Abstract
Context:
Although epidemiological studies have found that GH and IGF-1 normalization reduce the excess mortality of active acromegaly to expected rates, cross-sectional data report some cardiovascular (CV) risk markers to be less favorable in remission than active acromegaly.
Objective:
The objective of the study was to test the hypothesis that remission of acromegaly after surgical therapy increases weight and adiposity and some CV risk markers and these changes are paralleled by a rise in ghrelin.
Design:
Forty-two adults with untreated, active acromegaly were studied prospectively. Changes in outcome measures from before to after surgery were assessed in 26 subjects achieving remission (normal IGF-1) and 16 with persistent active acromegaly (elevated IGF-1) after surgery.
Setting:
The study was conducted at tertiary referral centers for pituitary tumors.
Main Outcome Measures:
Endocrine, metabolic, and CV risk parameters, anthropometrics, and body composition by dual-energy X-ray absorptiometry were measured.
Results:
Remission increased total ghrelin, body weight, waist circumference, C-reactive protein, homocysteine, high-density lipoprotein, and leptin and reduced systolic blood pressure, homeostasis model assessment score, triglycerides, and lipoprotein (a) by 6 months and for 32 ± 4 months after surgery. The ghrelin rise correlated with the fall in the levels of GH, IGF-1, and insulin and insulin resistance. Weight, waist circumference, and ghrelin did not increase significantly in the persistent active acromegaly group. Total body fat, trunk fat, and perentage total body fat increased by 1 year after surgery in 15 remission subjects: the increase in body fat correlated with the rise in total ghrelin.
Conclusions:
Although most markers of CV risk improve with acromegaly remission after surgery, some markers and adiposity increase and are paralleled by a rise in total ghrelin, suggesting that these changes may be related. Understanding the mechanisms and long-term implications of the changes that accompany treatment of acromegaly is important to optimizing management because some aspects of the postoperative profile associate with the increased metabolic and CV risk in other populations.
Epidemiological data report that biochemical remission of acromegaly is associated with a reduction of its excess mortality, including that due to cardiovascular disease, to population expected rates (1). On a patient level, however, cross-sectional studies suggest that some factors associated with increased cardiovascular (CV) risk, including adiposity, may be greater after remission than before. In support of this less favorable profile, we have observed in our clinical care of patients entering remission after surgery that some experience a clinically relevant and undesirable weight gain and inability to lose weight. Conclusions that can be drawn from prior cross-sectional studies are limited by the many determinants of the clinical and serum markers associated with CV risk that make comparisons with control groups difficult. Therefore, we have prospectively studied a cohort of newly diagnosed acromegaly patients before and over time after surgery to determine the consequences of surgical remission on anthropometrics, body composition, and CV risk markers as well as the potential mechanisms for these changes. Because our prior pilot data suggested that ghrelin levels rise after surgery for acromegaly (2), we also chose to test the hypothesis that a potential link exists between postoperative increases in the orexigenic factor ghrelin and the anthropometric changes we hypothesized would occur with surgical remission.
Materials and Methods
Study subjects
We prospectively studied 42 subjects (24 males, 18 females) with newly diagnosed, untreated acromegaly. At enrollment, the mean age was 44.5 ± 1.67 years (range 19–80 y), and 37 had a macroadenoma and five a microadenoma. Acromegaly was diagnosed by an IGF-1 level above the age-adjusted normal range, nadir GH after oral glucose greater than 1 μg/L in 41 subjects (0.44 μg/L in one subject), clinical characteristics of acromegaly, and pathological confirmation of a GH-secreting pituitary tumor removed by transsphenoidal surgery. None received medical therapy for acromegaly prior to surgery or medical therapy, radiotherapy, or additional surgery during the study observation period. Two subjects had secondary adrenal insufficiency that resolved within 1 month postoperatively and one had primary thyroid insufficiency on stable T4 therapy.
In males, gonadal function as assessed by serum T levels pre-/postoperatively was as follows: normal/normal, seven subjects; low/normal, nine subjects; low/low normal, four subjects; low/normal on treatment, one subject; and low/low (primary hypogonadism not treated), one subject. In females menstrual history pre/postoperatively was as follows: regular menses/regular menses, eight subjects; amenorrhea/regular menses, two subjects; irregular menses/regular menses, one subject; postmenopausal, no replacement therapy, six subjects; amenorrhea/amenorrhea, one subject; and regular menses/postmenopausal, one subject. Before surgery, seven subjects had hyperlipidemia treated with lipid-lowering therapy that was unchanged after surgery in all but one subject in remission, who could discontinue therapy. Two subjects, one in remission and one who had persistent active disease, developed new hyperlipidemia and began therapy after surgery. Three patients had type 2 diabetes mellitus preoperatively. One entered remission after surgery and metformin was discontinued. Two had persistent active disease and remained on their presurgery diabetes therapies, insulin, and glipizide. Sixteen subjects had a history of hypertension treated with medication before surgery. After surgery three subjects in remission had improvement with antihypertensive discontinuation. One in remission developed new hypertension and started medication 2 years postoperatively.
All were ambulatory with normal renal function and no liver disease. The study was approved by the Institutional Review Board of Columbia University Medical Center. All subjects gave written informed consent before participation.
Study design
Subjects were studied before and 1, 3, 6, and 12 months and yearly after surgery. At every visit, subjects underwent blood sampling, after an overnight fast, for IGF-I, GH, insulin, glucose, total ghrelin, leptin, C-reactive protein (CRP), homocysteine (HCY), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and lipoprotein (a) levels. Additionally, at the preoperative, 3 month, 12 month, and yearly visits, GH, glucose, insulin, and ghrelin were measured at 60, 90, and 120 minutes after a 100-g oral glucose tolerance test (OGTT) (Trutol 100*). Samples were frozen at −80°C in multiple aliquots until assayed. At every visit, subjects had a physical examination including anthropometric measurements, body weight by a digital scale to the nearest 0.01 kg, height to the nearest 0.5 cm, and waist circumference, and a detailed history was obtained. Before and 1 year postoperatively, 15 subjects also underwent whole-body and regional body composition estimation by dual-energy X-ray absorptiometry (software version 11.4; Lunar DPX) as previously described (3).
Hormone assays
GH, IGF-1, insulin, and CRP were measured by chemiluminescent immunometric assay and HCY by competitive immunoassay from IMMULITE (Siemens). In our laboratory, functional sensitivity for GH was 0.05 μg/L, CRP was 0.3 mg/L, and HCY was 0.5 μmol/L. IGF-1 levels were compared with their age-appropriate normal ranges. Total plasma ghrelin was measured by RIA (Phoenix Pharmaceuticals) with an intraassay coefficient of variation of 8.54%, interassay coefficient of variation of 11.3%, and a lower limit of detection of 20 pg/mL; glucose by the hexokinase method; leptin by a human RIA kit (LINCO Research); lipoprotein (a) by immunoprecipitin analysis (DiaSorin); total cholesterol (TC), HDL, and TGs were determined by the enzymatic, colorimetric method (COBAS; INTEGRA). LDL was calculated using the Friedewald equation: [LDL = TC − HDL − TGs (0.2)].
Statistical analysis
For analysis, subjects were divided into a remission group whose IGF-1 level normalized by 3 months after surgery and stayed normal for the duration of follow-up and a persistent active disease group, whose IGF-1 level remained elevated after surgery. Variables were compared by ANOVA with P values corrected for multiple comparisons, after log transformation if non-normally distributed, in each group from preoperative to each postoperative time point or peak postoperatively as specified. Preoperative values were compared in both the remission and persistent active groups with changes within their respective group at 6 months after surgery except in four subjects in the active group whose 3-month data points, the time of their last study visit, were used to determine the mean values reported in Table 1 for the persistent active group. In the remission group only, preoperative values were also compared with those in the long-term follow up at yearly visits. Ghrelin, insulin, glucose, and GH values were also determined from the OGTTs at the baseline and 3-month visits in both the remission and active groups and also the 12-month and yearly visits for the remission group. A Spearman correlation analysis was used to assess the relationships between changes in IGF-1, area under the curve (AUC) GH during the OGTT, and insulin sensitivity to changes in ghrelin AUC postoperatively, and the relationship of change in ghrelin AUC to change in body composition parameters. The area under the ghrelin or GH OGTT curve was determined by the trapezoid rule. Data were analyzed with GraphPad Prism 6.0. The values of P < .05 were significant. P values represent the comparison of the given time point to the preoperative values unless specified otherwise. Data are given as mean ± SE.
Table 1.
Clinical, Hormonal, and Metabolic Characteristic Before and by 6 Months After Surgery in the Remission and Persistent Active Disease Groups
| Remission Group |
Persistent Active Group |
|||||
|---|---|---|---|---|---|---|
| Preoperatively | Postoperatively | P Value | Preoperatively | Postoperatively | P Value | |
| Age, y, mean (range) | 43.6 ± 2.1 (19–64) | 46 ± 4.7 (27–80) | ||||
| Gender (M/F) | 13/13 | 11/5 | ||||
| Macro/micro | 24/2 | 13/3 | ||||
| IGF-1, ng/mL | 782 ± 50 | 253 ± 11 | <.0001 | 828 ± 54 | 481 ± 45 | .0017 |
| IGF-1, ULN, % | 290 ± 22 | 79 ± 5 | <.001 | 323 ± 28 | 130 ± 23 | .001 |
| GH fasting, μg/L | 26 ± 9.3 | 0.97 ± 0.18 | <.0001 | 36 ± 10 | 5.1 ± 1.0 | .0001 |
| GH nadir OGTT, μg/L | 19.2 ± 5.8 | 0.41 ± 0.09 | <.0001 | 25 ± 7.2 | 2.7 ± 0.6 | .0001 |
| HOMA-IR scorea | 3.683 ± 0.48 | 1.469 ± 0.25 | <.0001 | 5.63 ± 1.62 | 3.72 ± 0.86 | .09 |
| CRP, mg/L | 0.4 ± 0.9 | 2.5 ± 0.68 | .002 | 0.67 ± 0.22 | 1.33 ± 0.78 | .94 |
| HCY, μmol/L | 7.36 ± 0.38 | 9.28 ± 0.46 | .003 | 7.72 ± 0.38 | 9.65 ± 0.68 | .008 |
| Leptin, ng/mL | 11.8 ± 2.3 | 16.4 ± 2.8 | .0007 | 8.8 ± 2.5 | 12 ± 3.0 | .004 |
| Lipoprotein (a), mg/dL | 39.5 ± 7.56 | 27.79 ± 5.9 | .004 | 51.7 ± 10.5 | 57.9 ± 19.7 | .62 |
| TC, mg/dL | 184 ± 7.5 | 185.7 ± 7 | .8 | 184 ± 6.4 | 173 ± 4.9 | .9 |
| LDL, mg/dL | 119 ± 6.8 | 120 ± 6.6 | .78 | 115 ± 5.9 | 108 ± 3.4 | .38 |
| HDL, mg/dL | 43.6 ± 2.85 | 51.5 ± 2.1 | .02 | 41.9 ± 3.99 | 47.8 ± 5 5 | .58 |
| TGs, mg/dL | 106 ± 11 | 86 ± 7.6 | .004 | 134 ± 21 | 124 ± 19 | .84 |
| Weight, kg | 88 ± 4.0 | 91 ± 4.3 | .009 | 94 ± 6 | 96 ± 6.6 | .22 |
| Waist circumference (cm) | 91.4 ± 2.89 | 96.5 ± 2.84 | .04 | 96.5 ± 2.54 | 99 ± 3.7 | .17 |
| Systolic BP, mm Hg | 126 ± 2.86 | 120 ± 2.15 | .04 | 122 ± 3.4 | 117 ± 2.9 | .03 |
| Diastolic BP, mm Hg | 82.39 ± 1.79 | 79.96 ± 1.66 | .3 | 77 ± 3.3 | 75.86 ± 2.3 | .8 |
Abbreviations: macro, macroadenoma; micro, microadenoma; ULN, upper limit of normal for age for IGF-1 level. Most values reported in this table were obtained 6 months postoperatively, but for four subjects in the persistent active group who were followed up for only 3 months, these values were used in calculation of the means reported for this group.
Values for GH fasting, nadir GH OGTT, and HOMA-IR were from the 3-month postoperative visit and OGTT in all subjects. HOMA-IR scores = [fasting serum insulin (microunits per milliliter) × fasting plasma glucose (millimoles per liter)/22.5). Normal IGF-I ranges are as follows: age 19 years,141–483 ng/mL; 20 years, 127–424 ng/mL; ages 21–25 years, 116–358 ng/mL; 26–30 years, 117–329 ng/mL; 31–35 years, 115–307 ng/mL; 36–40 years, 109–284 ng/mL; 41–45 years, 101–267 ng/mL; 46–50 years, 94–252 ng/mL; 51–55 years, 87–238 ng/mL; 56–60 years, 81–225 ng/mL; 61–65 years, 75–212 ng/mL; 66–70 years, 69–200 ng/mL; 76–80 years, 59–177 ng/mL.
Results
Duration of follow-up
Patients who achieved remission were followed up after surgery for a mean of 32.5 ± 4 months (range 6–84 mo); the follow-up duration was 6 months in one subject, 12 months in five subjects, 24 months in nine subjects, 36 months in five subjects, 48 months in two subjects, 60 months in two subjects, and 84 months in two subjects. Of those subjects with persistent active disease, four were followed up for 3 months and 12 were followed up for 6 months after surgery.
IGF-1 and GH
Twenty-six subjects achieved remission and 16 had persistent active acromegaly (Table 1). GH, fasting and OGTT nadir, and IGF-1 fell by 6 months after surgery in both groups (Table 1). In the remission group, IGF-1 levels remained normal at the last follow-up visit, 79% ± 3% of the upper limit of normal (range 40th to 93th percentile of the normal range for age).
Ghrelin
Fasting and nadir ghrelin levels and percentage ghrelin suppression after oral glucose increased after surgery in the remission group and not the persistent active group (Table 2). In the remission group, fasting, nadir and percentage suppression after oral glucose did not differ between the 3-month, 12-month or last follow-up visits. The ghrelin AUC also increased in the remission group from 39 998 ± 3255 before to 51 325 ± 6535 by 3 months after surgery (P < .0001) but did not significantly increase in the persistent active group, 40 705 ± 4312 vs 44 139 ± 4415 (P = .15).
Table 2.
Total Ghrelin Fasting Levels and Percentage Suppression and Nadir During the OGTT Before and After Surgery in the Remission and Persistent Active Groups
| Fasting Ghrelin, pg/mL |
Nadir Ghrelin, pg/mL |
Percentage Suppression |
||||
|---|---|---|---|---|---|---|
| P Valuea | P Valuea | P Valuea | ||||
| Remission group | ||||||
| Preoperative | 323 ± 30 | 265 ± 22 | 17 ± 2.4 | |||
| 3 months | 456 ± 48 | <.0001 | 318 ± 26 | <.0001 | 27 ± 2.9 | .003 |
| 12 months | 464 ± 45 | <.0001 | 304 ± 15 | <.0001 | 24 ± 2.1 | .03 |
| Last visit | 484 ± 45 | <.0001 | 313 ± 19 | <.0001 | 26 ± 1.4 | .007 |
| Persistent active group | ||||||
| Preoperative | 367 ± 40 | 306 ± 34 | 15.6 ± 2.7 | |||
| 3 months | 376 ± 33 | .54 | 328 ± 33 | .2 | 13.5 ± 2.4 | .14 |
Nadir ghrelin is the lowest ghrelin level achieved during the OGTT. Percentage suppression is the suppression from fasting to nadir during the OGTT.
P value represents the test of significance of that time point to the preoperative value.
In all subjects combined, the rise in ghrelin AUC from before to 3 months after surgery correlated with the reduction from before to 3 months after surgery in IGF-1 level (r = 0.331, P = .03) and the AUC of GH during the OGTT (r = 0.392, P = .032). The rise in ghrelin AUC at 3 months after surgery correlated with the fall in homeostasis model assessment (HOMA) score (r = 0.344, P = .03) and the fall in fasting insulin levels (r = 0.367, P = .02) at 3 months after surgery.
Cardiovascular risk markers
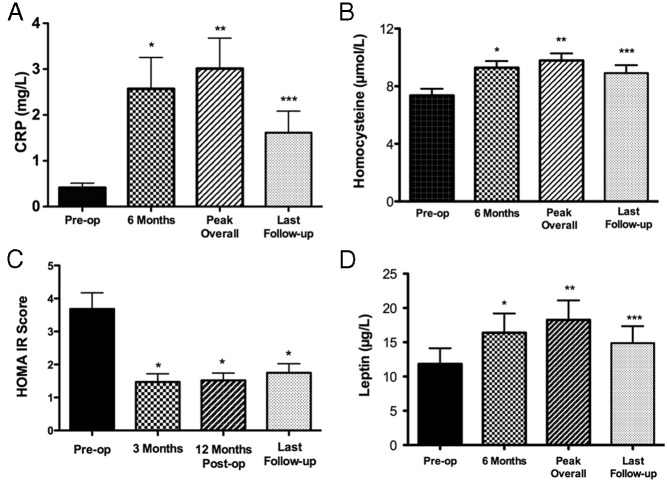
CRP levels rose after surgery in the remission but not the active group (Table 1). In the remission group, CRP peaked at 8.3 ± 1.9 months after surgery and remained higher than preoperatively in follow-up (Figure 1). Levels of HCY rose after surgery in both the remission and active groups (Table 1). In the remission group, HCY levels peaked at 10.6 ± 2.57 months after surgery and remained higher than before surgery levels in follow-up (Figure 1).
Figure 1.
CRP, HCY, HOMA-IR score, and leptin levels in the remission group before (preop.) and after surgery (postop.) at 6 months, overall peak, and at the last visit after surgery. CRP: at 6 months (*, P = .0001 vs preop); overall peak, 3.0 ± 0.66 mg/L (**, P < .0001 vs preop.); last follow-up, 1.613 ± 0.47 mg/L (***, P = .001 vs preop.). HCY: 6 months (*, P = .003 vs preop.); overall peak, 9.815 ± 0.486 μmol/L (**, P = .001 vs preop.); last visit, 8.8 ± 0.54 μmol/L (***, P = .004 vs preop.). HOMA-IR score: 3 months (*, P ≤ .0001 vs preop.); 12 months, 1.517 ± 0.22 (*, P = .0003 vs preop.); and last visit, 1.749 ± 0.27 (*, P = .0005 vs preop.) after surgery. Leptin: 6 months (*, P = .0007 vs preop.); overall peak, 18.3 ± 2.8 ng/ml (**, P = .0002 vs preop.); and last visit, 15 ± 2.5 (***, P = .04 vs preop.).
The HOMA index of insulin resistance (HOMA-IR) score fell significantly after surgery in the remission group (Table 1 and Figure 1). Fasting insulin levels in the remission group were 15.8 ± 1.7 μIU/mL before surgery, 6.68 ± 0.9 μIU/mL at 3 months (P < .0001), and 6.7 ± 0.7 μIU/mL at 12 months (P < .0001) and in the active group were 20.2 ± 4.2 μIU/mL before and 14.1 ± 2.2 μIU/mL (P = .06) after surgery.
TC and LDL cholesterol did not change after surgery in the remission or persistent active disease groups (Table 1). In the remission group, these levels were unchanged at 12 months after surgery, TC 185 ± 7.0 mg/dL (P = .82) and LDL 119 ± 6.4 mg/dL (P = .97), or at the last visit, TC 180 ± 7.5 mg/dL (P = .93) and LDL 108 ± 5.6 mg/dL (P = .36). HDL cholesterol rose after surgery in the remission group but not in the persistent active group (Table 1). In the remission group, HDL remained higher than presurgery levels at 12 months, 52 ± 2.3 mg/dL (P = .005), and at the last visit, 52.4 ± 2.15 mg/dL (P = .007).
TG levels fell in the remission group but not the persistent active group (Table 1). In the remission group, TG levels remained lower at 12 months, 75 ± 6.6 mg/dL (P = .0005), and at the last follow-up visit, 87 ± 12 mg/dL (P = .01). Lipoprotein (a) levels fell in the remission group after surgery (Table 1) and were 29.9 ± 8 mg/dL (P = .07) at the last study visit.
Systolic blood pressure (BP) was lower in both remission and persistent active diseases groups postoperatively (Table 1). In the remission group, systolic BP remained lower than before surgery at 1 year, 118 ± 2.3 mm Hg (P = .05), and was 119 ± 2.5 (P = .07) at the last study visit. Diastolic BP was not lower 6 months after surgery in either group but in the remission group was 78 ± 1.6 mm Hg (P = .03) 1 year after surgery and 79 ± 1.8 mm Hg (P = .36) at the last study visit.
Anthropometrics, body composition, and leptin
In the subjects overall, weight increased from 90.4 ± 3.4 kg before surgery to a peak of 93 ± 3.6 kg by 6 months (P = .001), an overall peak of 94 ± 3.7 kg (P = .0001) and 92.5 ± 3.6 kg (P = .006) at the last visit after surgery. In the remission group, weight increased in 22 of 26 and was 88 ± 4 kg before surgery, 91 ± 4.3 kg by 6 months (P = .009), 92.3 ± 4.5 kg at overall peak (P = .0001), and 93.7 ± 4.62 kg (P = .0036) at the last visit after surgery. Peak weight occurred at 21 ± 2.98 months after surgery. In the persistent active group, weight increased in 8 of 16 subjects and was 94 ± 6.6 kg before and 96 ± 6.6 kg by 6 months after surgery (P = .22) (Table 1).
Waist circumference increased in the remission but not significantly in the persistent active group (Table 1). Waist circumference increased in 21 of 26 of the remission and 10 of 16 in the active group. In the remission group, waist circumference remained greater at the last visit, 97.8 ± 3.5 cm, than before surgery (P = .003) .
Assessment of body composition by dual-energy X-ray absorptiometry before and 1 year after surgery in 15 subjects in the remission group demonstrated a significant increase in adiposity (Figure 2). The increase in percentage body fat correlated with the increase in ghrelin AUC during the OGTT (r = 0.576, P = .031) (Figure 3).
Figure 2.
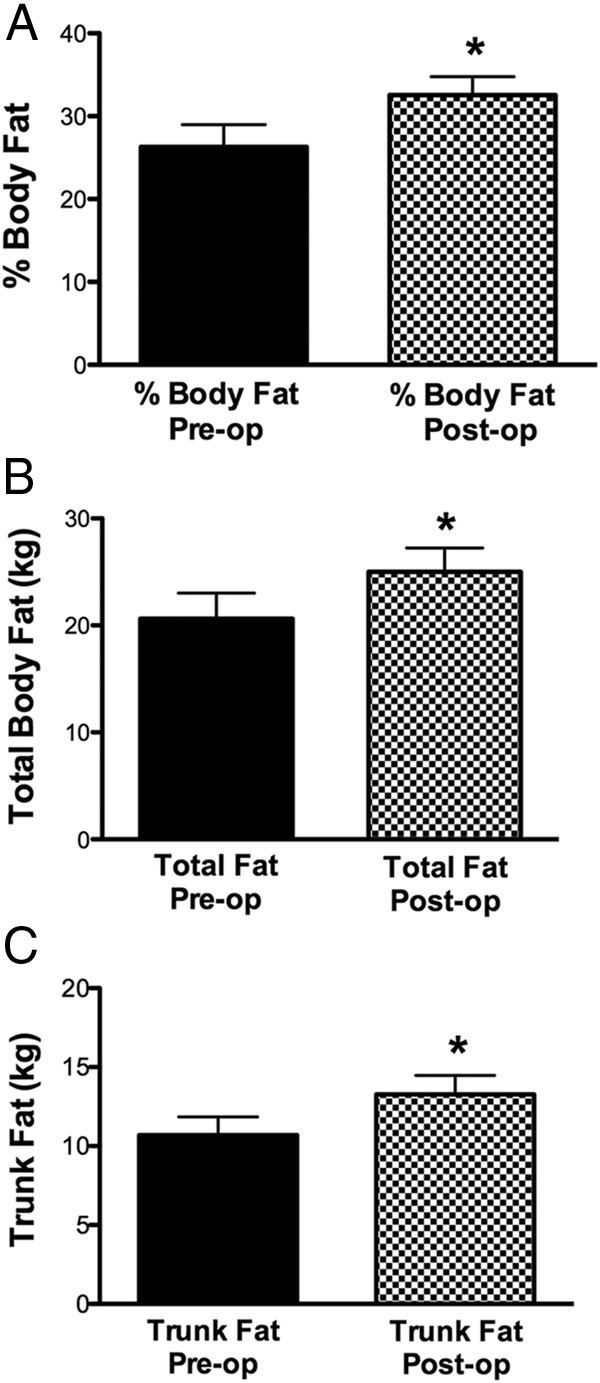
Percentage body fat, total body fat mass, and trunk fat mass before (preop) and 1 year after surgical remission (postop) in 15 patients with acromegaly. Preop vs postop were for body fat mass, 20.62 ± 2.397 kg vs 25.02 ± 2.2 kg (*, P = .0001); trunk fat mass, 10.69 ± 1.12 kg vs 13.27 ± 1.2 kg (*, P = .0001); total body percentage fat, 26.3% ± 2.7% vs 32.56% ± 2.2% (*, P < .0001).
Figure 3.
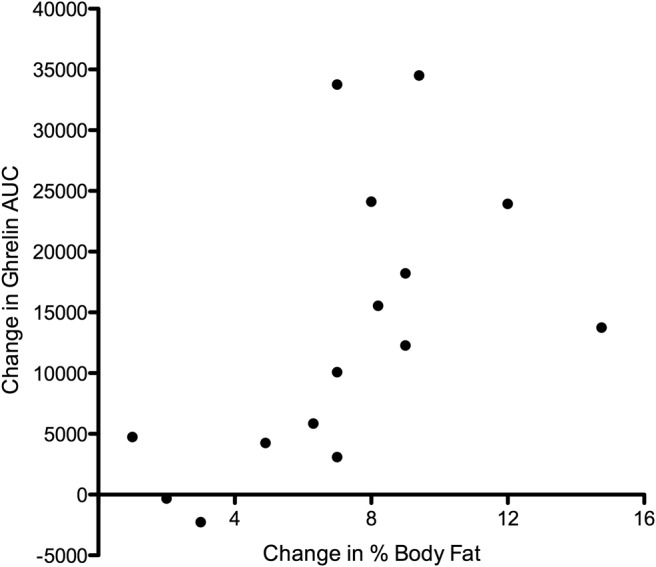
Correlation of the rise in percentage body fat after surgery in 15 patients in the remission group with the rise in AUC of total ghrelin during the OGTT by 1 year after surgery (r = 0.576, P = .031).
Leptin levels rose in both remission and persistent active disease groups after surgery (Table 1). In the remission group, leptin levels remained similar in follow-up to levels in the first 6 months (Table 1), peaking at 14 ± 2.9 months (range 3–60 mo) after surgery (Figure 1). In the subjects overall, leptin levels correlated with body mass index (BMI) both before (r = 0.443, P = .003) and after surgery (r = 0.494, P = .0009).
Fasting ghrelin and BMI did not correlate preoperatively (r = −0.162, P = .31) but did correlate negatively after surgery (r = −0.306, P = .048).
Discussion
In this prospective study, we have found that when patients with acromegaly achieve biochemical remission after surgical therapy, they develop a less favorable anthropometic and body composition profile and reductions in many but increases in other CV risk markers. These changes are paralleled by a rise in plasma ghrelin, raising the possibility that the rise in ghrelin contributes to the increase in adiposity that accompanies acromegaly remission.
In our study, patients with acromegaly who achieve long-term remission after surgery as their only therapy have increases in weight, waist circumference, and adiposity. Some prior retrospective and cross-sectional data support our findings, reporting BMI to be higher in patients in remission years after surgery compared with prior or surgery (4) and compared with controls (5, 6). However, in other studies, BMI did not increase 6–12 months after surgery (7, 8) and was not higher in remission than active disease patients (9). In our study, waist circumference increased with remission as did total body fat and trunk fat in 15 of these subjects, consistent with increasing central adiposity. Increases in waist circumference with remission after surgery has been shown in some but not all cross-sectional and retrospective studies (4, 6). In a cross-sectional study, percentage body fat was higher in remission than active acromegaly or controls (10), and in other studies fat mass increased by 6–12 months after surgery (8, 11, 12). Consistent with the increase in fat mass, we found a rise in leptin levels after surgery. Others have also found lower leptin levels in active acromegaly (13), but prior data conflict as to whether leptin changes may (14) or may not be explained by fat mass changes (15, 16). The forces driving increases in weight and adiposity after remission of acromegaly may be the lowering of the elevated resting energy expenditure and lipolysis rates of active acromegaly as GH excess is reduced (17), but we also investigated the relationship of these changes to those of the orexigenic factor, ghrelin.
We demonstrate, prospectively, a clear rise in plasma ghrelin that occurs within 3 months of remission of acromegaly after surgery and is sustained long term. Other cross-sectional studies have found total ghrelin and ghrelin AUC during a OGTT to be lower in active than inactive acromegaly (14, 18). In a longitudinal study, total ghrelin was lower in active acromegaly but had not yet recovered 7 days postoperatively (19), and our earlier data showed a rise in nine patients within the first year after surgery (2).
The factors that reduce circulating ghrelin levels in active acromegaly and those that account for the rise after surgery are not clear. In our study, the ghrelin rise correlated with the fall in GH and IGF-1, suggesting that GH excess could suppress ghrelin secretion. However, although ghrelin clearly stimulates pituitary GH secretion (20), evidence for a feedback of GH or its excess to directly suppress ghrelin secretion is sparse. In rodents, gastric ghrelin mRNA expression and plasma levels were decreased by GH (21, 22), but in transgenic mouse models of GH excess or blockade, these were not affected (23). Administration of high-dose GH has been shown to suppress ghrelin levels in humans (24). It has also been proposed that ghrelin levels may be suppressed in acromegaly by hyperinsulinemia and the rise after therapy may be due to insulin lowering because lower ghrelin levels in acromegaly have correlated with greater insulin resistance (18). In other populations, insulin and ghrelin levels correlate negatively (25) and insulin has been shown to suppress ghrelin (26–28). In our study the postoperative rise in ghrelin correlates with the fall in insulin resistance as measured by HOMA and fasting insulin levels. Ghrelin changes in acromegaly may also be due to changes in free fatty acids (FFA) levels because some but not all data suggest that FFAs suppress ghrelin (29, 30). FFAs are elevated in active acromegaly and could suppress ghrelin, and as FFAs fall with remission, ghrelin levels could rise (31). A limitation to our study is that we measured only total ghrelin because the differential effects of acylated vs unacylated ghrelin levels with regard to metabolic parameters have been reported (32, 33).
Our data also suggest a possible link between the ghrelin rise and the anthropometric changes that occur after acromegaly remission. Paralleling the rise in ghrelin levels after surgery were increases in weight, waist circumference, and adiposity and the rise in ghrelin correlated with the rise in body fat. It is known that active acromegaly is a state of increased lipolysis and energy expenditure, and the switch after remission to a state of lipogenesis and decreased energy expenditure (17, 31) could in part reflect accompanying ghrelin effects. In rodents, ghrelin induces lipogenesis, reduces fat utilization, and promotes weight gain (34–36). A rodent model of GH overexpression had increased energy expenditure and resisted diet-induced obesity (37), as could be seen in a reduced ghrelin model. In humans, ghrelin increased food intake (38) and promoted weight gain in patients with cancer-associated cachexia (39). In support of this association, GH administration to deficient patients lowered total ghrelin levels, which correlated with a reduction in body fat and leptin levels (40), the pattern opposite to acromegaly subjects achieving remission. Although a rise in ghrelin with increasing fat mass after surgery may seem paradoxical, active acromegaly is a state of ghrelin dysregulation, which may be reset to the expected negative correlations between ghrelin levels and BMI after surgical remission as we have seen.
A limitation of our study is that we did not test whether appetite stimulation vs peripheral effects of ghrelin on adipose tissue occurred, and our study was limited to measuring peripheral ghrelin levels when it is centrally acting ghrelin that seems most important to mediating its effects on appetite (41). Our finding of a correlation between rise in ghrelin and adiposity should be considered preliminary because this was investigated in only a relatively small number of subjects, and the results could have been influenced by large changes in a few subjects. However, further investigation into this novel potential association of the postoperative rise in ghrelin, we hypothesize via stimulation of appetite, reduction in lipolysis, and/or energy expenditure, with the postoperative weight gain and adiposity that occurs after acromegaly remission is warranted.
We have also demonstrated, prospectively, that some serum markers of CV risk rise after surgical remission along with the increase in weight and adiposity. CRP levels rose and remained above preoperative levels in long-term follow-up. In cross-sectional studies, CRP was higher in controlled than active acromegaly (5, 42) or controls (42). An inverse relationship between levels of CRP and GH has been shown with treatment of GH deficiency and other treatments for acromegaly (43), but the cause of this is unclear. CRP could be reduced in acromegaly because GH suppresses the hepatic acute-phase response of CRP through cross talk of cytokine signaling or through reduced fat mass production of IL-6, an important stimulator of CRP (44, 45). Whether this rise in CRP marks some increased inflammation and CV risk (46) in acromegaly is unknown. We also found a rise in HCY, also an independent marker of CV risk, in both remission and active disease groups after surgery, which is in contrast to cross-sectional data that found them comparable in active acromegaly and controls (42). GH administration has been shown to reduce HCY levels (47), but medical treatment of acromegaly has not been found to increase them (43).
Many of the other major parameters associated with cardiovascular risk, however, were lowered after surgery. Systolic blood pressure was lowered after surgery in our study and another study (7). Diastolic BP was reduced in another study (7), but we found it to be lowered only at 12 months after surgery. We also found, in the remission group, sustained reductions in lipoprotein (a) and TGs as well as a rise in HDL but no changes in TC or LDL after surgery. Cross-sectional studies have found lipoprotein (a) (48, 49) and TG (5) levels to be higher in active acromegaly vs controls, but TC, HDL, and LDL have generally not differed by disease status (5, 42, 48, 49). Similar to our results, another prospective study found lipoprotein(a) and TGs, but not TC, to decrease after surgery (50). Our study found, as have others, a reduction in insulin resistance with successful surgical therapy of acromegaly (7). Insulin resistance is likely to be an important contributor to the increased CV risk of active acromegaly. Insulin resistance did not deteriorate after the initial improvement postoperatively despite increasing weight and adiposity with time after surgery. Although based on epidemiological studies remission must correct the cause of increased CV mortality in active acromegaly, how the changes in favorable vs unfavorable characteristics after remission balance in the long term for treated acromegaly patients is unknown. Because the markers that improved such as insulin resistance, HDL, and systolic BP have strong associations with CV risk, the profile after surgery seems most consistent with the overall reduction of the CV risk. In cross-sectional data, the Framingham score was increased in active vs controlled acromegaly and controls (42). Reassuring data from the prospective follow-up of a small number of patients, across a spectrum of disease, showed no reduction in Framingham score but also no increase in CV events over time (51).
Importantly, even though changes in many of hormonal and clinical parameters measured in this study did not reach statistical significance in the persistent active group, it is likely that these changes fall along a spectrum of those due GH and IGF-1 reduction with surgery. Although we divided our subjects into remission and active groups to present changes that can occur with remission, even partial surgical resection may be accompanied by clinically relevant changes in metabolic, clinical, and CV risk markers. With a larger number of subjects in the persistent active group, significant changes in some of these measures may have been observed.
In conclusion, our prospective study found increases in weight and central adiposity after surgery for acromegaly along with reductions in many, but not all, markers of CV risk. Ghrelin levels rose in parallel with the less favorable anthropometric profile, novel evidence of a possible relationship of ghrelin to the increase in adiposity that follows surgical treatment of acromegaly. The increase in weight and adiposity is a clinically recognizable problem for many successfully treated patients. In an individual patient with acromegaly, it is unknown whether the changes represent a readjustment to the anthropometric and CV risk profile they would have were they not to have acromegaly. Further investigation into the mechanisms of these changes and their long-term implications is warranted.
Acknowledgments
This study was registered with the clinical trials with the number of NCT01809808.
This work was supported by National Institutes of Health Grants R01 DK 064720 and K24 DK 073040 (to P.U.F.) and in part by the Columbia University Clinical and Translational Science Awards Grant UL1 RR 024156 from the National Center for Research Resources/National Institutes of Health. C.C. was supported by the Doris Duke Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- BP
- blood pressure
- CRP
- C-reactive protein
- CV
- cardiovascular
- FFA
- free fatty acid
- HCY
- homocysteine
- HDL
- high-density lipoprotein
- HOMA
- homeostasis model assessment
- HOMA-IR
- HOMA index of insulin resistance
- LDL
- low-density lipoprotein
- OGTT
- oral glucose tolerance test
- TC
- total cholesterol
- TG
- triglyceride.
References
- 1. Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89(2):667–674. [DOI] [PubMed] [Google Scholar]
- 2. Freda PU, Reyes CM, Conwell IM, Sundeen RE, Wardlaw SL. Serum ghrelin levels in acromegaly: effects of surgical and long-acting octreotide therapy. J Clin Endocrinol Metab. 2003;88(5):2037–2044. [DOI] [PubMed] [Google Scholar]
- 3. Freda PU, Shen W, Reyes-Vidal CM, et al. Skeletal muscle mass in acromegaly assessed by magnetic resonance imaging and dual-photon x-ray absorptiometry. J Clin Endocrinol Metab. 2009;94(8):2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ronchi CL, Varca V, Beck-Peccoz P, et al. Comparison between six-year therapy with long-acting somatostatin analogs and successful surgery in acromegaly: effects on cardiovascular risk factors. J Clin Endocrinol Metab. 2006;91(1):121–128. [DOI] [PubMed] [Google Scholar]
- 5. Boero L, Manavela M, Merono T, Maidana P, Gomez Rosso L, Brites F. GH levels and insulin sensitivity are differently associated with biomarkers of cardiovascular disease in active acromegaly. Clin Endocrinol (Oxf). 2012;77(4):579–585. [DOI] [PubMed] [Google Scholar]
- 6. Dimopoulou C, Sievers C, Wittchen HU, et al. Adverse anthropometric risk profile in biochemically controlled acromegalic patients: comparison with an age- and gender-matched primary care population. Pituitary. 2010;13(3):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaffrain-Rea ML, Minniti G, Moroni C, et al. Impact of successful transsphenoidal surgery on cardiovascular risk factors in acromegaly. Eur J Endocrinol. 2003;148(2):193–201. [DOI] [PubMed] [Google Scholar]
- 8. Brummer RJ, Lonn L, Kvist H, Grangard U, Bengtsson BA, Sjostrom L. Adipose tissue and muscle volume determination by computed tomography in acromegaly, before and 1 year after adenomectomy. Eur J Clin Invest. 1993;23(4):199–205. [DOI] [PubMed] [Google Scholar]
- 9. Serri O, Beauregard C, Hardy J. Long-term biochemical status and disease-related morbidity in 53 postoperative patients with acromegaly. J Clin Endocrinol Metab. 2004;89(2):658–661. [DOI] [PubMed] [Google Scholar]
- 10. Sucunza N, Barahona MJ, Resmini E, et al. Gender dimorphism in body composition abnormalities in acromegaly: males are more affected than females. Eur J Endocrinol. 2008;159(6):773–779. [DOI] [PubMed] [Google Scholar]
- 11. Landin K, Petruson B, Jakobsson KE, Bengtsson BA. Skeletal muscle sodium and potassium changes after successful surgery in acromegaly: relation to body composition, blood glucose, plasma insulin and blood pressure. Acta Endocrinol (Copenh). 1993;128(5):418–422. [DOI] [PubMed] [Google Scholar]
- 12. Tominaga A, Arita K, Kurisu K, et al. Effects of successful adenomectomy on body composition in acromegaly. Endocr J. 1998;45(3):335–342. [DOI] [PubMed] [Google Scholar]
- 13. Ronchi CL, Corbetta S, Cappiello V, et al. Circulating adiponectin levels and cardiovascular risk factors in acromegalic patients. Eur J Endocrinol. 2004;150(5):663–669. [DOI] [PubMed] [Google Scholar]
- 14. Roemmler J, Otto B, Steffin B, Bidlingmaier M, Schopohl J. Serum leptin and ghrelin levels in active and inactive acromegalic patients during an oral glucose tolerance test. Exp Clin Endocrinol Diabetes. 2009;117(3):135–141. [DOI] [PubMed] [Google Scholar]
- 15. Miyakawa M, Tsushima T, Murakami H, Isozaki O, Demura H, Tanaka T. Effect of growth hormone (GH) on serum concentrations of leptin: study in patients with acromegaly and GH deficiency. J Clin Endocrinol Metab. 1998;83(10):3476–3479. [DOI] [PubMed] [Google Scholar]
- 16. Damjanovic SS, Petakov MS, Raicevic S, et al. Serum leptin levels in patients with acromegaly before and after correction of hypersomatotropism by trans-sphenoidal surgery. J Clin Endocrinol Metab. 2000;85(1):147–154. [DOI] [PubMed] [Google Scholar]
- 17. O'Sullivan AJ, Kelly JJ, Hoffman DM, Freund J, Ho KK. Body composition and energy expenditure in acromegaly. J Clin Endocrinol Metab. 1994;78(2):381–386. [DOI] [PubMed] [Google Scholar]
- 18. Cappiello V, Ronchi C, Morpurgo PS, et al. Circulating ghrelin levels in basal conditions and during glucose tolerance test in acromegalic patients. Eur J Endocrinol. 2002;147(2):189–194. [DOI] [PubMed] [Google Scholar]
- 19. Kawamata T, Inui A, Hosoda H, Kangawa K, Hori T. Perioperative plasma active and total ghrelin levels are reduced in acromegaly when compared with in nonfunctioning pituitary tumours even after normalization of serum GH. Clin Endocrinol (Oxf). 2007;67(1):140–144. [DOI] [PubMed] [Google Scholar]
- 20. Arvat E, Di Vito L, Broglio F, et al. Preliminary evidence that ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23(8):493–495. [DOI] [PubMed] [Google Scholar]
- 21. Lee HM, Wang G, Englander EW, Kojima M, Greeley GH., Jr Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143(1):185–190. [DOI] [PubMed] [Google Scholar]
- 22. Qi X, Reed J, Englander EW, Chandrashekar V, Bartke A, Greeley GH., Jr Evidence that growth hormone exerts a feedback effect on stomach ghrelin production and secretion. Exp Biol Med (Maywood). 2003;228(9):1028–1032. [DOI] [PubMed] [Google Scholar]
- 23. Nass R, Liu J, Hellmann P, et al. Chronic changes in peripheral growth hormone levels do not affect ghrelin stomach mRNA expression and serum ghrelin levels in three transgenic mouse models. J Neuroendocrinol. 2004;16(8):669–675. [DOI] [PubMed] [Google Scholar]
- 24. Vestergaard ET, Dall R, Lange KH, Kjaer M, Christiansen JS, Jorgensen JO. The ghrelin response to exercise before and after growth hormone administration. J Clin Endocrinol Metab. 2007;92(1):297–303. [DOI] [PubMed] [Google Scholar]
- 25. Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. [DOI] [PubMed] [Google Scholar]
- 26. Flanagan DE, Evans ML, Monsod TP, et al. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab. 2003;284(2):E313–E316. [DOI] [PubMed] [Google Scholar]
- 27. Mohlig M, Spranger J, Otto B, Ristow M, Tschop M, Pfeiffer AF. Euglycemic hyperinsulinemia, but not lipid infusion, decreases circulating ghrelin levels in humans. J Endocrinol Invest. 2002;25(11):RC36–R38. [DOI] [PubMed] [Google Scholar]
- 28. Saad MF, Bernaba B, Hwu CM, et al. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002;87(8):3997–4000. [DOI] [PubMed] [Google Scholar]
- 29. Gormsen LC, Nielsen C, Gjedsted J, et al. Effects of free fatty acids, growth hormone and growth hormone receptor blockade on serum ghrelin levels in humans. Clin Endocrinol (Oxf). 2007;66(5):641–645. [DOI] [PubMed] [Google Scholar]
- 30. Vestergaard ET, Hansen TK, Nielsen S, Moller N, Christiansen JS, Jorgensen JO. Effects of GH replacement therapy in adults on serum levels of leptin and ghrelin: the role of lipolysis. Eur J Endocrinol. 2005;153(4):545–549. [DOI] [PubMed] [Google Scholar]
- 31. Moller N, Schmitz O, Joorgensen JO, et al. Basal- and insulin-stimulated substrate metabolism in patients with active acromegaly before and after adenomectomy. J Clin Endocrinol Metab. 1992;74(5):1012–1019. [DOI] [PubMed] [Google Scholar]
- 32. Barazzoni R, Zanetti M, Ferreira C, et al. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(10):3935–3940. [DOI] [PubMed] [Google Scholar]
- 33. St-Pierre DH, Karelis AD, Coderre L, et al. Association of acylated and nonacylated ghrelin with insulin sensitivity in overweight and obese postmenopausal women. J Clin Endocrinol Metab. 2007;92(1):264–269. [DOI] [PubMed] [Google Scholar]
- 34. Thompson NM, Gill DA, Davies R, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145(1):234–242. [DOI] [PubMed] [Google Scholar]
- 35. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. [DOI] [PubMed] [Google Scholar]
- 36. Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(11):2540–2547. [DOI] [PubMed] [Google Scholar]
- 37. Olsson B, Bohlooly- YM, Fitzgerald SM, et al. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology. 2005;146(2):920–930. [DOI] [PubMed] [Google Scholar]
- 38. Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86(12):5992. [DOI] [PubMed] [Google Scholar]
- 39. Lundholm K, Gunnebo L, Korner U, et al. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer. 2010;116(8):2044–2052. [DOI] [PubMed] [Google Scholar]
- 40. Eden Engstrom B, Burman P, Holdstock C, Karlsson FA. Effects of growth hormone (GH) on ghrelin, leptin, and adiponectin in GH-deficient patients. J Clin Endocrinol Metab. 2003;88(11):5193–5198. [DOI] [PubMed] [Google Scholar]
- 41. Kirchner H, Heppner KM, Tschop MH. The role of ghrelin in the control of energy balance. Handb Exp Pharmacol. 2012(209):161–184. [DOI] [PubMed] [Google Scholar]
- 42. Vilar L, Naves LA, Costa SS, Abdalla LF, Coelho CE, Casulari LA. Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr Pract. 2007;13(4):363–372. [DOI] [PubMed] [Google Scholar]
- 43. Sesmilo G, Fairfield WP, Katznelson L, et al. Cardiovascular risk factors in acromegaly before and after normalization of serum IGF-I levels with the GH antagonist pegvisomant. J Clin Endocrinol Metab. 2002;87(4):1692–1699. [DOI] [PubMed] [Google Scholar]
- 44. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. [DOI] [PubMed] [Google Scholar]
- 45. Derfalvi B, Igaz P, Fulop KA, Szalai C, Falus A. Interleukin-6-induced production of type II acute phase proteins and expression of junB gene are downregulated by human recombinant growth hormone in vitro. Cell Biol Int. 2000;24(2):109–114. [DOI] [PubMed] [Google Scholar]
- 46. Albert MA, Ridker PM. The role of C-reactive protein in cardiovascular disease risk. Curr Cardiol Rep. 1999;1(2):99–104. [DOI] [PubMed] [Google Scholar]
- 47. Sesmilo G, Biller BM, Llevadot J, et al. Effects of growth hormone (GH) administration on homocyst(e)ine levels in men with GH deficiency: a randomized controlled trial. J Clin Endocrinol Metab. 2001;86(4):1518–1524. [DOI] [PubMed] [Google Scholar]
- 48. Maldonado Castro GF, Escobar-Morreale HF, et al. Effects of normalization of GH hypersecretion on lipoprotein(a) and other lipoprotein serum levels in acromegaly. Clin Endocrinol (Oxf). 2000;53(3):313–319. [DOI] [PubMed] [Google Scholar]
- 49. Wildbrett J, Hanefeld M, Fucker K, et al. Anomalies of lipoprotein pattern and fibrinolysis in acromegalic patients: relation to growth hormone levels and insulin-like growth factor I. Exp Clin Endocrinol Diabetes. 1997;105(6):331–335. [DOI] [PubMed] [Google Scholar]
- 50. Oscarsson J, Wiklund O, Jakobsson KE, Petruson B, Bengtsson BA. Serum lipoproteins in acromegaly before and 6–15 months after transsphenoidal adenomectomy. Clin Endocrinol (Oxf). 1994;41(5):603–608. [DOI] [PubMed] [Google Scholar]
- 51. Kawanami D, Maemura K, Takeda N, et al. C-reactive protein induces VCAM-1 gene expression through NF-κB activation in vascular endothelial cells. Atherosclerosis. 2006;185(1):39–46. [DOI] [PubMed] [Google Scholar]