 13) (see the Wnt Homepage, Nusse laboratory, Stanford University). Wnt proteins can signal through the canonical (Wnt/β-catenin) pathway. The Wnt/β-catenin signaling pathway is a regulator of cellular functions in embryonic development, homeostatic state, and tissue injury (14–16). The Wnt/β-catenin pathway is also activated in the process of kidney development and renal fibrosis (17–20). Wnt signals can also be transmitted through some additional pathway: non-canonical Wnt pathway, such as planar cell polarity (PCP)2 or Ca2+ pathway, which is β-catenin-independent (21, 22). The Wnt/PCP pathway transduces its signal by activating c-Jun N-terminal kinase (JNK). The disruption of the Wnt/PCP pathway is detrimental for oriented cell division and apicobasal polarity, but the function of non-canonical Wnt pathway is largely unknown in the process of kidney diseases.
The secreted Frizzled-related protein (Sfrp) is a secreted Wnt antagonist that interacts directly with Wnt ligand (23–28). Of the five Sfrp family members (Sfrp1 to Sfrp5), Sfrp1, Sfrp2, and Sfrp5 comprise Sfrp1 subfamily due to their sequence similarities (28). The phenotypes of single knock-out mice null for each Sfrp1, Sfrp2, or Sfrp5, were viable due to redundancy in their functions. The double or triple Sfrp knock-out mice were resulted in a lethal embryonic phenotype with reduction of anterior-posterior patterning (29, 30). Genetic and biochemical analyses revealed that Sfrp1/2/5 regulated Wnt/β-catenin and the Wnt/PCP pathways (30–32). Recently, some reports showed that Sfrp1 functioned as a mediator of senescence (33) and dysregulated glucose metabolism (34). However, little is still known about Sfrp1 function in the pathological events.
In the present study, we investigated the Sfrp1 function in the injured kidney. In a model of kidney injury, using the unilateral ureteral obstruction (UUO) model, the expression of Sfrp1 protein was increased. The kidneys from Sfrp1 knock-out mice showed significant increase in the renal fibrosis. Our results suggest that Sfrp1 is required for inhibition of renal tubulointerstitial fibrosis.
EXPERIMENTAL PROCEDURES
Mice
Sfrp1-deficient mice were maintained 129 and C57BL/6 mixed genetic background (29) and backcrossed for more than five generations onto C57BL/6 background.
Renal fibrosis was induced by ligation of the left ureter in male mice (35). Sfrp1+/+ and Sfrp1−/− mice (n = 3 per group) were used at 3, 7, and 14 days after the operation. The obstructed (UUO) and non-obstructed (Sham) kidneys were collected carefully and subjected to the analyses.
Cell Culture and Transfection
293T cells were grown in DMEM supplemented with 10% FBS. Mammalian expression vectors for Sfrp1-FLAG was constructed by insertion into pcDNA3 vectors (Invitrogen). Transfection was performed using Lipofectamine reagent (Invitrogen).
Preparation of Recombinant Proteins
His-tagged (for production of mouse Sfrp1, Sfrp2, and Sfrp5 antibodies) and MBP-tagged (for immunoblotting) Sfrp1–5 were expressed in BL21-CodonPlus-RP (Agilent Technologies, Santa Clara, CA) transformed with pET-28a (Invitrogen) and pMAL (New England Biolabs), respectively. Each His or MBP fusion protein was purified through affinity chromatography with TALON metal affinity resin (Clontech) or with amylose resin (New England Biolabs), respectively.
Antibody
We produced mouse monoclonal Sfrp1, rat monoclonal Sfrp2, and rat monoclonal Sfrp5 antibodies, as described previously (36).
The antibodies against the following proteins: vimentin (Progen, Heidelberg, Germany); Ca2+/calmodulin-dependent kinase II (EP1829Y), phospho-Smad3 (EP823Y), actinin 4 (EPR2533, Epitomics, Burlingame, CA); E-cadherin (no. 3195), active-β-catenin (no. 8814), Cyclin D1 (DCS6; no. 2926), phospho-JNK (no. 4668), JNK (no. 9252), phospho-Ca2+/calmodulin-dependent kinase II (no. 12716), Smad3 (no. 9523) phospho-c-Jun (no. 9261), c-Jun (no. 9165), phospho-p38 (no. 4511), p38 (no. 8960; Cell Signaling Technology); β-catenin (BD Transduction Laboratories), FLAG (M2), αSMA (1A4), phospho-histone H3 (Ser-10; Sigma), c-Myc (sc-764; Santa Cruz Biotechnology, Santa Cruz, CA), and MBP (New England Biolabs).
Tissue Extract Preparation and Immunoblotting
Mouse kidneys were homogenized directly in a SDS-PAGE sample buffer. Protein concentrations for cell extracts were determined by the Coomassie Brilliant Blue staining by SDS-PAGE gels. The lysates were loaded, transferred, and subjected to Western blotting with specific antibodies.
Histology and Immunohistochemistry
Mouse kidneys were fixed with 4% paraformaldehyde/PBS overnight at 4 °C, and embedded in paraffin. Three-μm-thick sections were prepared and mounted. Some slides were stained with hematoxylin and eosin. For immunohistochemistry, the slides were deparaffinized, and endogenous peroxidase was inactivated in 3% H2O2 in methanol for 30min, treated with 10 mm citrate buffer (pH 6.0) in a microwave for 15 min, and blocked in 5% serum in TBST for 1 h. Sections were incubated with primary antibodies overnight at 4 °C and then with appropriate biotinylated secondary antibodies (VECTOR Laboratories, Burlingame, CA) for 1 h at room temperature. The detection was carried out by using the VECTASTAIN ABC KIT and diaminobenzidine regent (VECTOR Laboratories).
The areas positive for αSMA, vimentin, E-cadherin, and actinin 4 were quantified by ImageJ software. Statistical significance, which was evaluated using Welch's t test, was defined as p < 0.05. Error bars indicate S.D.
TUNEL Assay
Apoptosis in the sham and UUO kidneys was assayed using the ApopTag Plus peroxidase kit (Chemicon, Temecula, CA) as described previously (37).
Ethics Statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal works were approved appropriately by the Shigei Medical Research Institute.
RESULTS
Sfrp1 Protein Is Increased in the Obstructed Kidney After UUO
To investigate the Sfrp1 function of the kidney disease, we produced monoclonal antibodies that specifically recognize Sfrp1 protein. Immunoblot analysis revealed that the monoclonal anti-Sfrp1 antibody immunoreacted specifically with a band corresponding to the position of the similar molecular weights in 293T cell lysates expressing mouse Sfrp1 (Fig. 1A). To explore the specificity of monoclonal anti-Sfrp1 antibody, Western blotting was performed using recombinant Sfrp proteins (mouse Sfrp1–5). As shown in Fig. 1B, this antibody was only reacted with Sfrp1, but not with other Sfrp proteins.
FIGURE 1.
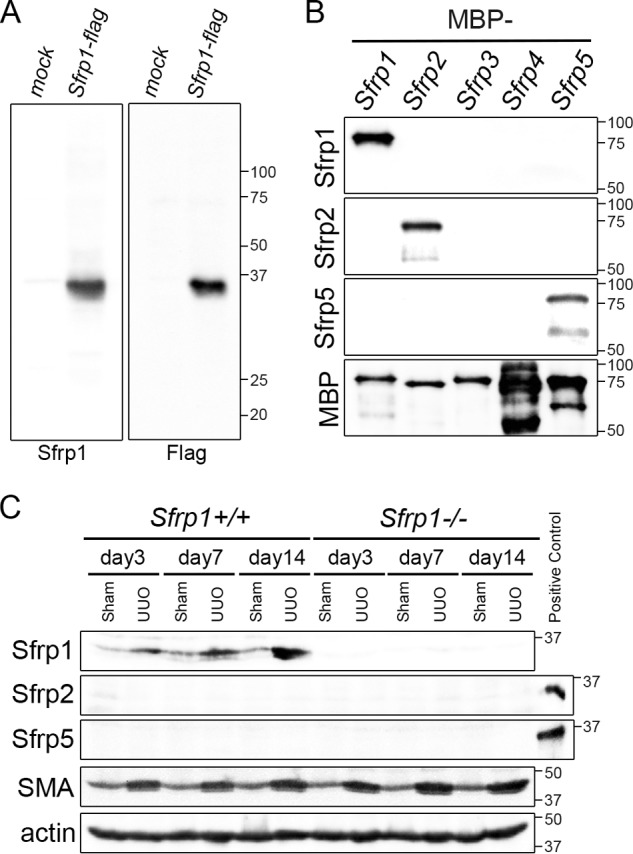
Sfrp1 increases after unilateral ureteral obstruction (UUO). A, the Sfrp1 antibody reacted specifically with a band corresponding to Sfrp1 in lysates of Sfrp1-FLAG-expressed 293T cells, but not 293T (mock) cells by Western blotting. B, characterization of an antibody specifically recognized Sfrp1, Sfrp2, and Sfrp5. Immunoreactivity was impaired specifically with Sfrp1, but not with other Sfrp protein families (Sfrp2–5). The Sfrp2 and Sfrp5 antibodies were also immunoreacted specifically with Sfrp2 and Sfrp5 proteins, respectively. C, the levels of the Sfrp1, Sfrp2, and Sfrp5 proteins were detected by Western blot analysis in the Sham and UUO kidneys in wild-type mice (Sfrp1+/+) or Sfrp1 knock-out mice (Sfrp1−/−) at different time points after UUO. Actin was evaluated as an internal control. His-Sfrp2 and His-Sfrp5 were used as a positive control to detect Sfrp2 and Sfrp5 proteins, respectively.
It is previously reported that Sfrp1 was highly expressed in the kidney of the newborn (38, 39). To determine whether Sfrp1 protein was changed after kidney damage, we performed Western blot analyses in the obstructed kidneys of wild-type and Sfrp1-deficient mice. After UUO, we found that Sfrp1 protein was increased at different time points in the obstructed kidneys (Fig. 1C). There were no Sfrp1 bands detectable in the sham-operated and UUO Sfrp1−/− kidney lysates when using the Sfrp1 antibody (Fig. 1C).
In previous reports, Sfrp1, Sfrp2, and Sfrp5 have been suggested on the basis of the similarity of their expression patterns during embryogenesis (29, 30). To examine the extent of functional redundancy between three Sfrp members, we investigated Sfrp1−/− UUO kidneys with graded levels of Sfrp2 and Sfrp5 protein expressions. Deletion of Sfrp1 did not result in a compensatory increase of the remaining gene product, Sfrp2 and Sfrp5 proteins (Fig. 1C).
Loss of Sfrp1 Is Associated with Increased Renal Fibrosis After UUO
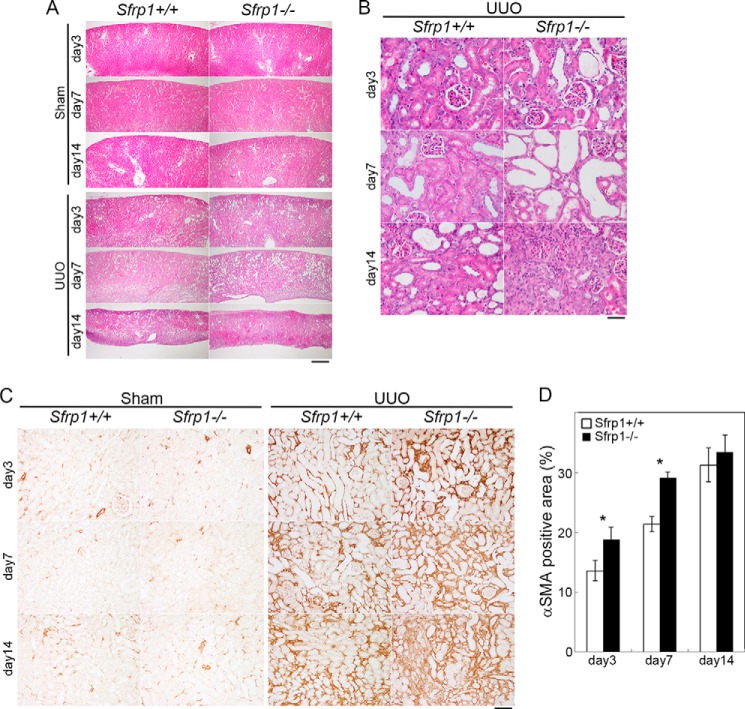
In the UUO injury model, Sfrp1 protein levels were significantly increased in the obstructed kidney. To analyze whether loss of Sfrp1 exacerbated the progression of fibrosis, UUO in the Sfrp1 knock-out mice was performed in the obstructed kidneys of Sfrp1+/+ and Sfrp1−/− mice. The kidney sections from sham-operated and UUO mice were stained with hematoxylin and eosin. In the wild-type (Sfrp1+/+) UUO-operated kidneys, fibrotic lesion in the cortex was observed after the surgery. In the heterozygous (Sfrp1+/−) UUO kidneys, there was no detectable difference of renal fibrosis compared with that of wild-type (data not shown). In contrast, the development of tubulointerstitial injury was progressively deteriorated in the homozygous UUO kidney (Fig. 2, A and B). Immunohistochemical analyses also revealed that the expression of αSMA, a marker of myofibroblast, was increased in the UUO kidneys (Fig. 2C). The αSMA-positive area was significantly higher in the Sfrp1-deficient kidneys compared those with wild-type kidneys at days 3 and 7 after UUO (Fig. 2, C and D).
FIGURE 2.
Loss of Sfrp1 exacerbates the progression of renal fibrosis after UUO. A and B, representative microscopic images of the sham and UUO kidneys in wild-type (Sfrp1+/+) and Sfrp1-deficient mice (Sfrp1−/−). These tissue sections were prepared and stained with hematoxylin and eosin. B, higher magnification is shown in A. C, αSMA immunostaining in the obstructed kidneys. D, graph shows analysis of the percentage of αSMA-positive area in the UUO kidneys. Scale bars, 500 μm (A), 50 μm (B), 100 μm (C). *, p < 0.05.
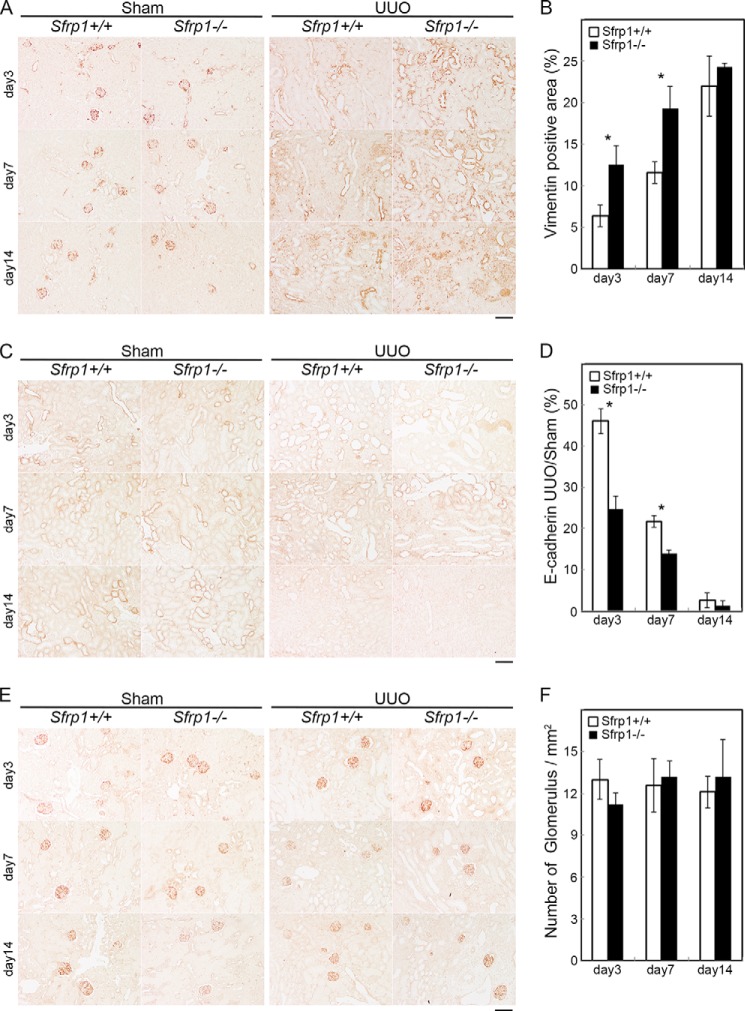
To further explore the effects of Sfrp1 disruption in the obstructed kidney, we examined the expression of vimentin and E-cadherin after UUO. Immunohistochemical analyses showed the expression level of vimentin was increased in the damaged kidneys from Sfrp1−/− mice when compared with those of Sfrp1+/+ littermates (Fig. 3, A and B). In contrast, the expression level of E-cadherin was decreased (Fig. 3, C and D). Actinin 4, a marker of renal glomerulus, the expression was not changed (Fig. 3, E and F). These results indicate that Sfrp1 maintain renal tubular epithelial cells during the fibrosis.
FIGURE 3.
Sfrp1-deficient mice are enhanced epithelial-to-mesenchymal transition after UUO. A, C, and E, immunohistochemical analyses of the mouse kidney sections with antibodies against vimentin (A), E-cadherin (C), and actinin 4 (E) in the sham and UUO kidneys after UUO. B, graph shows analysis of the percentage of vimentin-positive area in the UUO kidneys. D, the E-cadherin expression levels are given as a percentage of the UUO to sham-operated kidneys. F, renal glomerulus is unchanged in the obstructed Sfrp1−/− kidneys by counting actinin 4-positive glomerulus. Scale bars, 100 μm. *, p < 0.05.
Canonical Wnt/β-catenin Pathway in the Obstructed Sfrp1−/−Kidneys
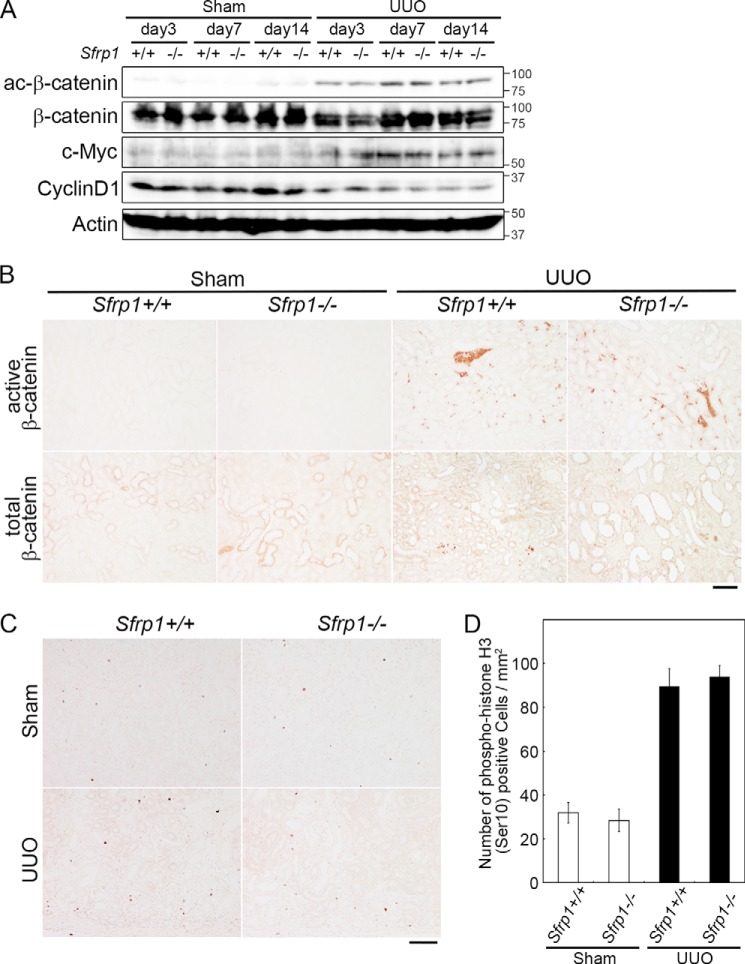
In the previous study, Sfrp regulated canonical and non-canonical Wnt pathway (30). We studied which Wnt pathway was regulated by Sfrp1 in the UUO kidney. As described previously (20), the canonical Wnt/β-catenin pathway was up-regulated after UUO. To investigate whether Sfrp1 protein during kidney damage-modulated canonical Wnt signaling, we performed Western blotting and immunohistochemistry of the UUO kidneys for measuring the amount of active β-catenin that is not phosphorylated on both Ser-37 and Thr-41 (40). The activity levels were not altered in the obstructed Sfrp1−/− kidneys in comparison with those in the Sfrp+/+ kidneys (Fig. 4, A and B).
FIGURE 4.
Wnt/β-catenin pathway in the Sfrp deficient obstructed kidneys. A, detection of active- and total β-catenin, c-Myc, and cyclin D1 by Western blotting of the UUO kidneys. Actin was evaluated as an internal control. B, immunohistochemical analyses of mouse kidney sections with antibodies against active and total β-catenin in the sham and UUO kidneys at 7 days after the surgery. C and D, number of phospho-HistonH3 (Ser-10) cells in the obstructed kidneys at 7 days after UUO. C, section of the Sham and UUO kidneys in Sfrp+/+ and Sfrp−/− mice were subjected to immunostaining with an anti-phospho-histone H3 (Ser-10) antibody. D, graph shows analysis of the percentage of phospho-histone H3 (Ser-10)-positive cells in the UUO kidneys. Scale bars, 100 μm.
We then observed the protein expression of several target genes of the Wnt/β-catenin pathway in the obstructed kidney. Western blot analyses showed that expression levels of the target genes, c-Myc and cyclin D1, were not altered (Fig. 4A). Furthermore, we determined whether cell proliferation ratio was changed in the Sfrp1−/− obstructed kidneys. No difference was immunohistochemically found in cell proliferation by counting phospho-histone H3-positive cells (Fig. 4, C and D). These data indicate that canonical Wnt/β-catenin pathway did not alter in the obstructed Sfrp1−/− kidneys.
Non-canonical Wnt/PCP Pathway Modulates the Maintenance of Renal Fibrosis
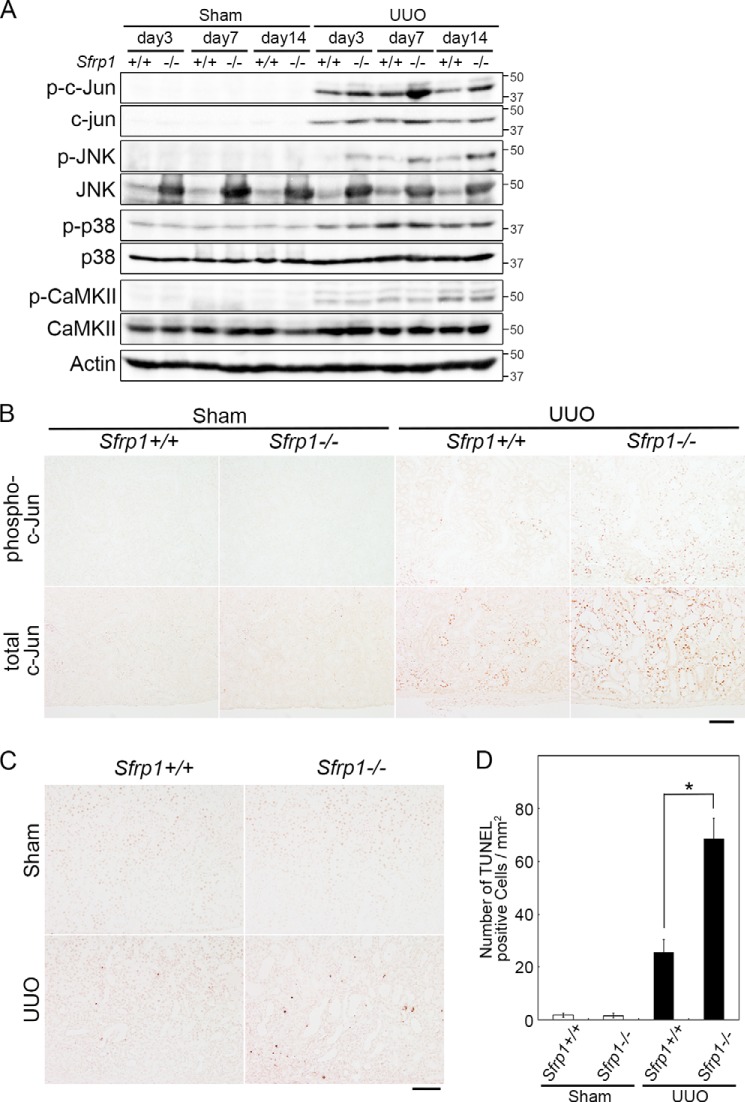
To study whether the non-canonical Wnt pathway was affected in the obstructed kidney of Sfrp1-deficient mice, we investigated activation of several putative mediators of the signaling (Fig. 5A). The JNK signaling pathway (Wnt/PCP pathway) is one branch of non-canonical Wnt pathway. We also performed Western blotting and immunohistochemistry of the UUO kidneys for measuring the levels of phospho- and total c-Jun, a downstream of JNK. We found that the levels of phospho- and total c-Jun were increased in the obstructed Sfrp1−/− kidneys (Fig. 5, A and B). In addition, we detected increased phospho-JNK levels by Western blotting (Fig. 5A). Surprisingly, the levels of total JNK were increased in Sham and UUO Sfrp1−/− kidneys (Fig. 5A).
FIGURE 5.
Non-canonical Wnt pathway in the Sfrp-deficient obstructed kidneys. A, detection of phospho- and total c-Jun, phospho- and total JNK, phospho- and total p38, and phospho- and total Ca2+/calmodulin-dependent kinase II (CaMKII) by Western blotting of the UUO kidneys. Actin was evaluated as an internal control. B, immunohistochemical analyses of mouse kidney sections with antibodies against phospho- and total c-Jun in the sham and UUO kidneys at 7 days after the surgery. C and D, number of TUNEL-positive cells in the obstructed kidneys at 7 days after UUO. C, section of the sham and UUO kidneys in Sfrp+/+ and Sfrp−/− mice were subjected to TUNEL staining. D, graph shows analysis of the percentage of TUNEL-positive cells in the UUO kidneys. Scale bars, 100 μm. *, p < 0.05.
Next, the calcium-dependent signaling pathway (Wnt/Ca2+ pathway) is a second branch of the non-canonical Wnt pathway. So, we asked whether the phosphorylation levels of Ca2+/calmodulin-dependent kinase II, which was activated in Wnt/Ca2+ pathway-mediated responses, was regulated. Western blot analyses indicated that this activity was unaltered in the obstructed Sfrp1−/− kidneys (Fig. 5A).
The activation of c-Jun was reported to regulate the diverse biological functions, including apoptosis, invasion, and metastasis, and cell polarity as well as non-canonical Wnt signaling. To evaluate the effect of apoptosis, we performed a TUNEL assay in the Sfrp1−/− obstructed kidneys. The numbers of TUNEL-positive apoptotic cells increased in the UUO kidneys of Sfrp1-deficient mice, compared with that of wild-type mice (Fig. 5, C and D). However, the level of phospho-p38, which regulates the apoptosis signal similar to the c-Jun pathway, showed no difference between the wild-type and homozygous mutant (Fig. 5A).
Finally, we focused the relationship between Wnt and TGF-β signaling in the Sfrp1-mediated UUO kidneys. To explore whether the TGF-β was affected in the obstructed kidney of Sfrp1-deficient mice, we examined Western blotting of the UUO kidneys for measuring the levels of phospho-Smad3, which are the most critical mediators in TGF-β signaling pathway. We found that the phosphorylation levels were not altered in the obstructed Sfrp1−/− kidneys in comparison with those in the Sfrp+/+ kidneys (Fig. 6). Thus, these observations suggest that the non-canonical Wnt/PCP pathway modulates the maintenance of renal fibrosis after kidney damage.
FIGURE 6.

TGF-β signaling is not altered in the obstructed Sfrp−/− kidneys. Detection of phospho- and total Smad3 by Western blotting of the UUO kidneys. Actin was evaluated as an internal control.
DISCUSSION
The present study demonstrated that Sfrp1 protein was increased in the obstructed kidney, and inactivation of Sfrp1 was associated with increased renal fibrosis during unilateral ureteral obstruction. Sfrp1 knock-out mice indicated that the renal tubules were disrupted, and the numbers of apoptotic cells were increased after UUO. Furthermore, phosphorylated c-Jun levels were significantly elevated in the obstructed Sfrp1−/− kidneys, indicated that Sfrp1 controlled renal damage through the non-canonical Wnt/PCP pathway.
It is well established that Wnt signaling is silenced in adult tissues (41, 42); however, it is activated in the injured tissues due to the progression of diseases (19, 20). The recent study showed that Wnt4 might be dispensable for myofibroblast transformation in the damaged kidney (18). In addition, Dkk-1 (Dickkopf-related protein 1), a ligand for Wnt antagonist, controls the myofibroblast progression of renal fibrosis (17). These studies indicate that Wnt/β-catenin signaling is important for the maintenance of kidney damage. Indeed, the amount of active β-catenin increases during progression of renal tubulointerstitial fibrosis (20). Dkk-1 blocks the increase of Wnt/β-catenin signaling, resulted in the reduction of kidney fibrosis (17). In the previous reports, recombinant Sfrp4, a secreted antagonist of Wnt signaling, also reduced the number of myofibroblasts and active β-catenin, indicated that Sfrp4 was able to interfere with kidney fibrosis through Wnt/β-catenin signaling (20). In this study, we demonstrated that loss of Sfrp1 led expansion of renal fibrosis in the obstructed kidney. However, the activity of β-catenin did not alter in the obstructed Sfrp1−/− kidneys. Distinct difference in the responses of Wnt/β-catenin signaling pathway might be due to the following possibilities.
Previous data of Ren et al. (17) suggested that Dkk-1 overexpression inhibited cell proliferation in the obstructed kidney after UUO. Notably, Wnt/β-catenin pathway is implicated in regulating cell proliferation. In contrast, our results indicated that cell proliferation was not altered in the obstructed kidneys between mouse genotypes. Alternatively, the numbers of apoptotic cells were increased in the UUO kidneys of Sfrp1-deficient mice. Renal tubular apoptosis causes progressive kidney disease such as fibrosis (43). The p38 mitogen-activated protein kinase (MAPK) cascade and JNK cascade play a critical role in renal fibrosis (44). Upon kidney damage, the levels of JNK and p38 phosphorylation are increased in UUO (43, 44).
In this study, we found that the levels of phosphorylated c-Jun and JNK were increased in the obstructed Sfrp1−/− kidneys. This observation is consistent with a recent report that in small intestine of Sfrp1−/−;Sfrp2−/−;Sfrp5+/− mice, phospho-c-Jun levels were elevated significantly in the epithelium in comparison with the control small intestine epithelium (31). Moreover, the Wnt/PCP pathway does not lead to β-catenin stabilization but activates JNK/c-Jun signaling thorough Frizzled and Dishevelled (31). Thus, it is understandable that Sfrp1 regulation of Wnt/PCP signaling controls renal interstitial fibrosis among chronic renal diseases. In recent study, Wnt11 signaling by TGF-β was activated in the renal fibrosis through the non-canonical Wnt/PCP pathway (45). We found no evidence that the levels of phospho-Smad3 altered in the obstructed Sfrp1−/− kidneys. However, further studies are needed to draw a definite conclusion concerning contribution of Wnt/PCP cascade to kidney pathology.
In conclusion, we identified that Sfrp1 regulated the progression of renal fibrosis in mouse unilateral ureteral obstruction. The relationship between kidney damage and the Wnt/non-canonical pathway definitely opens a new field to study mechanisms of renal diseases. Sfrp1 is a reliable candidate for the anti-fibrogenesis drug; however, further studies are needed to understand the underlying molecular mechanism(s).
Acknowledgements
We are grateful to Chieko Takahashi for technical assistance and Dr. Tohru Okigaki for critical comments on the manuscript. We thank Dr. Fumihiro Shigei, Chairman of the Board, for financial support.
This work was supported in part by the Ryobi Teien Memory Foundation.
- PCP
- planar cell polarity
- Sfrp
- secreted Frizzled-related protein
- UUO
- unilateral ureteral obstruction
- αSMA
- α-smooth muscle actin
- DKK-1
- Dickkopf-related protein 1.
REFERENCES
- 1. Zeisberg M., Neilson E. G. (2010) Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 21, 1819–1834 [DOI] [PubMed] [Google Scholar]
- 2. Farris A. B., Colvin R. B. (2012) Renal interstitial fibrosis: mechanisms and evaluation. Curr. Opin. Nephrol. Hypertens. 21, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boor P., Ostendorf T., Floege J. (2010) Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 6, 643–656 [DOI] [PubMed] [Google Scholar]
- 4. Tampe D., Zeisberg M. (2014) Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 10, 226–237 [DOI] [PubMed] [Google Scholar]
- 5. Meran S., Steadman R. (2011) Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 92, 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grande M. T., López-Novoa J. M. (2009) Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol. 5, 319–328 [DOI] [PubMed] [Google Scholar]
- 7. Kriz W., Kaissling B., Le Hir M. (2011) Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 121, 468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawakami T., Ren S., Duffield J. S. (2013) Wnt signaling in kidney diseases: dual roles in renal injury and repair. J. Pathol. 229, 221–231 [DOI] [PubMed] [Google Scholar]
- 9. Liu Y. (2010) New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 21, 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou D., Tan R. J., Zhou L., Li Y., Liu Y. (2013) Kidney tubular β-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci. Rep. 3, 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Böttinger E. P., Bitzer M. (2002) TGF-β signaling in renal disease. J. Am. Soc. Nephrol. 13, 2600–2610 [DOI] [PubMed] [Google Scholar]
- 12. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell. Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 13. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 14. Moon R. T., Kohn A. D., De Ferrari G. V., Kaykas A. (2004) WNT and β-catenin signaling: diseases and therapies. Nat. Rev. Genet. 5, 691–701 [DOI] [PubMed] [Google Scholar]
- 15. Huang H., He X. (2008) Wnt/β-catenin signaling: new (and old) players and new insights. Curr. Opin. Cell Biol. 20, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macdonald B. T., Semenov M. V., He X. (2007) SnapShot: Wnt/beta-catenin signaling. Cell 131, 1204. [DOI] [PubMed] [Google Scholar]
- 17. Ren S., Johnson B. G., Kida Y., Ip C., Davidson K. C., Lin S. L., Kobayashi A., Lang R. A., Hadjantonakis A. K., Moon R. T., Duffield J. S. (2013) LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc. Natl. Acad. Sci. U.S.A. 110, 1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DiRocco D. P., Kobayashi A., Taketo M. M., McMahon A. P., Humphreys B. D. (2013) Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J. Am. Soc. Nephrol. 24, 1399–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He W., Dai C., Li Y., Zeng G., Monga S. P., Liu Y. (2009) Wnt/β-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Surendran K., Schiavi S., Hruska K. A. (2005) Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 16, 2373–2384 [DOI] [PubMed] [Google Scholar]
- 21. Veeman M. T., Axelrod J. D., Moon R. T. (2003) A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 5, 367–377 [DOI] [PubMed] [Google Scholar]
- 22. Semenov M. V., Habas R., Macdonald B. T., He X. (2007) SnapShot: noncanonical Wnt signaling pathways. Cell 131, 1378. [DOI] [PubMed] [Google Scholar]
- 23. Finch P. W., He X., Kelley M. J., Uren A., Schaudies R. P., Popescu N. C., Rudikoff S., Aaronson S. A., Varmus H. E., Rubin J. S. (1997) Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc. Natl. Acad. Sci. U.S.A. 94, 6770–6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melkonyan H. S., Chang W. C., Shapiro J. P., Mahadevappa M., Fitzpatrick P. A., Kiefer M. C., Tomei L. D., Umansky S. R. (1997) SARPs: a family of secreted apoptosis-related proteins. Proc. Natl. Acad. Sci. U.S.A. 94, 13636–13641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cruciat C. M., Niehrs C. (2013) Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 5, a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawano Y., Kypta R. (2003) Secreted antagonists of the Wnt signaling pathway. J. Cell Sci. 116, 2627–2634 [DOI] [PubMed] [Google Scholar]
- 27. Mii Y., Taira M. (2011) Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev. Growth Differ. 53, 911–923 [DOI] [PubMed] [Google Scholar]
- 28. Bovolenta P., Esteve P., Ruiz J. M., Cisneros E., Lopez-Rios J. (2008) Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121, 737–746 [DOI] [PubMed] [Google Scholar]
- 29. Satoh W., Gotoh T., Tsunematsu Y., Aizawa S., Shimono A. (2006) Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development 133, 989–999 [DOI] [PubMed] [Google Scholar]
- 30. Satoh W., Matsuyama M., Takemura H., Aizawa S., Shimono A. (2008) Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/β-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis 46, 92–103 [DOI] [PubMed] [Google Scholar]
- 31. Matsuyama M., Aizawa S., Shimono A. (2009) Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet. 5, e1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsuyama M., Shimono A. (2012) The embryonic mouse gut tube as a model for analysis of epithelial polarity. Methods Mol. Biol. 839, 229–237 [DOI] [PubMed] [Google Scholar]
- 33. Elzi D. J., Song M., Hakala K., Weintraub S. T., Shiio Y. (2012) Wnt antagonist SFRP1 functions as a secreted mediator of senescence. Mol. Cell Biol. 32, 4388–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gauger K. J., Bassa L. M., Henchey E. M., Wyman J., Bentley B., Brown M., Shimono A., Schneider S. S. (2013) Mice deficient in sfrp1 exhibit increased adiposity, dysregulated glucose metabolism, and enhanced macrophage infiltration. PLoS One 8, e78320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chevalier R. L., Forbes M. S., Thornhill B. A. (2009) Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 36. Sado Y., Inoue S., Tomono Y., Omori H. (2006) Lymphocytes from enlarged iliac lymph nodes as fusion partners for the production of monoclonal antibodies after a single tail base immunization attempt. Acta Histochem. Cytochem. 39, 89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kusaka M., Katoh-Fukui Y., Ogawa H., Miyabayashi K., Baba T., Shima Y., Sugiyama N., Sugimoto Y., Okuno Y., Kodama R., Iizuka-Kogo A., Senda T., Sasaoka T., Kitamura K., Aizawa S., Morohashi K. (2010) Abnormal epithelial cell polarity and ectopic epidermal growth factor receptor (EGFR) expression induced in Emx2 KO embryonic gonads. Endocrinology 151, 5893–5904 [DOI] [PubMed] [Google Scholar]
- 38. Yoshino K., Rubin J. S., Higinbotham K. G., Uren A., Anest V., Plisov S. Y., Perantoni A. O. (2001) Secreted Frizzled-related proteins can regulate metanephric development. Mech. Dev. 102, 45–55 [DOI] [PubMed] [Google Scholar]
- 39. Trevant B., Gaur T., Hussain S., Symons J., Komm B. S., Bodine P. V., Stein G. S., Lian J. B. (2008) Expression of secreted frizzled related protein 1, a Wnt antagonist, in brain, kidney, and skeleton is dispensable for normal embryonic development. J. Cell Physiol. 217, 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Noort M., Meeldijk J., van der Zee R., Destree O., Clevers H. (2002) Wnt signaling controls the phosphorylation status of β-catenin. J. Biol. Chem. 277, 17901–17905 [DOI] [PubMed] [Google Scholar]
- 41. Major M. B., Camp N. D., Berndt J. D., Yi X., Goldenberg S. J., Hubbert C., Biechele T. L., Gingras A. C., Zheng N., Maccoss M. J., Angers S., Moon R. T. (2007) Wilms tumor suppressor WTX negatively regulates WNT/β-catenin signaling. Science 316, 1043–1046 [DOI] [PubMed] [Google Scholar]
- 42. He X. (2008) Cilia put a brake on Wnt signaling. Nat. Cell Biol. 10, 11–13 [DOI] [PubMed] [Google Scholar]
- 43. Ma F. Y., Flanc R. S., Tesch G. H., Han Y., Atkins R. C., Bennett B. L., Friedman G. C., Fan J. H., Nikolic-Paterson D. J. (2007) A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J. Am. Soc. Nephrol. 18, 472–484 [DOI] [PubMed] [Google Scholar]
- 44. Stambe C., Atkins R. C., Tesch G. H., Masaki T., Schreiner G. F., Nikolic-Paterson D. J. (2004) The role of p38α mitogen-activated protein kinase activation in renal fibrosis. J. Am. Soc. Nephrol. 15, 370–379 [DOI] [PubMed] [Google Scholar]
- 45. Zhang P., Cai Y., Soofi A., Dressler G. R. (2012) Activation of Wnt11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells. J. Biol. Chem. 287, 21290–21302 [DOI] [PMC free article] [PubMed] [Google Scholar]