Background: β-Glucan receptor Dectin-1 in dendritic cells and macrophages plays important roles in antifungal immunity.
Results: Dectin-1 is expressed in rat mast cells, and its tyrosine phosphorylation induces characteristic gene expression of transcription factors and cytokines through protein-tyrosine kinase Syk.
Conclusion: Dectin-1 functions in rat mast cells.
Significance: Dectin-1-mediated signaling in mast cells may contribute to antifungal immunity.
Keywords: Fungi, Host Defense, Innate Immunity, Mast Cell, Phosphotyrosine Signaling, Signal Transduction, Dectin-1, Syk
Abstract
Dectin-1 recognizes β-glucan and plays important roles for the antifungal immunity through the activation of spleen tyrosine kinase (Syk) in dendritic cells or macrophages. Recently, expression of Dectin-1 was also identified in human and mouse mast cells, although its physiological roles were largely unknown. In this report, rat mast cell line RBL-2H3 was analyzed to investigate the molecular mechanism of Dectin-1-mediated activation and responses of mast cells. Treatment of cells with Dectin-1-specific agonist curdlan induced tyrosine phosphorylation of cellular proteins and the interaction of Dectin-1 with the Src homology 2 domain of Syk. These responses depended on tyrosine phosphorylation of the hemi-immunoreceptor tyrosine-based activation motif in the cytoplasmic tail of Dectin-1, whereas they were independent of the γ-subunit of high-affinity IgE receptor. DNA microarray and real-time PCR analyses showed that Dectin-1-mediated signaling stimulated gene expression of transcription factor Nfkbiz and inflammatory cytokines, such as monocyte chemoattractant protein-1, IL-3, IL-4, IL-13, and tumor necrosis factor (TNF)-α. The response was abrogated by pretreatment with Syk inhibitor R406. These results suggest that Syk is critical for Dectin-1-mediated activation of mast cells, although the signaling differs from that triggered by FcϵRI activation. In addition, these gene expressions induced by curdlan stimulation were specifically observed in mast cells, suggesting that Dectin-1-mediated signaling of mast cells offers new insight into the antifungal immunity.
Introduction
Fungal infections are a major health threat and pose clinical problems due to increasing numbers of immunocompromised hosts because of the increase in immunosuppressive diseases, such as AIDS, and immunosuppressive therapies against chronic inflammatory diseases, autoimmune diseases, cancers, and transplant rejections. Antifungal immunity thus grows increasingly important and is initiated by recognition of fungal pathogens with innate immune receptors. Pattern recognition receptors, such as Toll-like receptors (TLRs)2 and C-type lectin receptors, play important roles in the innate antimicrobial immunity by recognition of pathogen-associated molecular patterns, including carbohydrates, lipids, nucleic acids, and proteins. β-Glucan, a major carbohydrate component of the fungal cell wall along with mannans and chitin, is known as a pathogen-associated molecular pattern recognized by Dectin-1.
Dectin-1 is a type II transmembrane receptor that recognizes β-glucan. Dectin-1 was first identified by Ariizumi et al. (1) and originally thought to be a DC-specific receptor, from which its name “dendritic cell-associated C-type lectin-1” was derived. However, the receptor is now known to be expressed by many other cell types, including macrophages, monocytes, neutrophils, and T cells (2, 3). Especially in DCs and macrophages, Dectin-1-mediated mechanisms of antifungal immunity have been studied. The following reviews the facts brought out by prior studies in DCs and macrophages.
Dectin-1 is composed of four domains, the carbohydrate recognition domain, stalk domain, transmembrane domain, and cytoplasmic domain, which possesses the hemi-immunoreceptor tyrosine-based activation motif (hemITAM). Alternative splicing produces two major isoforms, which vary by the inclusion or exclusion of the stalk region in rats (4), mice (5), and humans (6) (although Dectin-1 in humans has eight splicing variants in total). The carbohydrate recognition domain of Dectin-1 specifically recognizes soluble and particle β(1→3)- and β(1→6)-linked glucan (2). In contrast to classic Ca2+-dependent C-type lectin receptors, the carbohydrate recognition domain of Dectin-1 can recognize carbohydrate in a Ca2+-independent manner (2). Dectin-1 also recognizes impure particulate β-glucan zymosan, a stimulatory cell wall extract of Saccharomyces cerevisiae that is composed mainly of β-glucan but also mannan, chitin, protein, and lipid (7). A large number of receptors have been implicated in the recognition of zymosan, including mannose receptor (8), complement receptor 3 (9, 10), Dectin-1 (2), and TLR2 (11). Therefore, analysis using zymosan does not reflect the independent molecular mechanisms of Dectin-1, whereas zymosan acts as an ideal model of a complex microorganism displaying several pathogen-associated molecular patterns. Curdlan consists of purified β(1→3)-glucan polymer from Alcaligenes faecalis; therefore, curdlan was utilized as a specific agonist of Dectin-1 (12) in order to investigate the independent molecular mechanism of Dectin-1 in this study.
Upon ligand binding, hemITAM of Dectin-1 is phosphorylated by Src family protein-tyrosine kinases and recruits spleen tyrosine kinase (Syk) (10), which initiates a signaling cascade leading to nuclear factor-κB (NF-κB) (13, 14), nuclear factor of activated T-cells (NFAT) (15, 16), and MAPK activation (17–19). Traditional ITAM sequences, such as those found in Fc receptors, consist of a tandem repeat of YXX(I/L) sequences (where X is any amino acid), which, upon ligand binding and receptor clustering, become tyrosine-phosphorylated by Src kinases. In contrast to this, Dectin-1 has a single ITAM motif termed the hemITAM, and phosphorylation of this hemITAM sequence is sufficient to mediate the interaction with Syk (which normally requires two phosphotyrosines for binding) through an unknown mechanism (10, 20, 21). Syk kinase has two SH2 domains in tandem, which bind to specific phosphorylated tyrosine residues in protein and result in the assembly of signaling complexes (22). In the previous study using recombinant N-terminal (Syk-SH2(N)), C-terminal (Syk-SH2(C)), and tandem SH2 (Syk-SH2(NC)) domains of Syk to precipitate C-type lectin-like receptor 2 (CLEC2), both SH2 domains of Syk were required for binding and signaling downstream of CLEC2. This suggests that the mechanism of the binding of Syk to Dectin-1 is similar to that of CLEC2, because CLEC2 is a member of the C-type lectin receptor family and, like Dectin-1, possesses the hemITAM (23). Through the interaction with Syk, Dectin-1 activates a number of cellular responses, including phagocytosis (21) and reactive oxygen species production (21, 24) and the production of various cytokines (IL-1, IL-2, IL-6, IL-10, IL-12, IL-22, and TNF-α) and chemokines (CCL17 and CCL22) (25), leading to antifungal immunity.
Mast cells are now known to be critical effectors of not only allergic disease but also host defense (26, 27). Recently, it has been reported that Dectin-1 is expressed in mouse and human mast cells, and its activation elicits leukotriene release, reactive oxygen species production, and Dectin-1 expression, indicating the relationship between mast cells and antifungal immunity (24, 28, 29). However, the signaling pathway and physiological roles of Dectin-1 in mast cells are still largely unknown. The purpose of this study is to investigate the molecular mechanism of Dectin-1-mediated activation and responses of mast cells in order to analyze how Dectin-1 in mast cells contributes to innate antifungal immunity.
EXPERIMENTAL PROCEDURES
Antibodies and Materials
Anti-Tyr(P) (clone 4G10) and anti-GAPDH mAbs and anti-Tyr(P) (clone 4G10)-agarose conjugate were purchased from Millipore (Bedford, MA). Anti-dinitrophenyl (DNP) IgE mAb (clone SPE-7) was obtained from Sigma. Anti-mouse Dectin-1/CLEC7A polyclonal antibody was from R&D Systems (Minneapolis, MN). Anti-phospho-ERK and anti-ERK polyclonal antibodies were from Cell Signaling Technology (Danvers, MA). Syk polyclonal antibody was raised against rat Syk-specific peptide (EPTGGAWGPDRGLC), as described previously (30). Anti-phospholipase Cγ2 (PLCγ2) antibody was from Santa Cruz Biotechnology, Inc. Protein A-agarose was from Sigma. Curdlan was from Wako (Osaka, Japan). Antigen DNP-BSA (30 mol of DNP, 1 mol of BSA) was from LSL (Tokyo, Japan). Syk inhibitor R406 was from Selleck Chemicals (Houston, TX). BAY61-3606, PD98059, BAY11-7082, and cyclosporin A were from Wako. Curdlan was prepared as described previously (12). Curdlan is insoluble at neutral pH; therefore, it was once solubilized with 0.15 m NaOH solution at 10 mg/ml and then added to the culture medium to neutralize pH by diluting more than 100-fold.
cDNAs
The mouse Dectin-1A cDNA was obtained as follows. Total RNA from RAW 264.7 cells was isolated by using the RNeasy minikit (Qiagen, Valencia, CA), and the first-strand cDNA was generated by using Superscript III (Invitrogen) with oligo(dT) primers, according to the manufacturer's instructions. The mouse Dectin-1A cDNA was subsequently amplified by PCR using following primers: 5′-CAAGTGCTCTGCCTACCTAGGGCCCTGT-3′ (forward) and 5′-CACCATCTTTATATTCTCACATACATTTACAGTTCCTT-3′ (reverse). The PCR product was subcloned into pGEM-T easy vector (Promega, Madison, WI), and DNA sequence was verified. The cDNA fragment was inserted into pcDNA3.1(−) Myc-His expression vector (Invitrogen) to add epitope tag at the C-terminal site. The cDNA fragment encoding Myc-His-tagged Dectin-1A was transferred into pSVL expression vector (GE Healthcare). Substitution of Tyr15 to Phe (Y15F) by a point mutation of Dectin-1A cDNA was generated by the QuikChange Lightning site-directed mutagenesis kit (Stratagene, La Jolla, CA) using two primers, 5′-GAGAATCFTGGATGAAGATGGATTTACTCAATTAGACTTCAGCAC-3′ (forward) and 5′-GTGCTGAAGTCTAATTGAGTAAATCCATCTTCATCCAGATTCTC-3′ (reverse). The resulting point mutation was confirmed by DNA sequencing. The mutant cDNA (Dectin-1AY15F) was then transferred into the pSVL vector.
Cell Culture and Transfections
Rat basophilic leukemia RBL-2H3 cells were maintained as monolayer cultures in DMEM with 100 units/ml penicillin and 10% (v/v) heat-inactivated FCS. For stable transfection, 20 μg of linearized expression constructs and 2 μg of pSV2-neo vector were cotransfected into 5 × 106 RBL-2H3 cells by electroporation (950 microfarads, 310 V) using GenePulserXcell (Bio-Rad) as described (31). Stably transfected cell lines were selected with 0.4 mg/ml active G418 (Nacalai Tesque, Kyoto, Japan). Cell lines were screened by the level of protein expression by the immunoblotting of detergent-soluble lysates with anti-Dectin-1 and anti-FcϵRIβ antibodies (a gift from Dr. Reuben P. Siraganian, National Institutes of Health).
Preparation of Cell Lysates, Immunoprecipitation, and Immunoblotting
106 cells were incubated without or with 100 μg/ml curdlan in the medium for the indicated periods of time or cultured overnight with anti-DNP IgE mAb (1:5000) for sensitization and stimulated with 300 ng/ml DNP-BSA for 10 min at 37 °C. In some experiments, cells were pretreated with Syk inhibitor R406 prior to the stimulation. After the stimulation, cells were washed with ice-cold PBS twice and solubilized in the lysis buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 10 mm EDTA, 100 mm NaF, 1 mm Na3VO4, 1 mm PMSF, and 2 μg/ml aprotinin) containing 1% Triton X-100. Preparation of detergent-soluble cell lysates, immunoprecipitation, and immunoblotting were performed as described previously (32–34).
FACS Analysis
Parental or Dectin-1-expressing RBL-2H3 cells were reacted with anti-Dectin-1 antibody or goat anti-mouse IgG antibody (Jackson Immunoresearch, West Grove, PA) as a negative control for 30 min at 4 °C. After washing with PBS, cells were stained with Alexa Fluor 488 F(ab′)2 fragment of rabbit anti-goat IgG (Invitrogen). Data from stained cells were acquired by FACScantoII (BD Biosciences) and analyzed with FlowJo software (FlowJo, LLC, Ashland, OR).
Pull-down Assay
The GST-rat Syk-SH2 (both N- and C-terminal SH2 domains) expression construct was a gift from Dr. Reuben P. Siraganian. 5 × 106 cells were incubated without or with 100 μg/ml curdlan in medium and were solubilized with the lysis buffer containing 1% Triton X-100. In vitro binding experiments were performed as described previously (32–34).
Microarray Analysis
106 cells were pretreated without or with R406 (2 μm) for 5 min and subsequently stimulated without or with 100 μg/ml of curdlan for 2 h. Total RNAs were isolated, and the qualities were evaluated by Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The sense strand cDNAs were generated using the Ambion WT expression kit (Invitrogen), and the synthesized cDNAs were subjected to fragmentation and labeling by the GeneChip WT terminal labeling kit (Affymetrix, Santa Clara, CA) according to the manufacturer's instructions. Hybridization with the GeneChip Rat Gene 1.0 ST array, scanning, and generation of probe cell intensity data were carried out using an Affymetrix fluidics station 450 and GeneChip Scanner 3000 7G using Affymetrix GeneChip Command Console software (Affymetrix). The data were imported into the Subio platform version 1.16 (Subio, Kagoshima, Japan) for database management and quality control. All samples were assayed in two different biological replicates. Raw data were published in the GEO database (accession code GSE56246).
Quantitative Real-time PCR
106 cells were seeded in 35-mm dishes and cultured overnight. Cells were pretreated without or with R406, PD98059, BAY11-7082, or cyclosporin A and subsequently stimulated without or with 100 μg/ml curdlan for 2 h at 37 °C. Total RNA was extracted using the High Pure RNA isolation kit (Roche Applied Science), and the first-strand cDNAs were prepared using Superscript III with random primers. Real-time PCR was performed using the KAPA SYBR FAST Universal quantitative PCR kit (KAPA Biosystems, Wilmington, MA) according to the manufacturer's instructions. The primers used in this study were as follows: IL-3 (forward, 5′-ACAATGGTTCTTGCCAGCTCTAC-3′; reverse, 5′-AGGAGCGGGAGCAGCAT-3′), IL-4 (forward, 5′-CAGGGTGCTTCGCAAATTTTAC-3′; reverse, 5′-ACCGAGAACCCCAGACTTGTT-3′), IL-13 (forward, 5′-AGGAGCTGAGCAACATCACAC-3′; reverse, 5′-CCATAGCGGAAAAGTTGCTT-3′), TNF-α (forward, 5′-GTAGCCCACGTCGTAGCAA-3′; reverse, AAATGGCAAATCGGCTGAC-3′), MCP-1 (forward, CGGCTGGAGAACTACAAGAGA-3′; reverse, 5′-CTCTTGAGCTTGGTGACAAATACT-3′), Nfkbiz (forward, 5′-TGCTCCAGGCAATCCAGAAG-3′; reverse, GTTGCCTCCAGATCCACAAAC-3′), GAPDH (forward, 5′-TTCACCACCATGGAGAAGGC-3′; reverse, 5′-GGCATGGACTGTGGTCATGA-3′). The expression of the housekeeping gene gapdh was used as a reference for normalization. The data were developed by using StepOne software version 2.1 (Invitrogen), and the analyzed results were finally expressed as relative units.
Cytokine Production
106 cells were seeded in 35-mm dishes and cultured overnight without or with (for DNP-BSA) anti-DNP IgE mAb (1:5000) for sensitization. Cells were pretreated without or with R406 and then stimulated with 100 μg/ml curdlan or 30 ng/ml DNP-BSA for 6 h at 37 °C. Concentrations of MCP-1, IL-4, and TNF-α secreted into cell culture supernatants of untreated and stimulated cells were analyzed with plate-bound ELISA kits (Quantikine ELISA, R&D Systems) according to the manufacturer's recommendations.
Statistical Analysis
Significant differences were evaluated by the paired t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001 were considered significant).
RESULTS
Dectin-1 Stimulated with curdlan Triggers Tyrosine Phosphorylation of Cellular Proteins in RBL-2H3 Cells
Fungal cell wall component β-glucan induces tyrosine phosphorylation of cellular proteins in macrophages (17) or in DCs (13). Therefore, first we analyzed whether curdlan could induce tyrosine phosphorylation of cellular proteins in mast cell line RBL-2H3 cells (Fig. 1A). Tyrosine phosphorylation of cellular proteins gradually increased for 60 min after curdlan stimulation, although tyrosine phosphorylation was weaker and slower than that induced by FcϵRI engagement. Dose-response experiments showed that the response to curdlan reached a plateau at 1 μg/ml (Fig. 1B), suggesting that a functional receptor for curdlan is expressed in RBL-2H3 cells to trigger unidentified cellular responses.
FIGURE 1.
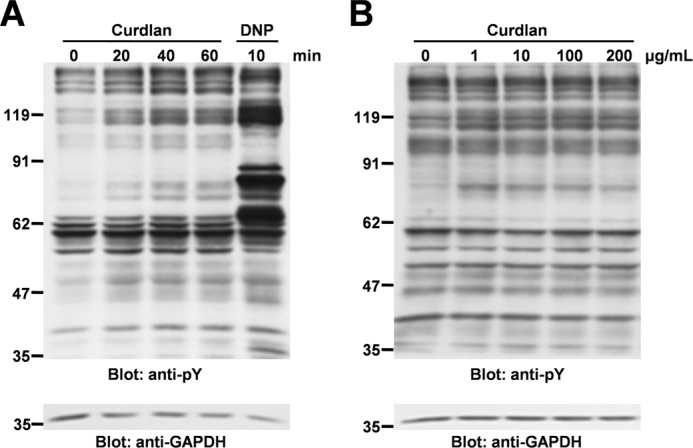
Curdlan induces tyrosine phosphorylation of cellular proteins in RBL-2H3 cells. A, analysis of time course. RBL-2H3 cells were stimulated without or with 100 μg/ml curdlan for the indicated times (Curdlan), or preincubated overnight with anti-DNP IgE and then stimulated with 300 ng/ml of antigen DNP-BSA for 10 min (DNP). Cells were solubilized with lysis buffer containing 1% Triton X-100. Detergent-soluble lysates were separated by SDS-PAGE and analyzed by immunoblotting with anti-Tyr(P) (pY) mAb, and anti-GAPDH mAb was used as an internal control. Incubation of cells with solvent (NaOH) alone had no effect on pH changes of culture medium and protein tyrosine phosphorylation in RBL-2H3 cells (data not shown). B, analysis of dose dependence. RBL-2H3 cells were stimulated with the indicated concentrations of curdlan for 40 min. Cells were solubilized with the lysis buffer containing 1% Triton X-100. Detergent-soluble lysates were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. Molecular size markers are indicated at the left in kDa. The results are representative of three independent experiments.
Expression of Dectin-1 in RBL-2H3 cells was detected by RT-PCR. Two differential isoforms, Dectin-1A and -1B, were confirmed by DNA sequencing (data not shown).
Generation of Stable RBL-2H3 Cell Lines Overexpressing Dectin-1 or Its Y15F Mutant
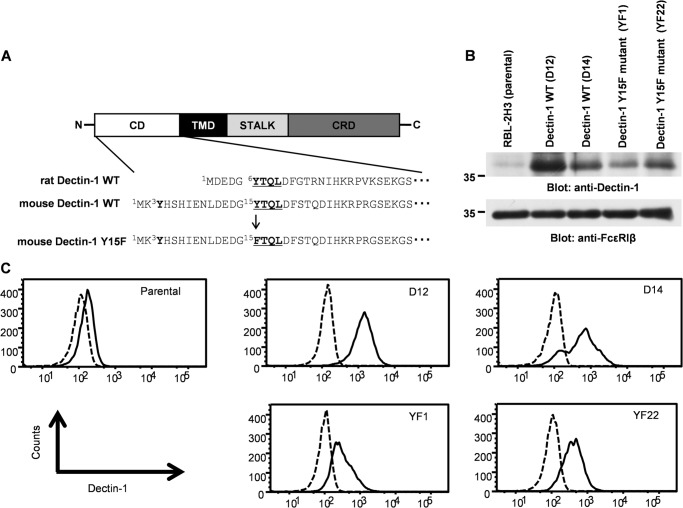
To analyze the function of Dectin-1 in mast cells, stable cell lines overexpressing Dectin-1 wild type (WT) or its inactive form were generated. Mouse Dectin-1 cDNA was transfected into RBL-2H3 cells (Fig. 2). A schematic diagram of Dectin-1 is shown in Fig. 2A. The cytoplasmic tail of mouse Dectin-1 possesses two tyrosine residues (Tyr3 and Tyr15); however, only Tyr15 which is the component of hemITAM is essential for the Dectin-1-mediated signaling in DCs (20). Furthermore, rat Dectin-1 does not possess a tyrosine residue corresponding to Tyr3 of the mouse Dectin-1 (Fig. 2A) (4). Therefore, Tyr15 was substituted with Phe (Y15F) to generate loss-of-function mutant of Dectin-1.
FIGURE 2.
Generation of stable RBL-2H3 cell lines overexpressing Dectin-1 or its Y15F mutant. A, schematic diagram of Dectin-1 WT and Y15F mutant used in this study. Dectin-1 is composed of four domains: the carbohydrate recognition domain (CRD), stalk domain (STALK), transmembrane domain (TMD), and cytoplasmic domain (CD), which possesses hemITAM. Underlines show amino acid sequence that constitutes the hemITAM motif. Arrow, amino acid substitution of Tyr15 to Phe (Y15F mutant). B and C, RBL-2H3 cells were stably transfected with pSVL-Myc-His-Dectin-1 WT or pSVL-Myc-His-Dectin-1 Y15F mutant, together with pSV2-neo, by electroporation. Clones resistant to G418 were selected and screened by the level of protein expression and surface expression. B, detergent-soluble lysates from the each cell line were separated by SDS-PAGE and analyzed by immunoblotting with anti-Dectin-1 and anti-FcϵRIβ antibodies, respectively. Molecular size markers are indicated at the left in kDa. C, analysis of cell surface expression of Dectin-1 in established cell lines by flow cytometry. Cells were incubated with anti-Dectin-1 antibody (solid line) or control goat antibody (dashed line) followed by staining with Alexa 488-labeled secondary antibody. The results are representative of three independent experiments.
pSVL-Myc-His-Dectin-1 or pSVL-Myc-His-Dectin-1 Y15F mutant was cotransfected with pSV2-neo into RBL-2H3 cells. Two clones of Dectin-1 WT or Y15F mutant-expressing cells in which the level of protein expression was highest were selected and utilized in the following study (Fig. 2B). Expression of the wild type and mutant form of Dectin-1 on the cell surface was confirmed by FACS analysis (Fig. 2C).
Tyr15 in hemITAM Is Required for Dectin-1-mediated Cellular Signaling in RBL-2H3 Cells
Curdlan stimulation induces phosphorylation of ERK in macrophages (17) and DCs (18). Therefore, we examined whether curdlan stimulation could induce tyrosine phosphorylation of cellular proteins and phosphorylation of ERK in the stable cell lines overexpressing Dectin-1 WT or Y15F mutant (Fig. 3A). As shown, treatment of cells overexpressing Dectin-1 WT with curdlan increased inducible tyrosine phosphorylation of cellular proteins as well as phosphorylation of ERK rather than parental cells, and those effects were not observed in the cells overexpressing Dectin-1 Y15F mutant (Fig. 3A). In addition, inducible phosphorylation was detectable when the cells were stimulated with 1 μg/ml curdlan and reached a maximum with 10–200 μg/ml curdlan (Fig. 3B). These results suggested that RBL-2H3 cells have a Dectin-1-mediated signaling pathway, and Tyr15 in hemITAM plays a critical role for the Dectin-1-mediated cellular signaling pathway. It is important to note that the pattern of protein tyrosine phosphorylation by curdlan stimulation was different from that induced by the engagement of FcϵRI with DNP, although the receptor for curdlan (Dectin-1) and FcϵRI recruit and activate Syk, resulting in transmission of a signal downstream (35). Therefore, the Dectin-1-mediated signaling pathway though Syk might be different from that induced by the engagement of FcϵRI in mast cells.
FIGURE 3.
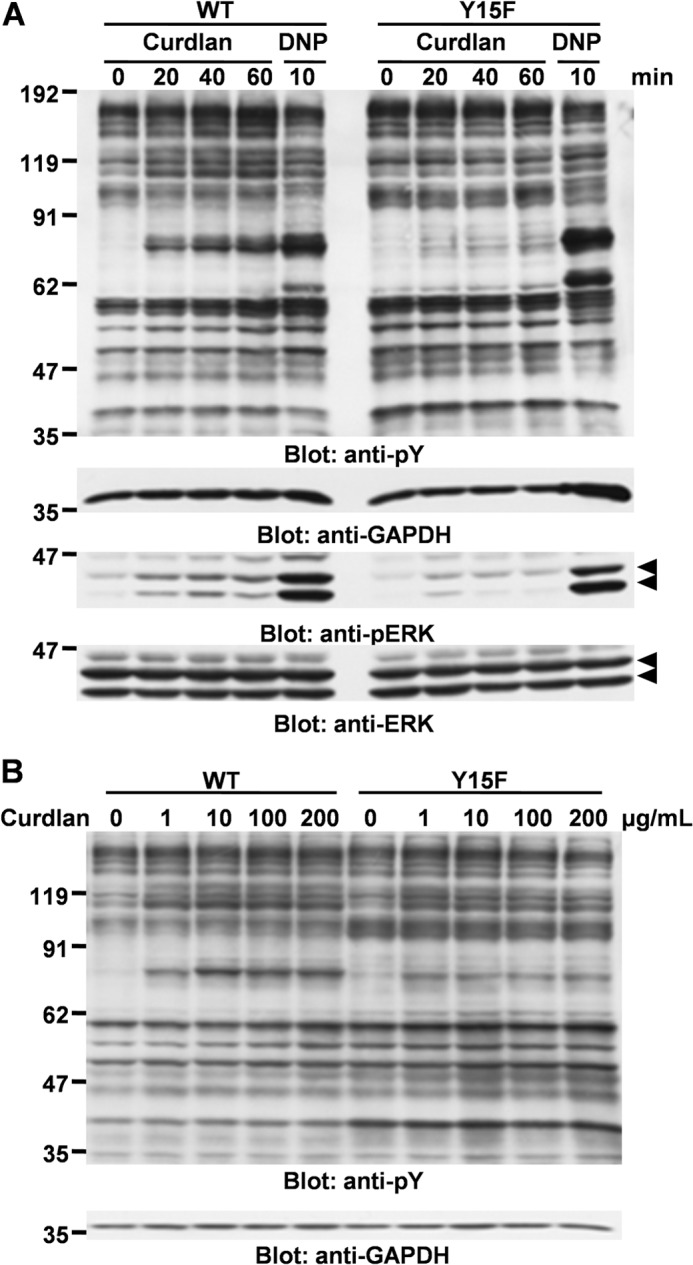
Overexpression of Dectin-1 increases curdlan-mediated protein tyrosine phosphorylation and ERK phosphorylation. Curdlan-induced tyrosine phosphorylation (pY) of cellular proteins and ERK phosphorylation. A, analysis of time course. Cell lines overexpressing Dectin-1 WT or Y15F mutant were stimulated without or with 100 μg/ml curdlan for the indicated times (Curdlan) or preincubated overnight with anti-DNP IgE and then stimulated with 300 ng/ml antigen DNP-BSA for 10 min (DNP). B, analysis of dose dependence. Cell lines overexpressing Dectin-1 WT and Y15F mutant were stimulated with the indicated concentrations of curdlan for 40 min. Cells were solubilized with the lysis buffer containing 1% Triton X-100. Detergent-soluble lysates were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. Molecular size markers are indicated at the left in kDa. Similar results were obtained when the other cloned cell lines were examined. Data shown were obtained by using D12 (WT) and YF22 (Y15F).
Curdlan Induces FcϵRIγ-independent Interaction of Dectin-1 with the SH2 Domain of Syk through Tyrosine Phosphorylation
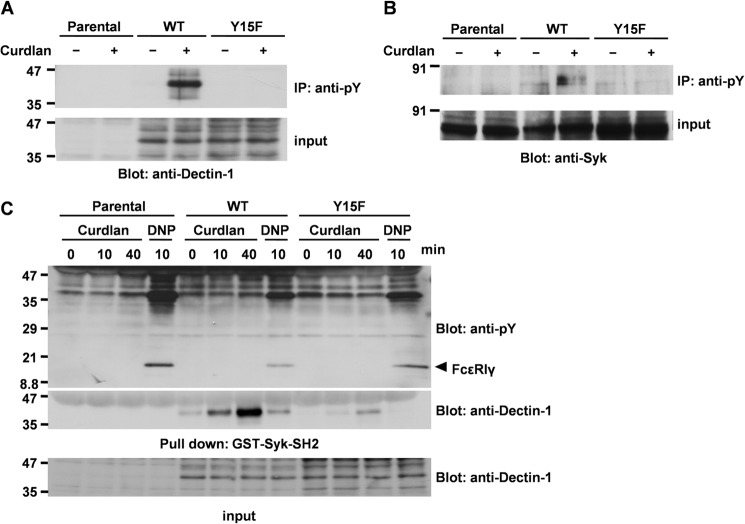
Previous studies demonstrated that stimulation with β-glucan induces tyrosine phosphorylation of Dectin-1 and Syk in macrophages (14, 21) and DCs (20), and tyrosine phosphorylation of these proteins is the key event for transmitting signal downstream (18, 20, 21, 36). Therefore, next we examined whether curdlan could induce tyrosine phosphorylation of Dectin-1 and Syk in mast cells. An immunoprecipitation study demonstrated that Dectin-1 and Syk were tyrosine-phosphorylated by curdlan stimulation in RBL-2H3 cells overexpressing Dectin-1 WT (Fig. 4, A and B). As expected, tyrosine phosphorylation of the Dectin-1 Y15F mutant was not detected, suggesting that Tyr15 is required for curdlan-induced tyrosine phosphorylation of Dectin-1 (Fig. 4A). In addition, consistent with the previous report (20), curdlan-mediated tyrosine phosphorylation of Syk was abrogated in Dectin-1 Y15F mutant cells (Fig. 4B).
FIGURE 4.
Curdlan induces tyrosine phosphorylation of Syk through the interaction of Dectin-1 with the SH2 domain of Syk in an FcϵRI-independent manner. A and B, analysis of tyrosine phosphorylation of Dectin-1 and Syk in generated RBL-2H3 cell lines. RBL-2H3 cells (parental) and cells overexpressing Dectin-1 WT and Y15F mutant were stimulated without or with 100 μg/ml curdlan for 40 min. Cells were solubilized with the denature lysis buffer (lysis buffer containing 1% Triton X-100, 0.1% SDS, and 0.5% deoxycholic acid), and cell lysates were immunoprecipitated with anti-Tyr(P) (pY) mAb-conjugated agarose beads, and then immunoprecipitates (IP) and the source of precipitation (input) were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-Dectin-1 (A) and anti-Syk (B) antibodies. C, pull-down assay. RBL-2H3 cells (Parental) and cells overexpressing Dectin-1 WT or Y15F mutant were stimulated without or with 100 μg/ml curdlan for the indicated times (Curdlan) or preincubated overnight with anti-DNP IgE and then stimulated with 300 ng/ml antigen DNP-BSA for 10 min (DNP). Detergent-soluble lysates were reacted with GST-Syk-SH2 prebound to glutathione-Sepharose 4B beads. The binding proteins (Pull down) and the source of precipitation (input) were separated by SDS-PAGE and analyzed by immunoblotting with anti-Tyr(P) and anti-Dectin-1 antibodies. Arrowhead, position of FcϵRIγ. A–C, molecular size markers are indicated at the left in kDa. Similar results were obtained when the other cloned cell lines were examined. A and C, considering the difference in expression level of Dectin-1 between D12 and YF22 (shown in Fig. 2B), 2 times more Y15F mutant cells (1 × 107 cells) than Dectin-1 WT cells (5 × 106 cells) were used in this experiment.
Because tyrosine phosphorylation of hemITAM (Tyr15) is essential for the interaction between Dectin-1 and the SH2 domain of Syk in DCs (20), we examined the existence of this interaction in mast cells. A pull-down assay using GST-Syk-SH2 demonstrated that Dectin-1 WT, but not the Y15F mutant, bound to the SH2 domain of Syk (Fig. 4C). The interaction between Dectin-1 and the SH2 domain of Syk was maximal at 40 min after curdlan stimulation (Fig. 4C). Endogenous rat Dectin-1 was unable to be detected because anti-mouse Dectin-1/CLEC7A antibody could not react with rat species expressing in RBL-2H3 cells. In addition, engagement of FcϵRI induces the interaction of FcϵRIγ with the SH2 domains of Syk in mast cells (22); hence, we examined whether the interaction between Dectin-1 and Syk was mediated by FcϵRIγ. Patterns of protein tyrosine phosphorylation show that FcϵRIγ was observed when the cells were stimulated with DNP through sensitized FcϵRI; however, this band was not detected in the cells stimulated with curdlan, suggesting that Dectin-1 associates with Syk in an FcϵRI-independent manner (Fig. 4C). These results demonstrated that signal transduction of Dectin-1 and that of FcϵRI through Syk are independent of each other, regardless of the similarity in the interaction between their hemITAM/ITAM and the SH2 domains of Syk.
Syk Plays a Critical Role in Dectin-1-mediated Signal Transduction in RBL-2H3 Cells
By using the Syk inhibitor R406, which binds to the ATP binding pocket of Syk and inhibits its kinase activity as an ATP-competitive inhibitor (37), the importance of Syk in Dectin-1-mediated signal transduction in mast cells was evaluated. Preincubation of cells overexpressing Dectin-1 WT with R406 decreased curdlan-induced tyrosine phosphorylation of cellular proteins and phosphorylation of ERK in a concentration-dependent manner (Fig. 5A). Because almost all of the protein tyrosine phosphorylation induced by curdlan stimulation was inhibited by R406, Syk plays a critical role in Dectin-1-mediated signal transduction in mast cells.
FIGURE 5.
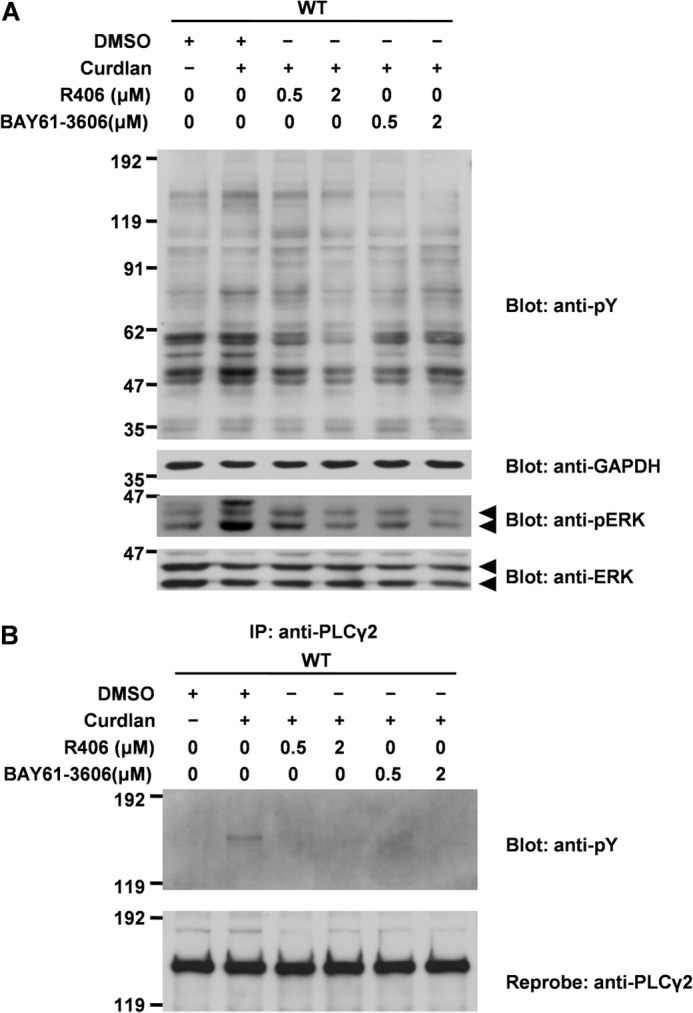
The effect of Syk inhibitors on Dectin-1-mediated tyrosine phosphorylation of cellular proteins. A, cell lines overexpressing Dectin-1 WT were preincubated with the indicated concentration of R406 or BAY61-3606 or an equal amount of solvent DMSO for 5 min and then stimulated without or with 100 μg/ml curdlan for 40 min. Detergent-soluble lysates were separated by SDS-PAGE and analyzed by immunoblotting with anti-Tyr(P) (pY), anti-GAPDH, anti-phospho-ERK (pERK), and anti-ERK antibodies. Arrowheads, phospho-ERK1/2 or ERK1/2. B, cell lines overexpressing Dectin-1 WT were preincubated with the indicated concentrations of R406 or BAY61-3606 for 5 min and then stimulated without or with 100 μg/ml curdlan for 40 min. Detergent-soluble lysates were immunoprecipitated with anti-PLCγ2 antibody, and then immunoprecipitates (IP) and the source of precipitation (input) were separated by 6% SDS-PAGE and analyzed by immunoblotting with anti-Tyr(P) mAb and anti-PLCγ2 antibody. A and B, similar results were obtained when the other cell lines were examined. Molecular size markers are indicated at the left in kDa.
Curdlan stimulation induces tyrosine phosphorylation of PLCγ2, which lies downstream of Syk, resulting in activation of ERK, JNK, and downstream transcription factors NFAT and NF-κB in DCs (15). As shown, tyrosine phosphorylation of PLCγ2 was induced by the stimulation with curdlan and was completely abrogated by the preincubation with R406 (Fig. 5B). These results demonstrated that Syk plays a critical role in Dectin-1-mediated protein tyrosine phosphorylation, including PLCγ2, and phosphorylation of ERK in RBL-2H3 cells.
Treatment of cells with BAY61-3606 as well as R406 completely inhibits curdlan-induced activation of ERK and PLCγ2 (Fig. 5, A and B). Therefore, we concluded that Syk is critical for curdlan-induced activation of ERK and PLCγ2.
Curdlan Stimulates the Expression of Characteristic Genes in Mast Cells, Different from Those in Macrophages or DCs
Microarray analysis was next performed to determine which transcription factors and cytokine genes were up-regulated by curdlan in mast cells. The list of the significantly up-regulated genes in RBL-2H3 cells overexpressing Dectin-1 WT after 2 h of curdlan stimulation is shown in Table 1. Consistent with previous studies in macrophages and DCs, gene expressions of TNF-α (12, 13, 15), IL-4/IL-13 (38, 39), early growth response transcription factor 3 (40, 41), and suppressor of cytokine signaling 1 (42) were also up-regulated in curdlan-stimulated mast cells. Interestingly, curdlan did not stimulate gene expressions that were previously reported in macrophages and DCs, such as IL-1β (43, 44), IL-2 (18), IL-10 (16, 18–20), and IL-12 (18, 20). Alternatively, gene expressions of IL-3, MCP-1, Nfkbiz were up-regulated by curdlan-stimulated mast cells, although those have not been reported in macrophages and DCs. These results demonstrated that Dectin-1-mediated signaling in mast cells plays a different role from those reported in macrophages and DCs. Therefore, Dectin-1-mediated signaling in mast cells may play novel roles in antifungal immunity.
TABLE 1.
Syk-regulated genes in mouse Dectin-1-transduced RBL-2H3 cells after 2 h of Curdlan stimulation
| Gene | Description | Change | p value |
|---|---|---|---|
| -fold | |||
| il-3 | Interleukin-3 | 17.37 | <0.01 |
| ccl2 | CCl2 (MCP-1) | 13.64 | 0.01 |
| nr4a2 | Transcription factor | 11.37 | 0.02 |
| nr4a3 | Transcription factor | 11.15 | 0.02 |
| egr3 | Transcription factor | 9.38 | <0.01 |
| il-13 | Interleukin-13 | 8.89 | <0.01 |
| vom2r3 | Vomeronasal 2 receptor, 3 | 5.06 | 0.04 |
| il-4 | Interleukin-4 | 4.77 | 0.01 |
| cish | Cytokine inducible SH2-containing protein | 4.54 | 0.01 |
| skil | Ski like oncogene | 4.26 | <0.01 |
| nfkbiz | Nfkbiz (IκBζ) | 3.67 | 0.02 |
| osm | Oncostatin M | 3.45 | 0.01 |
| ccl7 | CCl7 (MCP-3) | 3.18 | 0.02 |
| tnfrsf12a | TNF receptor | 3.11 | 0.01 |
| socs1 | Socs1 | 3.04 | 0.02 |
| gpr183 | G protein-coupled receptor 183 | 2.75 | 0.01 |
| csrnp1 | Carbohydrate (karatan sulfate Gal-6) sulfotransferase 1 | 2.74 | <0.01 |
| ler3 | Protocadherin 9 | 2.66 | 0.02 |
| tnf | TNF-α | 2.66 | <0.01 |
Dectin-1-mediated Signal Transduction in Mast Cells Leads to Characteristic Gene Expressions and Cytokine Secretion in a Syk-dependent Manner
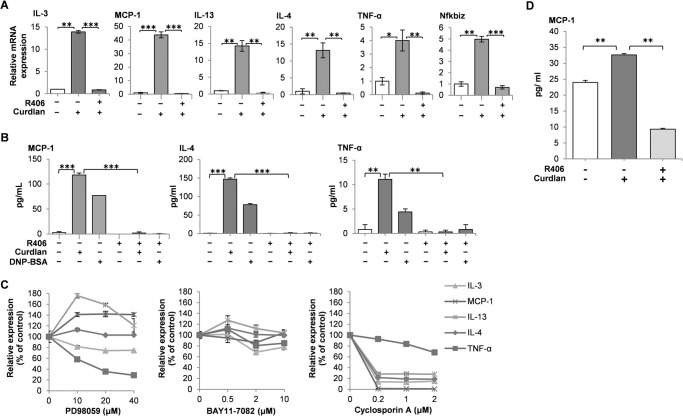
Finally, quantitative real-time PCR was performed to investigate curdlan-mediated gene expressions. Gene expressions of IL-3, MCP-1, IL-13, IL-4, TNF-α, and Nfkbiz were significantly up-regulated by 2 h of curdlan stimulation and potently inhibited by preincubation of cells with R406, suggesting that these gene expressions are regulated in a Syk-dependent manner (Fig. 6A). Among the inducible genes, curdlan-mediated secretion of MCP-1, IL-4, and TNF-α into the culture supernatants was examined using the cells overexpressing Dectin-1 WT (Fig. 6B). Consistent with the results of quantitative real-time PCR, secretion of MCP-1, IL-4, and TNF-α induced by curdlan was abrogated by pretreatment with Syk inhibitor R406 (Fig. 6B). These results demonstrated that Dectin-1-mediated signaling through Syk in mast cells stimulates characteristic gene expression of transcription factors and cytokines.
FIGURE 6.
Dectin-1-mediated signaling activates various gene expression and cytokine secretion through a Syk-dependent pathway in RBL-2H3 cells. A, quantitative analysis of curdlan-induced gene expression by real-time PCR. Cells overexpressing Dectin-1 WT were preincubated without or with 2 μm R406 for 5 min and then stimulated without or with 100 μg/ml curdlan for 2 h. Total RNA was isolated and reverse transcribed, and cDNA of the indicated genes was analyzed by quantitative real-time PCR. The expression of the housekeeping gene gapdh was used as a reference for normalization. All samples were assayed in three different biological replicates and are presented as mean ± S.D. (error bars). Significant differences were evaluated by Student's paired t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001 were considered significant). Similar results were obtained when the other cloned cell lines were examined. B, analysis of MCP-1, IL-4, and TNF-α secretions induced by curdlan. Cells overexpressing Dectin-1 WT were preincubated without or with 2 μm R406 for 5 min (R406) and then stimulated without or with 100 μg/ml curdlan (Curdlan) or preincubated overnight with anti-DNP IgE and then stimulated without or with 30 ng/ml of antigen DNP-BSA for 6 h (DNP-BSA). Cell culture supernatants were harvested, and ELISA was performed. Significant differences were evaluated by Student's paired t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001 were considered significant). All samples were assayed in triplicates and are presented as mean ± S.D. The results are representative of three independent experiments. C, effects of inhibitor for MEK, NF-κB, and NFAT on curdlan-induced gene expression. Cells overexpressing Dectin-1 WT (D12) were stimulated with 100 μg/ml curdlan for 2 h in the presence of the indicated concentrations of PD98059 (MEK inhibitor), BAY11-7082 (NF-κB inhibitor) or cyclosporin A (inhibitor of the calcineurin/NFAT pathway). Gene expression was quantified by real-time PCR and is expressed relative to DMSO control (100%). All samples were assayed in triplicates and are presented as mean ± S.D. The results are representative of three independent experiments. D, analysis of MCP-1 secretion from parental RBL-2H3 cells. RBL-2H3 cells were preincubated without or with 2 μm R406 for 5 min (R406) and then stimulated without or with 100 μg/ml curdlan (Curdlan). Cell culture supernatants were harvested, and MCP-1 ELISA was performed. Significant differences were evaluated by Student's paired t test (**, p < 0.01). All samples were assayed in triplicates and are presented as mean ± S.D. The results are representative of three independent experiments.
To further investigate the Syk-mediated cellular signaling, we next examined the effects of inhibitor for MEK, NF-κB, and NFAT on curdlan-induced mRNA expression of various cytokines. The treatment of curdlan-stimulated cells with MEK inhibitor PD98059 dramatically suppressed mRNA expression of TNF-α (29% of control). Interestingly, the expressions of MCP-1 and IL-13 were up-regulated (142 and 176% of control, respectively) (Fig. 6C). Although we could not observe any significant effects of NF-κB inhibitor BAY11-7082, the treatment of cells with cyclosporin A, a calcineurin/NFAT pathway inhibitor, caused dramatic suppression of mRNA expressions of IL-3, MCP-1, IL-4, and IL-13 but not TNF-α. These results suggest that Syk-dependent mRNA expressions of MCP-1, IL-3, IL-4, and IL-13 in curdlan-stimulated mast cells is dependent on the activation of NFAT, and that of TNF-α is dependent on the activation of ERK.
Finally, we examined whether treatment of parental RBL-2H3 cells with curdlan stimulates secretion of MCP-1. As shown in Fig. 6D, curdlan-mediated increase of secretion of MCP-1 from parental RBL-2H3 cells was observed. As expected, R406 strikingly suppressed the secretion of MCP-1.
DISCUSSION
Previously, Dectin-1 expression was reported in human cord blood-derived mast cells (28), human peripheral blood-derived mast cells (29), and mouse bone marrow-derived mast cells (24). Crucially, to begin with, Dectin-1 expressed in rat mast cell line RBL-2H3 was identified. With RT-PCR and DNA sequence, two functional isoforms expressing in RBL-2H3 cells were identified (data not shown), named A and B (4). In addition, cellular proteins in RBL-2H3 cells are tyrosine-phosphorylated by curdlan stimulation (Fig. 1). These results demonstrated that RBL-2H3 cells express Dectin-1 and have a Dectin-1-mediated signal transduction pathway.
The importance of Syk in Dectin-1-mediated signal transduction has been demonstrated in macrophages and DCs. When hemITAM of Dectin-1 is tyrosine-phosphorylated, Syk is recruited, and its SH2 domain binds to hemITAM of Dectin-1, resulting in tyrosine phosphorylation of Syk and signal transduction to downstream (13, 20). Consistent with these facts, tyrosine phosphorylation of Dectin-1 and Syk and the interaction between Dectin-1 and the SH2 domain of Syk were detected after curdlan stimulation of RBL-2H3 cells overexpressing Dectin-1 WT, whereas those in the Y15F mutant were abrogated (Fig. 4). Interestingly, FcϵRI does not have the interaction between its ITAM and SH2 domains of Syk after Dectin-1 stimulation with curdlan (Fig. 4C). This indicates that the signal transductions of Dectin-1 and FcϵRI through Syk are completely independent each other, regardless of their similarity in the interaction between their hemITAM/ITAM and SH2 domains of Syk. Syk is a non-receptor type protein-tyrosine kinase and plays critical roles in B cell receptor, T cell receptor, FcϵRI, Dectin-1, and CLEC2 to transmit those activation signals downstream (45, 46). In this report, we found that Syk is important not only in macrophages and DCs but also in mast cells for Dectin-1-mediated signal transduction, by using R406 and BAY61-3606 (Fig. 5, A and B). Furthermore, gene up-regulations of IL-3, MCP-1, IL-4, IL-13, Nfkbiz, and TNF-α by curdlan stimulation were also abrogated with R406 and BAY61-3606 (Fig. 6A), indicating that the Dectin-1-mediated regulations of these genes also depend on Syk.
As shown in Fig. 3A, we found that the pattern of protein tyrosine phosphorylation is different between FcϵRI- and Dectin-1-stimulated cells. Although activation of Syk is essential in these signaling pathways, it is reported that FcϵRI activates Fyn kinase independently of activation of Syk (47). In addition, it is reported that protein-tyrosine phosphatase SHP-1 could activate JNK in response to aggregation of FcϵRI (48). These Syk-independent signaling pathways through FcϵRI, but not Dectin-1, could account for the differences in gene expression triggered by these receptors.
With DNA microarray analysis, various up-regulated genes were detected in mast cells following curdlan stimulation (Table 1). Six genes (il-3, mcp-1, il-4, il-13, tnf-α, and nfkbiz) were focused on because they were well analyzed in FcϵRI signaling in mast cells and possibly correspond to antifungal immunity. Quantitative real-time PCR analysis revealed that curdlan could induce gene expressions of IL-3, MCP-1, IL-4, IL-13, TNF-α, and Nfkbiz in a Syk-dependent manner (Fig. 6A). MCP-1 is a CC chemokine and plays a critical role in the recruitment of monocytes into the site of inflammatory responses (49). Previous studies show that zymosan stimulates MCP-1 production, resulting in monocyte recruitment into inflammatory sites (50, 51). However, zymosan stimulates not only Dectin-1 but also TLR2 (14); therefore, it has remained unknown whether MCP-1 is induced by only the Dectin-1-mediated signaling pathway. In this study, it was first revealed that MCP-1 was produced through Dectin-1-mediated signal transduction by using curdlan, a specific agonist of Dectin-1, in mast cells in a TLR2-independent manner (Fig. 6B) (52, 53). IL-4 and IL-13, canonical type 2 cytokines, are closely related cytokines required for the generation of high-affinity IgE antibodies, mucus overproduction, and smooth muscle alterations (54). IL-4 and IL-13 highly up-regulate Dectin-1 expression on the cell surface through STAT6 activation in macrophages (54, 55). We demonstrated that mast cells stimulated through Dectin-1-mediated signal transduction produce IL-4 and IL-13 (Fig. 6). TNF-α, a well known cytokine induced by Dectin-1 stimulation by curdlan (12, 13, 15), is an essential cytokine required for the successful control of many fungal pathogens (56–59). TNF-α production induced by curdlan in mast cells indicates that mast cells contribute to innate antifungal immunity.
nfkbiz, the IκBζ-coding gene, is rapidly induced by various inflammatory stimuli; however, its physiological function remains largely unknown (60, 61). Recently, it has been reported in a macrophage study that IκBζ is a transcriptional key regulator of MCP-1 following TLR stimulation (62). However, very importantly, it has never been reported whether Nfkbiz is up-regulated through Dectin-1-mediated signal transduction and exists in mast cells. In addition, although IκBζ is inducible in the MyD88-dependent part of the TLR/IL-1R signaling pathway (61), its regulatory mechanism remains unclear. Dectin-1-mediated signal transduction stimulates Nfkbiz transcription in mast cells, and it was also pharmacologically identified that Syk is a key molecule for Dectin-1-mediated transcription of Nfkbiz, indicating the possibility of elucidation of a novel mechanism of IκBζ induction (Fig. 6A). With immunoblotting using IκBα, IκB kinase, and phospho-IκB kinase antibodies, NF-κB is not activated with curdlan stimulation in RBL-2H3 cells (data not shown). Goodridge et al. (63) demonstrated that although Dectin-1 signals directly activate NF-κB in mouse DCs, Dectin-1 signaling alone does not activate NF-κB in mouse macrophages (14, 42, 63). According to this, Dectin-1-mediated activation of NF-κB in mast cells may not activate in the same manner as that in macrophages. These results, new to this study, are summarized in Fig. 7.
FIGURE 7.
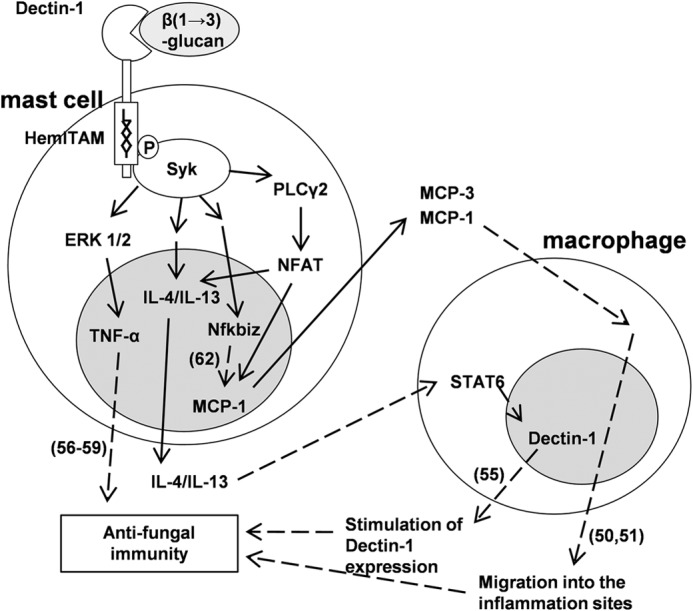
Dectin-1-mediated signaling in mast cells may directly and indirectly contribute to antifungal immunity. This schema shows dectin-1-mediated signal transduction and subsequent increase of transcription factor and cytokines revealed in this study. Solid arrows, results revealed in this study; dashed arrows, results revealed in past reports (reference numbers shown in parenthesis). Dectin-1 is expressed in mast cells and recognizes β(1→3)-glucan. Tyr15 in hemITAM of Dectin-1 is required for subsequent recruitment of the SH2 domains of Syk, resulting in tyrosine phosphorylation of Syk. Dectin-1/Syk signaling activates intracellular signal transducers involving PLCγ2, NFAT, and ERK1/2, resulting in stimulation of gene expression of cytokines and transcription factors, such as MCP-1, IL-3, IL-4, IL-13, TNF-α, and Nfkbiz. MCP-1, IL-4/IL-13, Nfkbiz, and TNF-α may assume induction of macrophage migration into the inflammation sites together with MCP-3, stimulation of Dectin-1 expression in macrophages, stimulation of MCP-1 gene expression, and inflammatory response against fungi, respectively. These events lead an individual to the successful control of fungal infections.
It is well known that the administration of zymosan into the peritoneal cavity of mice induced massive infiltration of neutrophils with an increased level of chemoattractants and inflammatory cytokines, including MCP-1 and TNF-α. Given the fact that these effects of zymosan were significantly impaired in mast cell-deficient W/WV mice (64–66), mast cells might play important roles in the host defense against fungal infection. In addition to our present study, it was demonstrated that the treatment of bone marrow-derived mast cells with zymosan enhanced cell surface expression of Dectin-1 and stimulated reactive oxygen species production, although these effects were significantly reduced in mast cells derived from TLR2-deficient mice (24). Further studies are required to understand how Dectin-1 and other innate immunoreceptors activated by the fungal cell wall differently or cooperatively regulate mast cell activation.
In conclusion, these results strongly suggest that mast cells contribute innate antifungal immunity. In addition, it has been reported that Dectin-1 has relationships with not only innate immunity but also autoimmune diseases (67–69), allergic diseases (29, 70), and metabolic diseases (71), indicating that mast cells may contribute to and offer new insights into these diseases.
Acknowledgments
We thank Dr. Reuben P. Siraganian for providing the reagents. We thank Satomi Yamamoto and the Life Science Research Laboratory of the University of Fukui for assistance.
This work was supported in part by research funding from grants-in-aid from the Japan Society for the Promotion of Science and research funding from the University of Fukui (to S. F. and K. S.).
- TLR
- Toll-like receptor
- DC
- dendritic cell
- SH2
- Src homology 2
- ITAM
- immunoreceptor tyrosine-based activation motif
- hemITAM
- hemi-immunoreceptor tyrosine-based activation motif
- FcϵRIγ
- γ-subunit of high-affinity IgE receptor
- MCP-1
- monocyte chemoattractant protein-1
- Syk
- spleen tyrosine kinase
- NF-κB
- nuclear factor-κB
- NFAT
- nuclear factor of activated T-cells
- CLEC2
- C-type lectin-like receptor 2
- DNP
- dinitrophenyl
- PLC
- phospholipase C.
REFERENCES
- 1. Ariizumi K., Shen G.-L., Shikano S., Xu S., Ritter R., 3rd, Kumamoto T., Edelbaum D., Morita A., Bergstresser P. R., Takashima A. (2000) Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275, 20157–20167 [DOI] [PubMed] [Google Scholar]
- 2. Brown G. D., Gordon S. (2001) Immune recognition: a new receptor for β-glucan. Nature 413, 36–37 [DOI] [PubMed] [Google Scholar]
- 3. Taylor P. R., Brown G. D., Reid D. M., Willment J. A., Martinez-Pomares L., Gordon S., Wong S. Y. (2002) The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169, 3876–3882 [DOI] [PubMed] [Google Scholar]
- 4. Kato Y., Adachi Y., Ohno N. (2008) Characterization of rat β-glucan receptor dectin-1. Microbiol. Immunol. 52, 418–428 [DOI] [PubMed] [Google Scholar]
- 5. Heinsbroek S. E., Taylor P. R., Rosas M., Willment J. A., Williams D. L., Gordon S., Brown G. D. (2006) Expression of functionally different dectin-1 isoforms by murine macrophages. J. Immunol. 176, 5513–5518 [DOI] [PubMed] [Google Scholar]
- 6. Willment J. A., Gordon S., Brown G. D. (2001) Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 276, 43818–43823 [DOI] [PubMed] [Google Scholar]
- 7. Di Carlo F. J., Fiore J. V. (1958) On the composition of zymosan. Science 127, 756–757 [DOI] [PubMed] [Google Scholar]
- 8. Taylor P. R., Brown G. D., Herre J., Williams D. L., Willment J. A., Gordon S. (2004) The role of SIGNR1 and the β-glucan receptor (Dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J. Immonol. 172, 1157–1162 [DOI] [PubMed] [Google Scholar]
- 9. Thornton B. P., Vĕtvicka V., Pitman M., Goldman R. C., Ross G. D. (1996) Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 156, 1235–1246 [PubMed] [Google Scholar]
- 10. Brown G. D. (2006) Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6, 33–43 [DOI] [PubMed] [Google Scholar]
- 11. Brown G. D., Herre J., Williams D. L., Willment J. A., Marshall A. S., Gordon S. (2003) Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kataoka K., Muta T., Yamazaki S., Takeshige K. (2002) Activation of macrophages by linear (1→3)-β-d-glucans. J. Biol. Chem. 277, 36825–36831 [DOI] [PubMed] [Google Scholar]
- 13. Strasser D., Neumann K., Bergmann H., Marakalala M. J., Guler R., Rojowska A., Hopfner K.-P., Brombacher F., Urlaub H., Baier G., Brown G. D., Leitges M., Ruland J. (2012) Syk kinase-coupled C-type lectin receptors engage protein kinase C-δ to elicit Card9 adaptor-mediated innate immunity. Immunity 36, 32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gantner B. N., Simmons R. M., Canavera S. J., Akira S., Underhill D. M. (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu S., Huo J., Lee K.-G., Kurosaki T., Lam K.-P. (2009) Phospholipase Cγ2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J. Biol. Chem. 284, 7038–7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly E. K., Wang L., Ivashkiv L. B. (2010) Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J. Immunol. 184, 5545–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olsson S., Sundler R. (2007) The macrophage β-glucan receptor mediates arachidonate release induced by zymosan: essential role for Src family kinases. Mol. Immunol. 44, 1509–1515 [DOI] [PubMed] [Google Scholar]
- 18. Slack E. C., Robinson M. J., Hernanz-Falcón P., Brown G. D., Williams D. L., Schweighoffer E., Tybulewicz V. L., Reis e Sousa C. (2007) Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur. J. Immunol. 37, 1600–1612 [DOI] [PubMed] [Google Scholar]
- 19. Elcombe S. E., Naqvi S., Van Den Bosch M. W., MacKenzie K. F., Cianfanelli F., Brown G. D., Arthur J. S. (2013) Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS One 8, e60086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., Reis e Sousa C. (2005) Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 [DOI] [PubMed] [Google Scholar]
- 21. Underhill D., Rossnagle M., E., Lowell C. A., Simmons R. M. (2005) Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kihara H., Siraganian R. P. (1994) Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J. Biol. Chem. 269, 22427–22432 [PubMed] [Google Scholar]
- 23. Fuller G. L., Williams J. A., Tomlinson M. G., Eble J. A., Hanna S. L., Pöhlmann S., Suzuki-Inoue K., Ozaki Y., Watson S. P., Pearce A. C. (2007) The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J. Biol. Chem. 282, 12397–12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang Z., Marshall J. S. (2009) Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology 214, 321–330 [DOI] [PubMed] [Google Scholar]
- 25. Vautier S., MacCallum D. M., Brown G. D. (2012) C-type lectin receptors and cytokines in fungal immunity. Cytokine 58, 89–99 [DOI] [PubMed] [Google Scholar]
- 26. Galli S. J., Nakae S., Tsai M. (2005) Mast cells in the development of adaptive immune responses. Nat. Immunol. 6, 135–142 [DOI] [PubMed] [Google Scholar]
- 27. Voehringer D. (2013) Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 13, 362–375 [DOI] [PubMed] [Google Scholar]
- 28. Olynych T. J., Jakeman D. L., Marshall J. S. (2006) Fungal zymosan induces leukotriene production by human mast cells through a dectin-1-dependent mechanism. J. Allergy Clin. Immunol. 118, 837–843 [DOI] [PubMed] [Google Scholar]
- 29. Ribbing C., Engblom C., Lappalainen J., Lindstedt K., Kovanen P. T., Karlsson M. A., Lundeberg L., Johansson C., Nilsson G., Lunderius-Andersson C., Scheynius A. (2011) Mast cells generated from patients with atopic eczema have enhanced levels of granule mediators and an impaired Dectin-1 expression. Allergy 66, 110–119 [DOI] [PubMed] [Google Scholar]
- 30. Benhamou M., Ryba N. J., Kihara H., Nishikawa H., Siraganian R. P. (1993) Protein-tyrosine kinase p72syk in high affinity IgE receptor signaling. J. Biol. Chem. 268, 23318–23324 [PubMed] [Google Scholar]
- 31. Sada K., Miah S. M., Maeno K., Kyo S., Qu X., Yamamura H. (2002) Regulation of FcϵRI-mediated degranulation by an adaptor protein 3BP2 in rat basophilic leukemia RBL-2H3 cells. Blood 100, 2138–2144 [DOI] [PubMed] [Google Scholar]
- 32. Shukla U., Hatani T., Nakashima K., Ogi K., Sada K. (2009) Tyrosine phosphorylation of 3BP2 regulates B cell receptor-mediated activation of NFAT. J. Biol. Chem. 284, 33719–33728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogi K., Nakashima K., Chihara K., Takeuchi K., Horiguchi T., Fujieda S., Sada K. (2011) Enhancement of B-cell receptor signaling by a point mutation of adaptor protein 3BP2 identified in human inherited disease cherubism. Genes Cells 16, 951–960 [DOI] [PubMed] [Google Scholar]
- 34. Chihara K., Kimura Y., Honjoh C., Yamauchi S., Takeuchi K., Sada K. (2014) Tyrosine phosphorylation of 3BP2 is indispensable for the interaction with Vav3 in chicken DT40 cells. Exp. Cell Res. 322, 99–107 [DOI] [PubMed] [Google Scholar]
- 35. Sada K., Zhang J., Siraganian R. P. (2001) SH2 domain-mediated targeting, but not localization, of Syk in the plasma membrane is critical for FcϵRI signaling. Blood 97, 1352–1359 [DOI] [PubMed] [Google Scholar]
- 36. Leibundgut-Landmann S., Osorio F., Brown G. D., Reis e Sousa C. (2008) Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 112, 4971–4980 [DOI] [PubMed] [Google Scholar]
- 37. Braselmann S., Taylor V., Zhao H., Wang S., Sylvain C., Baluom M., Qu K., Herlaar E., Lau A., Young C., Wong B. R., Lovell S., Sun T., Park G., Argade A., Jurcevic S., Pine P., Singh R., Grossbard E. B., Payan D. G., Masuda E. S. (2006) R406, an orally available spleen tyrosine kinase inhibitor blocks Fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 319, 998–1008 [DOI] [PubMed] [Google Scholar]
- 38. Rand T. G., Sun M., Gilyan A., Downey J., Miller J. D. (2010) Dectin-1 and inflammation-associated gene transcription and expression in mouse lungs by a toxic (1,3)-β-d-glucan. Arch. Toxicol. 84, 205–220 [DOI] [PubMed] [Google Scholar]
- 39. Rand T. G., Robbins C., Rajaraman D., Sun M., Miller J. D. (2013) Induction of Dectin-1 and asthma-associated signal transduction pathways in RAW 264.7 cells by a triple-helical (1,3)-β-d-glucan, curdlan. Arch. Toxicol. 87, 1841–1850 [DOI] [PubMed] [Google Scholar]
- 40. Goodridge H. S., Simmons R. M., Underhill D. M. (2007) Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 178, 3107–3115 [DOI] [PubMed] [Google Scholar]
- 41. Tassi I., Cella M., Castro I., Gilfillan S., Khan W. N., Colonna M. (2009) Requirement of phospholipase C-γ2 (PLCγ2) for Dectin-1-induced antigen presentation and induction of TH1/TH17 polarization. Eur. J. Immunol. 39, 1369–1378 [DOI] [PubMed] [Google Scholar]
- 42. Eberle M. E., Dalpke A. H. (2012) Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. J. Immunol. 188, 5644–5654 [DOI] [PubMed] [Google Scholar]
- 43. Kankkunen P., Teirilä L., Rintahaka J., Alenius H., Wolff H., Matikainen S. (2010) (1,3)-β-Glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. 184, 6335–6342 [DOI] [PubMed] [Google Scholar]
- 44. Gringhuis S. I., Kaptein T. M., Wevers B. A., Theelen B., van der Vlist M., Boekhout T., Geijtenbeek T. B. (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat. Immunol. 13, 246–254 [DOI] [PubMed] [Google Scholar]
- 45. Sada K., Takano T., Yanagi S., Yamamura H. (2001) Structure and function of Syk protein-tyrosine kinase. J. Biochem. 130, 177–186 [DOI] [PubMed] [Google Scholar]
- 46. Mócsai A., Ruland J., Tybulewicz V. L. (2010) The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parravicini V., Gadina M., Kovarova M., Odom S., Gonzalez-Espinosa C., Furumoto Y., Saitoh S., Samelson L. E., O'Shea J. J., Rivera J. (2002) Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 3, 741–748 [DOI] [PubMed] [Google Scholar]
- 48. Xie Z. H., Zhang J., Siraganian R. P. (2000) Positive regulation of c-Jun N-terminal kinase and TNF-α production but not histamine release by SHP-1 in RBL-2H3 mast cells. J. Immunol. 164, 1521–1528 [DOI] [PubMed] [Google Scholar]
- 49. Lu B., Rutledge B. J., Gu L., Fiorillo J., Lukacs N. W., Kunkel S. L., North R., Gerard C., Rollins B. J. (1998) Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187, 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ajuebor M. N., Flower R. J., Hannon R., Christie M., Bowers K., Verity A., Perretti M. (1998) Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J. Leukoc. Biol. 63, 108–116 [DOI] [PubMed] [Google Scholar]
- 51. Takahashi M., Galligan C., Tessarollo L., Yoshimura T. (2009) Monocyte chemoattractant protein-1 (MCP-1), not MCP-3, is the primary chemokine required for monocyte recruitment in mouse peritonitis induced with thioglycollate or zymosan A. J. Immunol. 183, 3463–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McIntosh M., Stone B. A., Stanisich V. A. (2005) Curdlan and other bacterial (1→3)-β-d-glucans. Appl. Microbiol. Biotechnol. 68, 163–173 [DOI] [PubMed] [Google Scholar]
- 53. Ferwerda G., Meyer-Wentrup F., Kullberg B.-J., Netea M. G., Adema G. J. (2008) Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 10, 2058–2066 [DOI] [PubMed] [Google Scholar]
- 54. Van Dyken S. J., Locksley R. M. (2013) Interleukin-4 and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu. Rev. Immunol. 31, 317–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Willment J. A., Lin H.-H., Reid D. M., Taylor P. R., Williams D. L., Wong S. Y., Gordon S., Brown G. D. (2003) Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. 171, 4569–4573 [DOI] [PubMed] [Google Scholar]
- 56. Kawakami K., Qifeng X., Tohyama M., Qureshi M. H., Saito A. (1996) Contribution of tumour necrosis factor-α (TNF-α) in host defense mechanism against Cryptococcus neoformans. Clin. Exp. Immunol. 106, 468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Netea M. G., van Tits L. J., Curfs J. H., Amiot F., Meis J. F., van der Meer J. W., Kullberg B. J. (1999) Increased susceptibility of TNF-α lymphotoxin-α double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J. Immunol. 163, 1498–1505 [PubMed] [Google Scholar]
- 58. Mehrad B., R. Strieter R. M., Standiford T. J. (1999) Role of TNF-α in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162, 1633–1640 [PubMed] [Google Scholar]
- 59. Allendoerfer R., Deepe G. S., Jr. (1998) Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 160, 6072–6082 [PubMed] [Google Scholar]
- 60. Yamazaki S., Muta T., Takeshige K. (2001) A novel IκB protein, IκB-ζ, induced by proinflammatory stimuli, negatively regulates nuclear factor-κB in the nuclei. J. Biol. Chem. 276, 27657–27662 [DOI] [PubMed] [Google Scholar]
- 61. Yamamoto M., Yamazaki S., Uematsu S., Sato S., Hemmi H., Hoshino K., Kaisho T., Kuwata H., Takeuchi O., Takeshige K., Saitoh T., Yamaoka S., Yamamoto N., Yamamoto S., Muta T., Takeda K., Akira S. (2004) Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 430, 218–222 [DOI] [PubMed] [Google Scholar]
- 62. Hildebrand D. G., Alexander E., Hörber S., Lehle S., Obermayer K., Münck N.-A., Rothfuss O., Frick J.-S., Morimatsu M., Schmitz I., Roth J., Ehrchen J. M., Essmann F., Schulze-Osthoff K. (2013) IκBζ is a transcriptional key regulator of CCL2/MCP-1. J. Immunol. 190, 4812–4820 [DOI] [PubMed] [Google Scholar]
- 63. Goodridge H. S., Shimada T., Wolf A. J., Hsu Y. M., Becker C. A., Lin X., Underhill D. M. (2009) Differential use of CARD9 by Dectin-1 in macrophages and dendritic cells. J. Immunol. 182, 1146–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rao T. S., Currie J. L., Shaffer A. F., Isakson P. C. (1994) In vivo characterization of zymosan-induced mouse peritoneal inflammation. J. Pharmacol. Exp. Ther. 269, 917–925 [PubMed] [Google Scholar]
- 65. Kolaczkowska E., Seljelid R., Plytycz B. (2001) Role of mast cells in zymosan-induced peritoneal inflammation in Balb/c and mast cell-deficient WBB6F1 mice. J. Leukoc. Biol. 69, 33–42 [PubMed] [Google Scholar]
- 66. Takeshita K., Sakai K., Bacon K. B., Gantner F. (2003) Critical role of histamine H4 receptor in leukotriene B4 production and mast cell-dependent neutrophil recruitment induced by zymosan in vivo. J. Pharmacol. Exp. Ther. 307, 1072–1078 [DOI] [PubMed] [Google Scholar]
- 67. Yoshitomi H., Sakaguchi N., Kobayashi K., Brown G. D., Tagami T., Sakihama T., Hirota K., Tanaka S., Nomura T., Miki I., Gordon S., Akira S., Nakamura T., Sakaguchi S. (2005) A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Plantinga T. S., Fransen J., Takahashi N., Stienstra R., van Riel P. L., van den Berg W. B., Netea M. G., Joosten L. A. (2010) Functional consequences of DECTIN-1 early stop codon polymorphism Y238X in rheumatoid arthritis. Arthritis Res. Ther. 12, R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salazar-Aldrete C., Galán-Díez M., Fernández-Ruiz E., Niño-Moreno P., Estrada-Capetillo L., Abud-Mendoza C., Layseca-Espinosa E., Baranda L., González-Amaro R. (2013) Expression and function of dectin-1 is defective in monocytes from patients with systemic lupus erythematosus and rheumatoid arthritis. J. Clin. Immunol. 33, 368–377 [DOI] [PubMed] [Google Scholar]
- 70. Lilly L. M., Gessner M. A., Dunaway C. W., Metz A. E., Schwiebert L., Weaver C. T., Brown G. D., Steele C. (2012) The β-glucan receptor Dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 189, 3653–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cortez-Espinosa N., García-Hernández M. H., Reynaga-Hernández E., Cortés-García J. D., Corral-Fernández N. E., Rodríguez-Rivera J. G., Bravo-Ramírez A., González-Amaro R., Portales-Pérez D. P. (2012) Abnormal expression and function of Dectin-1 receptor in type 2 diabetes mellitus with poor glycemic control (HbA1c>8%). Metabolism 61, 1538–1546 [DOI] [PubMed] [Google Scholar]