Abstract
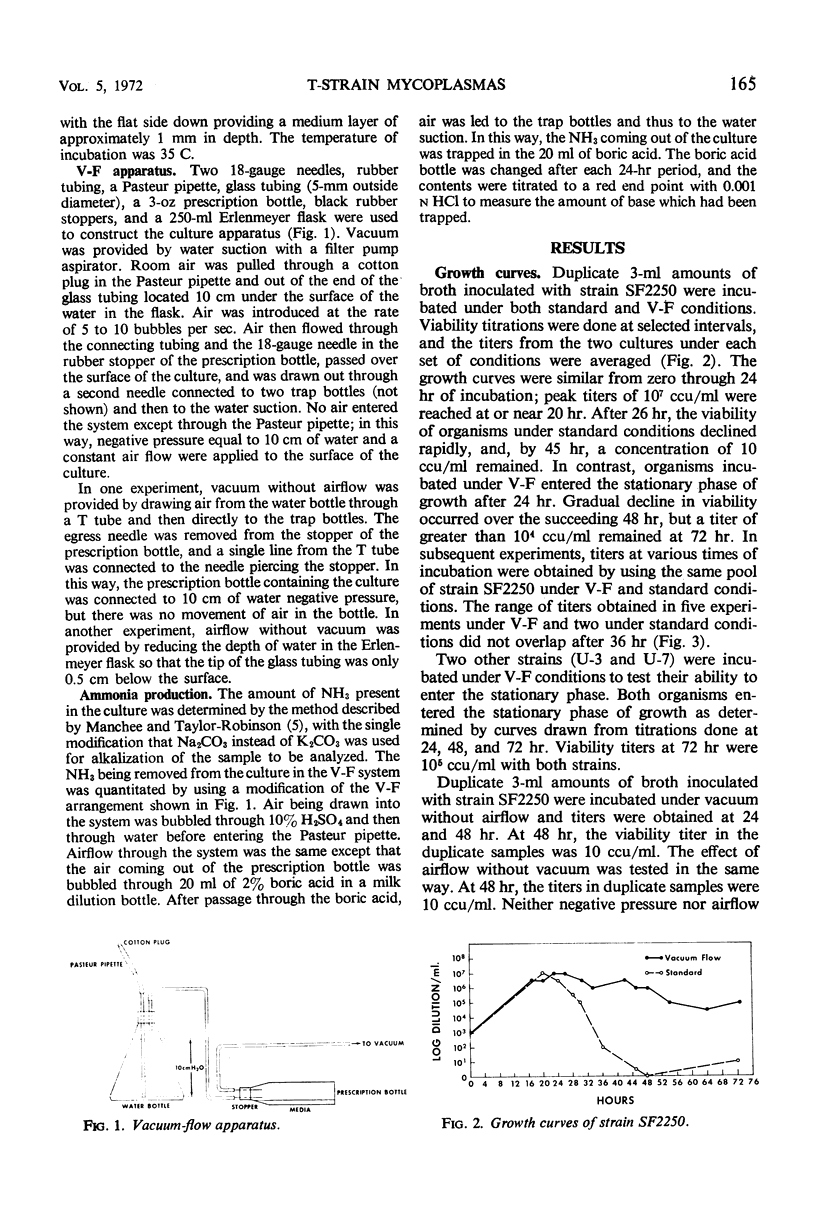
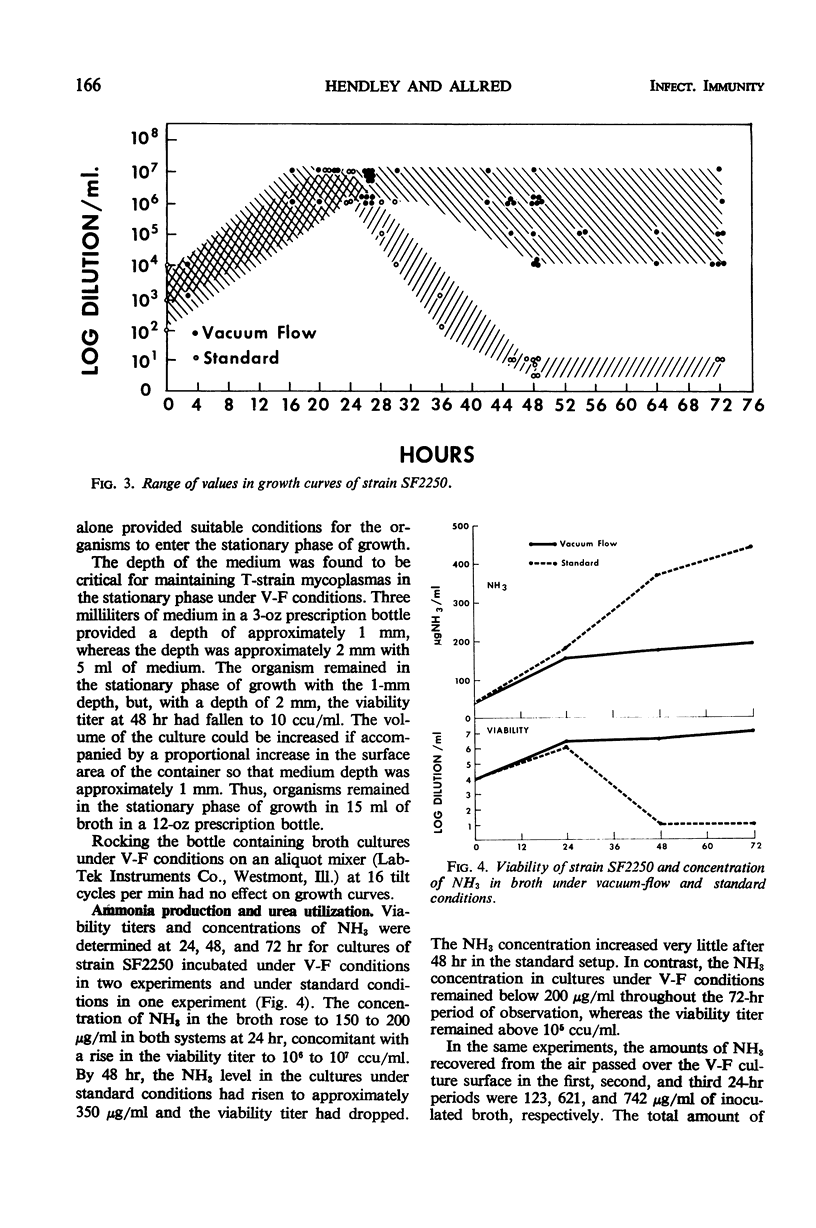
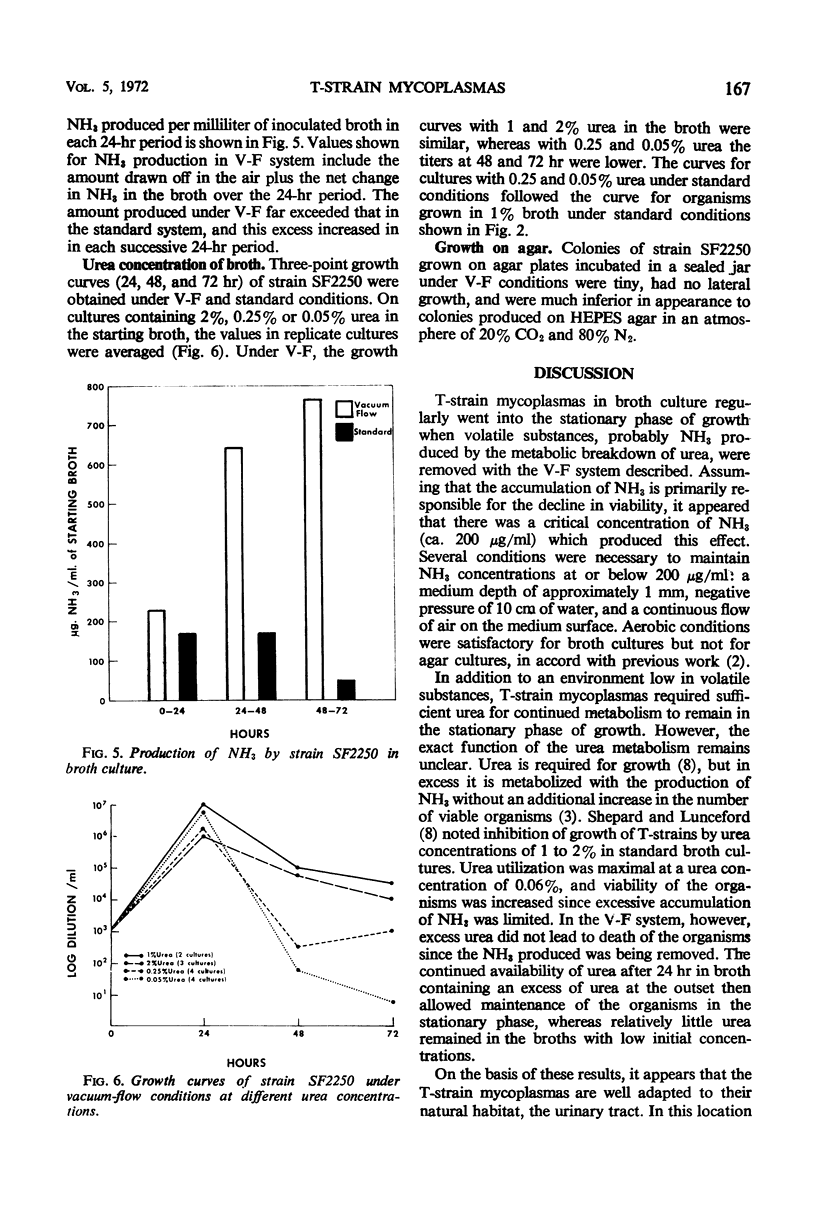
Growth of T-strain mycoplasmas in standard broth cultures has been characterized by rapid growth to peak titers of 106 to 107 color change units/ml at 20 to 24 hr, followed by a sharp decline in the viability over the next few hours. In a vacuum-flow (V-F) system utilizing negative pressure equal to 10 cm of water and air flow on the surface of broth, T strains were seen to enter the stationary phase of growth after 24 hr, and viability titers after 72 hr were ≥ 104 color change units/ml. Maintenance of organisms in the stationary phase required both vacuum and air flow, a medium depth of 1 mm, and 1% urea in the broth. Concentration of ammonia in broth cultures under V-F remained below 200 μg/ml during 72 hr of observation. Ammonia levels in standard broth cultures exceeded 200 μg/ml after 24 hr, coincident with the decline in viability. Air passed over the medium surface in the V-F system contained large amounts of ammonia; the amount increased during each succeeding 24-hr period of observation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun P., Klein J. O., Lee Y. H., Kass E. H. Methodologic investigations and prevalence of genital mycoplasmas in pregnancy. J Infect Dis. 1970 Apr;121(4):391–400. doi: 10.1093/infdis/121.4.391. [DOI] [PubMed] [Google Scholar]
- Ford D. K. CULTURE OF HUMAN GENITAL "T-STRAIN" PLEUROPNEUMONIA-LIKE ORGANISMS. J Bacteriol. 1962 Nov;84(5):1028–1034. doi: 10.1128/jb.84.5.1028-1034.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. K., MacDonald J. Influence of urea on the growth of T-strain mycoplasmas. J Bacteriol. 1967 May;93(5):1509–1512. doi: 10.1128/jb.93.5.1509-1512.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendley J. O., Jordan W. S., Jr Mycoplasma pharyngeal flora in civilians. Am Rev Respir Dis. 1968 Apr;97(4):524–532. doi: 10.1164/arrd.1968.97.4.524. [DOI] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Enhanced growth of T-strain mycoplasmas with N-2-hydroxyethylpiperazone-N'-2-ethanesulfonic acid buffer. J Bacteriol. 1969 Oct;100(1):78–85. doi: 10.1128/jb.100.1.78-85.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Effect of pH on the immunogenicity of Mycoplasma pneumoniae. J Bacteriol. 1969 Feb;97(2):612–619. doi: 10.1128/jb.97.2.612-619.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD M. C. The recovery of pleuropneumonia-like organisms from Negro men with and without nongonococcal urethritis. Am J Syph Gonorrhea Vener Dis. 1954 Mar;38(2):113–124. [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Occurrence of urease in T strains of Mycoplasma. J Bacteriol. 1967 May;93(5):1513–1520. doi: 10.1128/jb.93.5.1513-1520.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



